Abstract
Postprandial hyperglycemia due to paradoxical hyperglucagonemia is a major challenge of diabetes treatment despite the use of the artificial pancreas. We postulated that adjunctive therapy with pramlintide or exenatide would attenuate hyperglycemia in the postprandial phase through glucagon suppression, thereby optimizing the functioning of the closed-loop (CL) system. Subjects with type 1 diabetes (T1DM) on insulin pump therapy were recruited to participate in a 27-hour hospitalized admission on 3 occasions (2-4 weeks apart) and placed on the insulin delivery via CL system in random order to receive (1) insulin alone (control), (2) exenatide 2.5 µg + insulin, (3) pramlintide 30 µg + insulin. Medications were given prior to lunch and dinner, which was a standardized meal of 60 grams of carbohydrates. Insulin delivery was as per the ePID algorithm via the Medtronic CL system and continuous subcutaneous glucose monitoring via Medtronic Sof-sensors. Ten subjects age 23 ± 1 years with a HbA1c of 7.29 ± 0.3% (56 ± 1 mmol/mol) and duration of T1DM 10.6 ± 2.0 years participated in the 3-part study. Exenatide was found to be significantly better in attenuating postprandial hyperglycemia as compared to insulin monotherapy (P < .03) and pramlintide (P > .05). Glucagon suppression was statistically significant with exenatide (P < .03) as compared to pramlintide. Insulin requirements were lower with adjunctive therapy, but statistically insignificant. Insulin monotherapy results in postprandial hyperglycemia in T1DM in the CL setting and adjunctive therapy with exenatide reduces postprandial hyperglycemia effectively and should be considered as adjunctive therapy in T1DM.
Keywords: artificial pancreas, closed loop, ePID algorithm, exenatide, pramlintide, type 1 diabetes
Artificial pancreas closed-loop (CL) systems utilize continuous glucose monitoring in subcutaneous interstitial tissue and controller algorithms to deliver insulin in a glucose responsive manner in real time and aims to have postprandial glucose concentrations that are comparable to those of healthy controls. Nocturnal hypoglycemia is improved with this system, which was shown previously,1,2 and also recently in diabetes camp setting.3 Despite advancements in these systems, postprandial hyperglycemia continues to be a challenge.4-6
T1DM management in the open-loop or CL setting relies mainly on insulin delivery to normalize glucose concentrations. In diabetes, there is failure of glucagon suppression in the immediate postprandial period coupled with the failure of glucagon response to hypoglycemia in the late postprandial period. This may be one of the reasons for blood glucose fluctuations seen in T1DM.7 With adjunctive therapy using glucagon suppressors such as pramlintide, an amylin analogue, we and others have shown that postprandial hyperglycemia is attenuated. However, our studies showed that pramlintide resulted in a right shift of the glucose curve without adequate reduction in peak glucose concentrations. Furthermore, if higher doses of pramlintide were used with insulin, it caused immediate postprandial hypoglycemia.7,8
In this study, we aimed to test 2 glucagon suppressors in the CL system and determine which one would be most effective in attenuating postprandial glucose excursions. Pramlintide is an analogue of amylin and is approved for use in subjects with type 1 and type 2 diabetes of 16 years and above. Exenatide is a short acting glucagon-like peptide-1 (GLP-1) receptor agonist used extensively for the treatment of type 2 diabetes as mono and adjuvant therapy with oral hypoglycemic agents. Both pramlintide and exenatide exert their action through glucagon suppression and prolongation of gastric emptying. Our earlier studies demonstrated an effective glucose-lowering dose of exenatide (2.5 mcg) in type 1 diabetes.9 This is the first study to our knowledge comparing exenatide versus pramlintide in type 1 diabetes in a CL setting.
Methods
Subjects
The study was approved by the Einstein Institutional Review Board and the US Food and Drug Administration (FDA) gave an investigational device exemption. Subjects were recruited both through general advertising and from the diabetes clinic at the Children’s Hospital at Montefiore. Informed consent was obtained prior to study start and screening was performed prior to the start of study procedures. Subjects enrolled were between 18 and 30 years of age, were diagnosed with T1DM at least 1 year previously, were on continuous subcutaneous insulin infusion (CSII) therapy, and had no other chronic condition (except controlled hypothyroidism). Subjects were included if they had a normal hemoglobin (> 12 gm/dl), had HbA1c < 8.5% (69 mmol/mol), had a weight of ≥ 50 kg, and were taking no other medications (apart from insulin) that could alter the blood glucose concentration or gastric emptying. Subjects were excluded if they had a prior history of gastroparesis, pancreatitis, or impaired renal function, history of QT prolongation on EKG, hypoglycemic unawareness, or hypersensitivity to 5-HT3 receptor antagonists or the study medications pramlintide or exenatide. For female subjects, a serum human chorionic gonadotropin (HCG) pregnancy test was done to rule out pregnancy. Serum amylase levels were monitored prior to all study visits. A total of 10 subjects completed the study with the following demographics (Table 1).
Table 1.
Demographics and Clinical Characteristics.
| Age (years) | 23.24 ± 1 |
| Sex (M/F) | 5/5 |
| Duration of diabetes (years) | 10.64 ± 2 |
| BMI (kg/m2) | 25.2 ± 0.9 |
| HbA1C (%) | 7.29 ± 0.3 |
| HbA1C (mmol/mol) | 56 ± 1 |
Data are mean ± SEM.
Study Procedures
Screening
A complete history, physical exam, and laboratory tests that included complete blood count, serum amylase, serum electrolytes, HbA1C, and serum HCG pregnancy test for women were completed to evaluate for inclusion. Baseline glycemic status was assessed using open-loop subcutaneous continuous glucose monitoring for 3 days the Medtronic iPro Professional Continuous Glucose Monitor (CGM) with Sof-sensor electrodes. If they met inclusion criteria, study subjects were enrolled in a 3-period crossover design to receive (1) control—CL with insulin monotherapy (Con), (2) CL with insulin and pramlintide (CL+P), (3) CL with insulin and exenatide (CL+E), in random order.
Inpatient studies
Subjects were admitted to the research unit around 7 pm on day 1. Two subcutaneous Medtronic Sof-sensors (MMT 7002/7003) were inserted. Subjects’ own usual insulin regimen was programmed into the study insulin pump when they arrived on site.
Open-loop phase
Sensors inserted measured glucose in the interstitial fluid and were connected to MiniLink REAL-Time Transmitters (MMT 7703), which were specially modified to transmit every 1 minute, instead of the commercially available ones that transmit every 5 minutes. Insulin Aspart or NovoLog® (Novo Nordisk, Bagsvaerd, Denmark) was administered via a Paradigm Series Insulin Pump (MMT 715). An intravenous catheter was placed in the antecubital vein for blood sampling.
Hourly plasma glucose measurements were done at the bedside using an Analox GM 9 analyzer® (Analox Instruments, London, UK), and these values were used to calibrate the sensors. Capillary blood glucose checks were done whenever necessary using OneTouch Ultra 2 glucometer (LifeScan, Inc, Milpitas, CA). The glucose measurements were used to calibrate the sensors prior to the start of the CL control portion of the protocol.
CL phase
Subjects were started on the CL phase of automated insulin delivery at 6 am on day 2. During this phase insulin was delivered automatically through the pump based on the glucose sensor data delivered to the control tool software. The glucose data were transmitted every minute via radiofrequency signaling. A laptop with the Control Tool software version 5.1, which uses Medtronic external Physiological Insulin Delivery (ePID) algorithm5 with the Insulin Feedback (IFB)10 feature, provided the automated insulin delivery during the CL phase and collected the glucose sensor data during the open-loop phase of the study. Although 2 Medtronic Sof-sensors were used, control of the CL software was set by the researcher to the sensor, which performed better and was switched to the other sensor as deemed necessary. Sensors were calibrated if the values were 20-30% off from the reference plasma glucose value. At 7 am, breakfast consisting of a standard liquid meal of Boost High Protein Drink 9.6 oz. (360 ml 50 g of carbohydrate) was given to the subject and was consumed over a period of 10 to 15 minutes. Of their bolus insulin requirement, 25% was based on their usual insulin: carbohydrate ratio was administered manually prior to drinking the Boost drink. The rationale for this bolus was to calibrate glucose sensors more effectively and large variations in glucose concentrations were avoided right before study start. The breakfast time period of 7 am to 11 am was not included in the final analysis to allow the CL to equilibrate from a fasting to postprandial state and also to ensure the sensors and the system were optimally communicating prior to the administration of study medication at lunchtime.
Data were collected and analyzed from an hour before lunch, which was served at 12 pm and dinner at 5 pm. Each meal consisted both of solids and liquids and was approximately 60 grams of carbohydrates with approximately 20 grams of protein and 15 grams of fat. The same meal was served to subjects at the same time during all 3 parts of the protocol. Subjects were on the CL control phase of the study until 10 pm on day 2 and were discharged after determining they were stable.
Ten subjects participated in all 3 inpatient visits in which they received insulin monotherapy in one, and pramlintide 30 µg dose or exenatide 2.5 µg dose as adjuvant therapy in the rest. Fixed doses of the medication were determined based on our earlier published and unpublished data involving the use of these medications in subjects with type 1 diabetes. The order of these 3 visits was randomized. Data were not analyzed from the 2 subjects who dropped out of the study citing scheduling difficulties. Both pramlintide and exenatide were given as a subcutaneous injection before lunch and dinner. Each inpatient visit was spaced 2-4 weeks apart. Subjects did not receive any other meal or snack apart from the previously mentioned lunch and dinner during the CL control part of the study. During the study, blood samples were drawn every 15 minutes for the first 2 hours after meals and then every half an hour for the next 3 hours to measure insulin and glucagon concentrations before and after the meals.
During the study, if the subject’s plasma glucose values were less than 70 mg/dl, oral glucose (5-15 g) was administered to achieve euglycemia (90-130 mg/dl). Only 3 corrections to hypoglycemia were allowed for 1 study period. The patients had no meals other than prescribed in the study protocol. If the plasma glucose was more than 300 mg/dl, blood ketones were measured using Precision Xtra Blood Glucose & Ketone Monitoring System (Abbott Diabetes Care, Abbott Park, IL). Ondansetron (Zofran) was ordered for subjects as needed for unbearable nausea or if they had an episode of vomiting.
Hormonal analysis
Glucagon and insulin were measured on a Wizard 2 gamma counter (Perkin Elmer Corporation, Waltham, MA) using a radioimmunoassay method with human specific antibodies using commercially available kits (Millipore Corporation, Billerica, MA). Lower limit of quantification (LLOQ) for insulin was 3 uU/mL and glucagon was 25 pg/mL.
Statistical analysis
Glucose control between the insulin monotherapy (control) and the arms with adjuvant pramlintide or exenatide therapy were compared using reference plasma glucose concentrations and sensor glucose values used by the control algorithm for the lunch and dinner periods. Data are expressed as mean ± SEM. Sensor accuracy was calculated as the mean absolute relative deviation (MARD) of the sensor glucose level from the reference venous glucose level for all paired points. Statistical comparisons were done using analysis of variance for the effect of drugs and time for plasma glucose, insulin, and glucagon concentrations. In addition, if significance was found at P < .05, then post hoc analyses using paired t tests were done between the control, pramlintide, and exenatide arms. GraphPad Prism 6 software (GraphPad Software, Inc, San Diego, CA) was used for statistical analysis.
Results
Fourteen subjects were screened; of them, 12 qualified and 10 completed all study visits. Two study subjects dropped out after 1 study visit citing scheduling difficulties and their data were not included. There was loss of data for 1 of the subject visits due to software malfunction, and it was promptly reported to the FDA and Medtronic. Data are presented on 10 subjects. Half of the study subjects were males, and all were in good glycemic control (Table 1).
Table 2 shows the reference and sensor glucose variations during the lunch and dinner period (12 pm-10 pm) while subjects were on the CL during the control visit with insulin monotherapy (Control), CL + pramlintide (CL+P), and CL + exenatide (CL+E). When exenatide/pramlintide were compared to control the percentage time wherein the subjects’ glucose was greater than 180 mg/dL was significantly less with exenatide as compared to insulin alone and insulin + pramlintide. Exenatide therapy also resulted in subjects staying in range 80-180 mg/dl or 4.4-10 mmol/L for the longest time period. Hypoglycemia was under 10 minutes for all treatment arms and there was no increase in hypoglycemia with either pramlintide or exenatide.
Table 2.
Percentage of Time Within Various Glucose Ranges During Control and Adjunctive Treatment With Pramlintide and Exenatide.
| Con | CL+P | CL+E | ||
|---|---|---|---|---|
| Percentage time (min) > 180 mg/dL (10 mmol/L) | ||||
| (12 pm-10 pm) | ||||
| Reference | 29 ± 2 | 28 ± 5 (P = .84) | 16 ± 5 (P = .02)* | |
| Sensor | 30 ± 3 | 26 ± 3 (P = .42) | 16 ± 5 (P = .03)* | |
| Percentage time (min) between 80 and 180 mg/dL (4.43-10 mmol/L) | ||||
| (12 pm-10 pm) | ||||
| Reference | 61 ± 5 | 62 ± 4 (P = .83) | 77 ± 6 (P = .03)* | |
| Sensor | 62 ± 5 | 66 ± 4 (P = .5) | 76 ± 6 (P = .1) | |
| Percentage time (min) < 80 mg/dL (4.43 mmol/L) | ||||
| (12 pm-10 pm) | ||||
| Reference | 10 ± 4 | 9 ± 2 (P = .9) | 7 ± 2 (P = .5) | |
| Sensor | 9 ± 3 | 8 ± 2 (P = .9) | 8 ± 2 (P = .9) | |
Data are mean ± SEM. Con, closed loop with insulin monotherapy (control); CL+P, closed loop with pramlintide; CL+E, closed loop with exenatide. P < .05 considered significant.
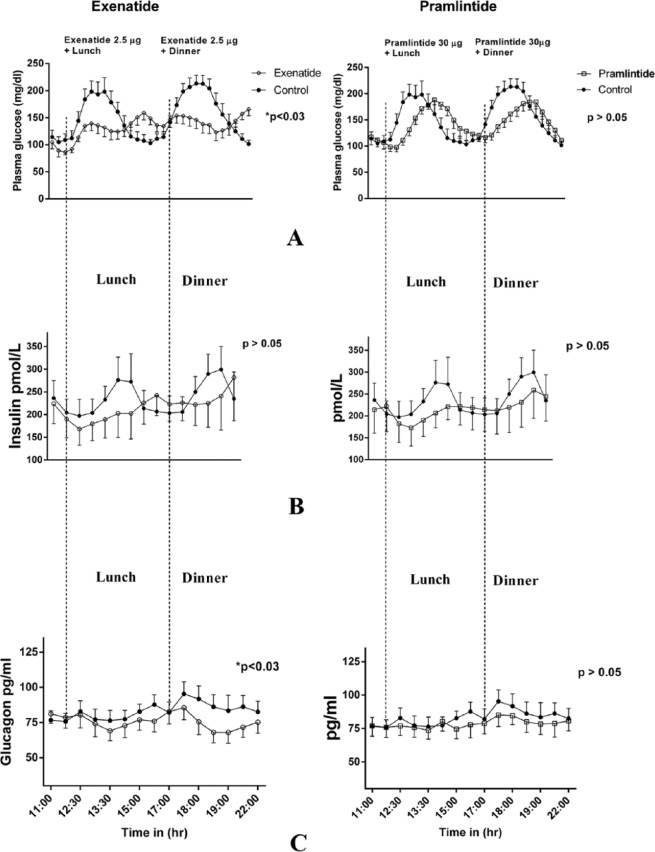
Figure 1 demonstrates plasma glucose profiles in the top panel when exenatide and pramlintide were administered to the same subjects compared to insulin monotherapy. Exenatide was very effective in reducing blood glucose concentrations (P < .03) both after lunch and dinner (A). This is also reflected in Tables 2 and 3. However, we failed to see a statistical difference in average glucose concentrations for the entire study period (Table 3). Insulin concentrations tended to be lower with exenatide and pramlintide but they were not statistically significant (B). In the lowermost panel (C), glucagon concentrations showed a more robust decrease with exenatide (P < .03) than pramlintide, and glucagon reduction with pramlintide was statistically insignificant. The CL delivered an average of 0.35 ± 1.2 units of insulin/kg for the control visits with insulin monotherapy, 0.32 ± 0.08 (P > .6) units of insulin/kg for the visits with adjuvant pramlintide therapy, and 0.3 ± 0.15 (P > .6) for the visits with adjuvant exenatide therapy.
Figure 1.
(A) Plasma glucose comparison between adjunctive therapy with exenatide and pramlintide for lunch and dinner. (B) Plasma insulin comparison. (C) Plasma glucagon comparison.
Table 3.
Glucose Ranges During Control and Adjunctive Treatment With Pramlintide and Exenatide.
| Con | CL+P | CL+E | |
|---|---|---|---|
| AUC glucose (mmmol/dL*hrs) | |||
| Reference | 6.8 ± 1.7 | 4 ± 1 (ns) | 2.9 ± 1.1 (P < .04) |
| Sensor | 7 ± 2 | 4 ± 1 (ns) | 2.6 ± 1 (ns) |
| Average glucose 7 am-10 pm (mmol) | |||
| Reference | 8.4 ± 2.7 | 8.2 ± 2.6 (ns) | 7.7 ± 2.4 (ns) |
| Sensor | 8.6 ± 2.8 | 8.3 ± 2.6 (ns) | 7.6 ± 2.4 (ns) |
Data are mean ± SEM. Con, closed loop with insulin monotherapy (control); CL+P, closed loop with pramlintide; CL+E, closed loop with exenatide. P < .05 considered significant.
Sensor Accuracy Analysis
MARD between the sensor glucose value used for control and the Analox plasma glucose analyzer was 11.9% for insulin monotherapy, 11.1% for adjuvant therapy with pramlintide, and 8.4% for adjuvant therapy with exenatide.
Adverse Events
One subject experienced nausea with pramlintide and 3 subjects experienced nausea with exenatide. One of these subjects also had an episode of vomiting during the exenatide study visit, but responded to Zofran and the remainder of the study period was uneventful. No subjects reported any adverse events after discharge.
Discussion
In this study, we compared exenatide and pramlintide in reducing postprandial glucose excursions in the CL setting. Since the mechanism of action of both drugs is similar, we wanted to be able to clinically use one that was best at reducing postprandial glucose. Glucagon suppression was concomitant with glucose attenuation and was greater with exenatide compared to pramlintide. These data are novel because such a comparison has not been previously demonstrated. We were the first to show that 24-hour pramlintide infusion improves overall glucose excursions in patients with type 1 diabetes.11 Despite the positive outcomes of our study, ultimately translating it to clinical care was thought to be cumbersome until automated dual insulin and pramlintide infusion systems are developed. Hence, we continued to examine other glucagon suppressors and found exenatide in our initial dose seeking studies to be remarkably effective in postprandial glucose lowering without causing immediate postprandial hypoglycemia. These data are consistent with GLP-1 infusions that demonstrated improvement in glucose excursions in both type 1 and type 2 diabetes patients.12,13
Weinzimer et al administered pramlintide in CL with a similar algorithm and reported improvement in glucose excursions.14 However, the statistical analysis used in his study was unclear, with a time effect seen and the effect of the drug nonstatistical with an average reduction of only 5 mg/dl with pramlintide. In a recent meta-analysis of pramlintide, only a modest reduction in HbA1c of 0.2-0.4%15 was noted. Hence, examining new glucagon suppressors such as exenatide and liraglutide is important to find the one that is most effective in glucose lowering with the least amount of side effects. All subjects in this study were drug naïve to both pramlintide and exenatide. Side effects associated with glucagon suppressor’s pramlintide and exenatide are higher with initial use and improve over time.16,17 Despite side effects, such as occasional nausea and 1 case of vomiting, the subjects did well and there were no after-effects that were reported upon study completion. We analyzed the data with and without the subject that had vomiting and found no change in statistical significance.
Liraglutide, a longer acting GLP-1 agonist when compared to exenatide, was recently used in T1DM and adjunctive therapy resulted in decreased basal insulin requirements and a significant improvement in glycemic control.18 Liraglutide data lend credence to the concept of using it as an adjuvant therapy to treat T1DM, which has long been thought to be a bihormonal disease, that is, of insulin deficiency and glucagon deregulation. Most recently, leptin has been used as adjunctive therapy in the treatment of BB rats with T1DM with a beneficial effect.19 These studies demonstrate the need to look beyond insulin and examine alternate hormonal targets that are dysregulated in T1DM.
Sensor accuracy in studies has utilized MARD as an indicator. MARD between sensor glucose values and self-monitoring blood glucose data in the past has been in the range of 15-17%.20 The accuracy achieved in our study was better due to several reasons: (1) the calibration was performed with and compared to the plasma glucose measurements, which have better accuracy than the home blood glucose meters, (2) the sensor was calibrated whenever it deviated significantly from the plasma glucose measurement, (3) the better of 2 sensors was used in the CL, and (4) sensors that did not track the blood glucose were not used for control. Sensor accuracy improves with better glucose concentrations and that may have occurred in our patients when on exenatide. Furthermore, on control days there seemed to be a delay in insulin delivery and the rise in glucose concentrations may have been further exacerbated.
The limitation of our study was that it was only done for 1 day. More data are required to study the long-term use of adjunctive treatment with exenatide in the CL setting. However, new treatments such as pramlintide, exenatide, and others may make it possible for patients to have normal postprandial glucose values and thus prolong the euglycemic period, which is critical for better glycemic control. This in turn may help prevent diabetes-related complications in the future. Studies in patients with type 1 diabetes examining the role of pramlintide and exenatide for a 3 month period are currently underway.
Conclusions
We have shown that pramlintide and exenatide are safe and viable adjunctive therapy options for patients with type 1 diabetes, and are effective in the CL setting with exenatide being better than pramlintide in its postprandial glucose lowering effect. A larger sample size is needed to definitively show whether adjuvant therapy with exenatide across a wider cross-section of patients with T1DM.
Footnotes
Abbreviations: CGM, continuous glucose monitor; CL, closed loop; CL+E, CL with insulin and exenatide; CL+P, CL with insulin and pramlintide; Con, CL with insulin monotherapy; CSII, continuous subcutaneous insulin infusion; FDA, Food and Drug Administration; GLP-1, glucagon-like peptide-1; MARD, mean absolute relative deviation; T1DM, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MC is an employee of Medtronic Minimed, Inc and also a shareholder.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by an award from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), grant R01-DK085597, to RAH. The Albert Einstein College of Medicine Clinical Research Center (CRC), which supported our study, is funded by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant number UL1TR000086. All of the technical support including training, data extraction, and medical devices used in the study including pumps, sensors, and laptop computers with the algorithm software utilized in the study were provided by Medtronic Minimed, Inc.
References
- 1. Bruttomesso D, Farret A, Costa S, et al. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3:1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kovatchev B, Cobelli C, Renard E, et al. Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Technol. 2010;4:1374-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824-833. [DOI] [PubMed] [Google Scholar]
- 4. Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabetes Med. 2006;23:1-12. [DOI] [PubMed] [Google Scholar]
- 5. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934-939. [DOI] [PubMed] [Google Scholar]
- 6. Wilinska ME, Budiman ES, Taub MB, et al. Overnight closed-loop insulin delivery with model predictive control: assessment of hypoglycemia and hyperglycemia risk using simulation studies. J Diabetes Sci Technol. 2009;3:1109-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heptulla RA, Rodriguez LM, Bomgaars L, Haymond MW. The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes. 2005;54:1100-1107. [DOI] [PubMed] [Google Scholar]
- 8. Chase HP, Lutz K, Pencek R, Zhang B, Porter L. Pramlintide lowered glucose excursions and was well-tolerated in adolescents with type 1 diabetes: results from a randomized, single-blind, placebo-controlled, crossover study. J Pediatr. 2009;155:369-373. [DOI] [PubMed] [Google Scholar]
- 9. Raman VS, Mason KJ, Rodriguez LM, et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care. 2010;33:1294-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruiz JL, Sherr JL, Cengiz E, et al. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. 2012;6:1123-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heptulla RA, Rodriguez LM, Mason KJ, Haymond MW. Twenty-four-hour simultaneous subcutaneous Basal-bolus administration of insulin and amylin in adolescents with type 1 diabetes decreases postprandial hyperglycemia. J Clin Endocrinol Metab. 2009;94:1608-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 1999;22:1137-1143. [DOI] [PubMed] [Google Scholar]
- 13. Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual beta-cell function. Diabetes. 2011;60:1599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35:1994-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee NJ, Norris SL, Thakurta S. Efficacy and harms of the hypoglycemic agent pramlintide in diabetes mellitus. Ann Fam Med. 2010;8:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pencek R, Roddy T, Peters Y, et al. Safety of pramlintide added to mealtime insulin in patients with type 1 or type 2 diabetes: a large observational study. Diabetes Obes Metab. 2010;12:548-551. [DOI] [PubMed] [Google Scholar]
- 17. Tobin GS, Cavaghan MK, Hoogwerf BJ, McGill JB. Addition of exenatide twice daily to basal insulin for the treatment of type 2 diabetes: clinical studies and practical approaches to therapy. Int J Clin Pract. 2012;66:1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varanasi A, Bellini N, Rawal D, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol. 2011;165:77-84. [DOI] [PubMed] [Google Scholar]
- 19. Kruger AJ, Yang C, Lipson KL, et al. Leptin treatment confers clinical benefit at multiple stages of virally induced type 1 diabetes in BB rats. Autoimmunity. 2011;44:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mastrototaro J, Shin J, Marcus A, Sulur G, Investigators SCT. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther. 2008;10:385-390. [DOI] [PubMed] [Google Scholar]