Abstract
The most recent (2011) National Diabetes Fact Sheet states the combined diagnosed and undiagnosed number of diabetes cases in the United States is approaching 25 million, and another 79 million are prediabetic. Of the diabetes patients, 60-70% suffer from mild to severe neuropathy. This combined loss of sensory and motor control in diabetic limbs is usually considered an irreversible, progressive process. Patients suffering from these losses are at a significantly higher risk for development of foot ulceration, frequently leading to infection and partial or major limb amputation. However, a review of focal nerve entrapment surgical decompression literature suggests that several diabetic sensorimotor polyneuropathy (DSPN) symptoms and complications are potentially partially reversible or preventable. Decompression surgery represents a paradigm shift in treatment protocols because it both relieves pain and restores protective sensation, while providing significant protection against a cascade of serious foot complications. This review surveys current research regarding the biological basis for diabetic focal entrapment neuropathy. Metabolic dysfunction related to aldose reductase, oxidative stress, and advanced glycation end products are considered and correlated to peripheral nerve enlargement and entrapment. In addition, observational studies correlated to that biological basis are presented as well as surgical outcomes illustrating the effect of decompression on DSPN symptomatic relief, nerve function, and protection against complications.
Keywords: diabetic sensorimotor polyneuropathy, nerve decompression surgery, aldose reductase, oxidative stress, advanced glycation end products, ultrasonographic nerve enlargement
The most recent (2011) National Diabetes Fact Sheet states the tally of diagnosed and undiagnosed diabetes cases in the United States is approaching 25 million, and another 79 million are prediabetic. Of the diabetes patients, 60-70% suffer from mild to severe neuropathy.1 This combined loss of sensory and motor control in diabetic limbs is usually considered an irreversible, progressive process. Patients suffering from these losses are at significantly higher risk for development of foot ulceration, frequently leading to infection, and minor or major limb amputation.2-8
However, a review of surgical decompression literature addressing focal nerve entrapment suggests that several diabetic sensorimotor polyneuropathy (DSPN) symptoms and complications are potentially partially reversible or preventable. The following review surveys current research regarding the biological basis for diabetic focal entrapment neuropathy, observational studies correlated to that biological basis, and the clinical rationale and outcomes related to nerve decompression (ND) surgery.
Background
It is important to note that diabetic neuropathies are heterogeneous disorders. This discussion is focused on the somatic subset described as distal symmetric peripheral polyneuropathy, classically known as “stocking-glove anesthesia.” Determinants affecting the rate of development and ultimate severity of the neuropathy include: duration of diabetes and adequacy of glucose control,9 a conclusion morphologically confirmed by Perkins et al.10
Two separate types of focal neuropathies are recognized: mononeuropathies and entrapment.11 Mononeuropathies are felt to be the consequence of vascular injury, and are said to resolve with only supportive care management. Entrapment neuropathies (the focus of this discussion) are said to occur in up to 30% of all diabetic neuropathy12 and are postulated to occur as the result of a “double crush” phenomena.
Double Crush Syndrome
“Double crush” syndrome is a condition that was originally described by Upton and McComas13 in regard to carpal tunnel syndrome (CTS). Their research indicated an additive effect of subclinical nerve entrapment in both the cervical region (radiculopathy) and the carpal tunnel as it related to developing CTS. The theory suggests that if subclinical impairment of a nerve occurs at 1 site, the nerve is vulnerable to dysfunction if a second such impairment occurs at another site.
To broadly corroborate Upton’s double crush theory in the context of DSPN, it has been noted that the general population has an incidence of 2.7%14 (diagnosis confirmed by clinical and electrophysiology findings) in developing CTS, whereas an incidence of 20% or more is found in diabetes.11,15
Dellon and Mackinnon16 help to demonstrate the double crush hypothesis experimentally by compressing sciatic nerves in rats at 1 or 2 locations. Their experimental model aimed to determine if a second site of minimal nerve compression would reduce nerve function more than expected. Electrophysiologically, they concluded that function was significantly reduced in rats with a double site compression.
In diabetes-induced nerve swelling, double crush can be an important factor as nerves pass through anatomically constrained channels. Investigators have used such observations to postulate a potential relationship between the phenomena of double crush syndrome and lower extremity focal entrapment neuropathy—demonstrated by enlarged nerve trunks passing through size-constrained fibro-osseous tunnels.17,18
Biochemical Basis
Aldose Reductase
To understand how diabetes-induced nerve damage could contribute to focal entrapment neuropathy, one must appreciate at the molecular level how biochemical pathways can affect the neuron structurally and functionally.
First, it is important to note the neuronal response to hyperglycemia through the sorbitol-aldose reductase or polyol pathway. The sorbitol pathway begins with aldose reductase, an enzyme needed to process cellular glucose for energy production. Under normal conditions, a higher affinity hexokinase enzyme utilizes the bulk of the intracellular glucose to convert it to glucose-6-phosphate, while aldose reductase makes only small amounts of sorbitol from residually available glucose (Figure 1).
Figure 1.
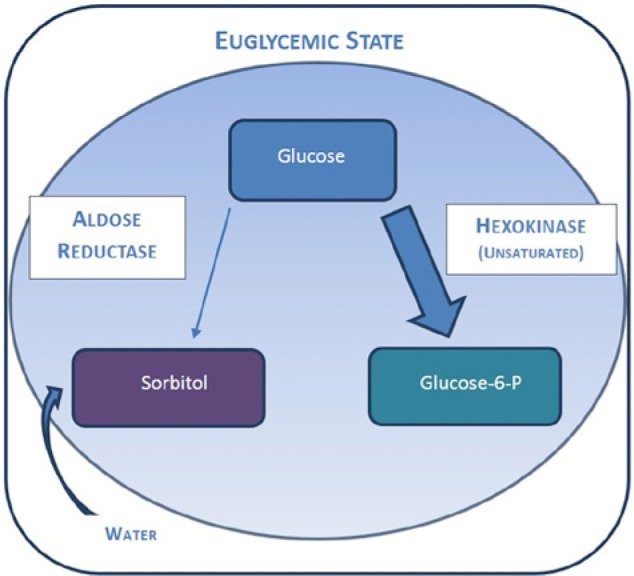
Diagram showing nerve glucose metabolism in euglycemic state.
In chronic hyperglycemia, lack of insulin action allows the neuron to take up, via proposed insulin-independent GLUT 1 transporters,19 greater amounts of glucose than it normally does under euglycemic conditions. With elevated intracellular glucose levels, the hexokinase enzyme becomes fully saturated and excess glucose is left to be converted to sorbitol by aldose reductase.
Because sorbitol has low plasma membrane permeability, it acts as an osmotic driver, pulling extracellular fluid into the neuron, causing axonal and nerve trunk swelling. Schwann cells are also rich in aldose reductase and exhibit a similar response. Both the nerve and Schwann cells contribute to a disordered peripheral nerve or “glucose neurotoxicity” (Figure 2).19-22
Figure 2.
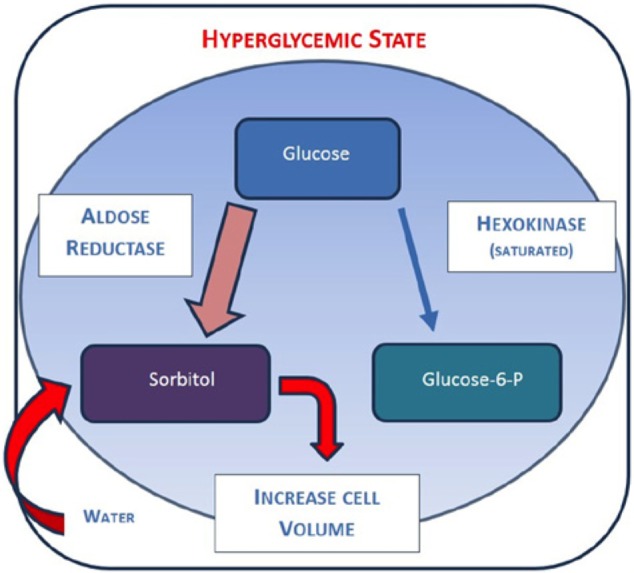
Diagram showing nerve glucose metabolism in hyperglycemia state.
Oxidative Stress
Not only do the neuron and Schwann cells become swollen from the sorbitol osmotic gradient, but these cells also struggle to manage ATP energy production, which in itself leads to enlargement. Hyperglycemia initially provides the cell more fuel to produce ATP. However, as the electron transport chain (ETC) becomes more active, it begins to be hampered by an inability to replenish free radical scavengers such as glutathione. When radical scavengers are recharged too slowly, a rate-limiting bottleneck forms and obstructs energy production.23 Furthermore, the mitochondria, which house the ETC activity, become the first structures to be damaged by elevated reactive oxygen species. Since the axonal region has a large population of mitochondria as well as a high surface-to-volume ratio, axons are particularly vulnerable to oxidative damage from glucose overload.24
Experimentally, this “double cellular crisis” of energy failure and oxidative damage has been demonstrated in Schwann cells and suggested in peripheral nerve studies.25 In addition, oxidative stress has been shown experimentally to be reversible in some degree with administration of local antioxidants, thus supporting the microbiologic breakdown hypothesis.26 This “double cellular crisis” contributes to macroscopic changes in the nerve as evidenced by swelling prior to neuron death.
Advanced Glycation End Products
In addition to the osmotic and energy-based degeneration, the nerves and surrounding tissues are also subject to degeneration by advanced glycation end products (AGEs). These AGEs, accumulating by nonenzymatic glycosylation of proteins, have been linked to other serious clinical complications of diabetes, including retinopathy and nephropathy. AGEs are notably problematic in nerve pericytes. Pericytes serve as small contractile cells of the basement membrane and maintain a close association to endothelial cells conducting blood to the nerve. Shimizu et al27 illustrated that pericytes significantly increase type IV collagen and fibronectin production as a result of AGE exposure. Consequently, the basement membrane at the blood-nerve-barrier hypertrophies, forcing pericytes to increase vascular endothelial growth factor (VEGF) to overcome compromised blood flow to the nerve.
Compromised blood flow becomes amplified by the external pressures on the small vasa nervorum blood vessels supplying the nerve at entrapment sites due to mechanical stiffening of connective tissue. Rosenbloom and Silverstein28 explain that AGE-driven changes in the associated skin and periarticular joint tissues lead to limited joint mobility, Dupuytrens disease, flexor tenosynovitis, CTS, stiff-hand syndrome, and shoulder-hand reflex dystrophy. These ubiquitous, inelastic fibrous restrictions are problematic where the peripheral nerve passes through constrained, AGE-shrunken tunnels. Ultimately, AGEs limit the degree of nerve compliance and excursion, while at the same time diminishing the nutrient blood supply to the nerve.
Returning to the concept of double crush, nerve enlargement becomes an impediment when the nerve trunks travel through anatomically restricted fibro-osseous locations, such as the carpal tunnel, cubital tunnel, around the neck of the fibula, tarsal tunnel, and medial and lateral plantar tunnels. Because these nerves are vulnerable to dysfunction from internal and external compression forces at multiple sites, this can be considered double crush syndrome.
Observational Research
Ultrasound Nerve Enlargement Studies
Substantiating the concept that metabolic dysfunction in diabetes leads to enlarged nerves, several researchers have conducted diagnostic ultrasound studies to characterize diabetic nerves, particularly near entrapment sites. Pertinent works by Watanabe et al,29,30 Liu et al,31 Riazi et al,32 Lee et al,33,34 and Zhang et al35 demonstrate that diabetes patients, especially neuropathic diabetes patients, exhibit significantly larger nerve cross-sectional areas when compared to healthy populations. Lee and Dauphinee33 found that cross-sectional tibial nerve area was double that of non-neuropathic diabetes cases and nondiabetic controls. Riazi et al,32 in an ultrasound study of 98 diabetes cases, 55 with DSPN, measured cross-sectional areas of the posterior tibial nerve at incremental locations proximal to the medial malleolus. They report a significant differential in patients with DSPN, a result agreeing closely with Lee et al’s results.33
Hobson-Webb et al,36 however, did not find significant sonographic size differences in DSPN for sural or peroneal nerve branches at several location between the knee and ankle. However, in the context of nerve entrapment, the Hobson-Webb et al study does not consider 4 key elements. First and significantly, the study did not measure the posterior tibial nerve, a nerve that has demonstrated enlargement in several previous articles.32 Second, Hobson-Webb et al note the difficulty of studying the axial cross sectional area of the common peroneal nerve in its tortuous course near the fibular neck where entrapment can occur. Third, the sural nerve has no known anatomic entrapment sites. Fourth, they also speculate that proximity to sites of entrapment might be a necessary condition for nerve caliber enlargement.
Consistent with this is the reported resolution of increased size found by Zhang et al.35 They report that in 560 DSPN patients there was a significant reduction in posterior tibial nerve size demonstrated at 18 month follow-up after ND, which also correlated to increased nerve conduction velocity—a finding that had not previously been demonstrated. Interestingly, Zhang et al also were unable to demonstrate enlargement in the tortuous common peroneal nerve proximal to the peroneal tunnel under the peroneus longus muscle.
Nerve Compression Functional Studies
Several laboratory studies provide scientific justification for ND. The Mosconi and Kruger37 nerve cuff study placed polyethylene cuffs ranging in size from 0.028 inches to 0.030 inches around the sciatic or sural nerve of rats and correlated nerve fiber changes with clinically established pain response protocols. “Large myelinated axons underwent an early and sustained numerical depletion. Both the thinly myelinated and unmyelinated axon populations were initially diminished, but later rose to levels significantly greater than control values.” The late axon augmentation was postulated to have occurred as a result of regeneration and/or adjacent uninjured axon sprouting. Microscopically, the fibers exhibited “edematous swelling, hypertrophy of the perineurial sheath, infiltration of fibroblasts and collagen into the intraneural compartment, increasing interaxonal space and decreasing order and density of axonal packing.” The rats suffered from gait and postural mismatch in response to compression and cold at the cuff site. Barac et al38 demonstrated a similar result with compression of rats’ sciatic nerve, finding a lengthened withdrawal time from painful thermal stimulus.
Dahlin et al39 demonstrated on a molecular level that in streptozotocin-induced diabetic rats localized compression of the sciatic nerve leads to significant reduction in fast axonal transport. Using a graded compression, it was noted that at pressures of 20-30 mmHg, axonal transport was blocked. According to Rosson et al,40 perineural tissue pressures of 26 mmHg are recorded in diabetes patients with focal nerve entrapment in the foot. Additional work by Dahlin and McLean41 in a rabbit model reported similar effects in both slow and fast axonal transport.
Kale et al42 added to Mosconi and Kruger’s work by investigating the degree to which ND yields performance-based results. Kale et al treated 3 groups of streptozotocin-induced diabetic rats. Control rats had only the sciatic nerve dissected. The second group received a tarsal tunnel release with epineurotomy of both the sciatic nerve and the branching peroneal and tibial nerves. A third group received epineurotomy plus intrafascicular neurolysis to further decompress fascicles. Later gait analysis found the third group performed best. Electron microscopy images of all 3 groups showed degeneration, but less pronounced degeneration in the 2 treatment groups. Conclusively, Kale et al reported that combined internal and external decompression of the nerves gave the best clinical result.
Clinical Results
Clinical decompression of nerves to restore function is not a new concept. Hansen and Looft43 described in 1895 the value of incising nerves by opening the nerve sheath to reduce the dysfunction of leprosy neuritis. Since that time, surgical ND by epineurotomy has become a common procedure, especially in the context of CTS. However, appreciation of its value in diabetic focal entrapments, particularly in the lower extremity of DSPN patients, is relatively new. This approach is based on the neurologic effects of nerve enlargement seen in streptozotocin-induced rat diabetes, which include gait abnormalities44 and could be prevented by prophylactic release of the rat tarsal tunnel analogue.45
Clinical effects of surgical ND have been measured by both subjective and objective outcomes. Dellon46 reported in 1992 subjective results of external neurolysis in DSPN on a total of 154 peripheral nerves in 51 upper and 31 lower extremities. He found that 80% of the patients reported subjective, measured improvement in pain and sensibility following decompression. Untreated limbs were also monitored, and 50% reported progressive worsening of the neuropathic condition while the treated limb maintained its improved state.
Other investigators’ surgical reports are consistently confirmatory. Valdivia et al47 found in a series of 200 peripheral ND surgeries that 87% of patients subjectively had increased sensation and 83% of patients report sensibility recovery post-decompression. Baltodano et al48 report in a meta-analysis of ND studies that 91% of 875 patients (1053 lower extremities) experienced visual analog score (VAS) pain relief > 4 points. Dellon’s meta-analysis49 found that 80% of patients improved VAS pain scores from a mean of 8.5 to 2. In carpal tunnel decompression, Mondelli et al50 note 99% success in eliminating pain, reduction in paresthesia, and improved function postoperatively.
Dellon’s recommended approach51 is to decompress all sites of potential anatomic entrapment involving common peroneal tunnel, tarsal tunnel, medial, and lateral plantar nerves at the abductor tunnel, medial calcaneal nerve, and deep peroneal nerve at extensor hallucis brevis tendon. This generally involves incisions in 3 sites, the fibular neck, medial ankle and foot, and dorsal foot near the medial cuneiform-first metatarsal joint. External neurolysis is achieved by division of the constricting fibro-osseous tunnel tissue. Since the origin of entrapment is systemic and metabolic, all potential entrapment sites are addressed with ND.
Objective results of ND mirror the encouraging subjective improvements reported. First, decompression facilitates significant improvement in measured nerve function. Zhang et al35 characterized 560 DSPN patients with bilateral ND procedures of the lower extremity both prior to decompression and in follow-up 18 months later. That study determined that nerve conducting velocities, 2-point discrimination and subjective, quantitative thermal sensory testing improved, in some cases performing at the same level as non-neuropathy diabetes patients. Zhang et al specifically attribute these improvements to the decompression, supported by the work of Rosson et al,40 who demonstrated that patients experienced high perineural tissue pressure prior to decompression within the medial plantar tunnel with foot plantar flexion and pronation (26.5 mmHg). Following decompression, the medial plantar tunnel pressure drops significantly (as it does in all decompressed compartments) to 7 mmHg.
Second, decompression reduces postural imbalance and associated fall risk. Ducic et al52 measured the sway characteristics of 14 elderly, diabetes patients with neuropathy. One group had unilateral ND and the second had bilateral procedures. Postoperative sway measurements revealed that patients in the unilateral decompression group had a sway reduction of 5% with their eyes open and 31% with eyes closed. The bilateral decompression group had a significantly larger reduction of 23% with eyes open and 145% with eyes closed. Significant functional improvement in postural control presumably translates to reduced risk of fall and associated injury.
Third, decompression has been shown to reduce the risk of ulceration, reulceration, hospitalization for infection, and amputation. In a landmark multicenter prospective registry study of 628 patients (839 operated limbs) and 38 surgeons performing neurolysis of chronically compressed tibial nerves, Dellon et al53 report that 0.2% of patients with no previous ulceration history developed new ulcers, 3.8% of patients with a past ulceration had recurrent ulcerations, and 1 patient underwent amputation. Aszmann et al54 reported that 50 DSPN cases who had unilateral ND for pain relief subsequently had 3 amputations and 12 ulcers in 4.5 years, all in the contralateral leg that had not been operated. Nickerson and Rader55 describe retrospectively a durable 80% reduction in diabetic foot ulceration (DFU) recurrence risk lasting at least 5 years. A 3 year prospective study of unilateral ND after DFU finds the non-operated intact legs to have 5.5 times the risk of ulceration.56 Zhang et al35 reinforce these findings in their prospective study that included 208 prior ulcer DSPN patients who underwent bilateral decompression, reporting that no patient had new ulcers, reulceration, wound infections, or amputation in an 18 month follow-up.
Conclusion
DSPN is a growing concern because of its skyrocketing incidence. The severity and frequency of DSPN is associated with a cascade of complications including ulcers, amputations, and early mortality. An appreciation of the molecular origin of metabolically induced nerve enlargement should lead us to understand that frequent focal nerve entrapments can accompany diabetes. Fortunately there seems to be a safe and reliable therapy—ND. Decompression both relieves pain and restores protective sensation, while providing significant protection against a cascade of serious DSPN foot complications.
Peripheral ND is a particularly useful modality and a much broader appreciation of its therapeutic potential is warranted. The significant beneficial outcomes of surgical decompression therapy should signal to all clinicians that there is a need for considering a paradigm shift in current treatment algorithms. Empowering DSPN patients, who might otherwise remain with their “irreversible” sensorimotor loss and complication risk, is a reward worth our focused attention.
Footnotes
Abbreviations: CTS, carpal tunnel syndrome; DFU, diabetic foot ulceration; DSPN, diabetic sensorimotor polyneuropathy; ETC, electron transport chain; ND, nerve decompression; VEGF, vascular endothelial growth factor.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. CDC.gov. Center for Disease Control: National diabetes fact sheet, 2011. Available from: http://www.cdc.gov/diabetes.
- 2. Reiber G, Vileikyte L, Boyko E. Causal pathways for incident lower extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22:157-162. [DOI] [PubMed] [Google Scholar]
- 3. Apelqvist J, Agardh C. The association between clinical risk factors and outcome of diabetic foot ulcers. Diabetes Res Clin Pract. 1992;18:43-53. [DOI] [PubMed] [Google Scholar]
- 4. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 5. McNeeley M, Boyko E, Ahroni J. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. Diabetes Care. 1995;18:216-219. [DOI] [PubMed] [Google Scholar]
- 6. Rith-Najarian S, Stolusky T, Gohdes D. Identifying diabetic patients at high risk for lower extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care. 1992;15:1386-1389. [DOI] [PubMed] [Google Scholar]
- 7. Veves A, Uccioli L, Manes C. Comparison of risk factors for foot problems in diabetic patients attending teaching hospital outpatient clinics in four different European states. Diabetes Med. 1994;11:709-713. [DOI] [PubMed] [Google Scholar]
- 8. Ramsey S, Newton K, Blough D. Incidence, outcomes and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382-387. [DOI] [PubMed] [Google Scholar]
- 9. Pinzur M. Diabetic peripheral neuropathy. Foot Ankle Clin N Am. 2011;16:345-349. [DOI] [PubMed] [Google Scholar]
- 10. Perkins BA, Greene DA, Bril V. Glycemic control is related to the morphological severity of diabetic sensorimotor polyneuropathy. Diabetes Care. 2001;24:748-752. [DOI] [PubMed] [Google Scholar]
- 11. Vinik AI. Advances in diabetes for the millennium: new treatment for diabetic neuropathies. Strelitz Diabetes Institutes, Department of Internal Medicine, Eastern Virginia Medical School. Med Gen Med. 2004;6(3 suppl):13. [PMC free article] [PubMed] [Google Scholar]
- 12. Vinik AI. Diabetic neuropathy: pathogenesis and therapy. Strelitz Diabetes Institutes, Department of Internal Medicine, Eastern Virginia Medical School. Am J Med. 1999;107(2B):17S-26S. [DOI] [PubMed] [Google Scholar]
- 13. Upton A, McComas A. The double crush in nerve entrapment syndromes. Lancet. 1973;2(7825):359-362. [DOI] [PubMed] [Google Scholar]
- 14. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;281(2):153-158. [DOI] [PubMed] [Google Scholar]
- 15. Ramchurn N, Mashamba C, Leitch E, et al. Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Int Med. 2009;20(7):718-721. [DOI] [PubMed] [Google Scholar]
- 16. Dellon AL, Mackinnon SE. Chronic nerve compression model for the double crush hypothesis. Ann Plast Surg. 1991;26(3):259-264. [DOI] [PubMed] [Google Scholar]
- 17. Nemoto K, Matsumoto N, Tazaki K, Horiuchi Y, Uchinishi K, Mori Y. An experimental study on the “double crush” hypothesis. J Hand Surg Am. 1987;12(6):1011. [DOI] [PubMed] [Google Scholar]
- 18. Hurst L, Weissberg D, Carroll R. The relationship of the double crush to carpal tunnel syndrome (an analysis of 1,000 cases of carpal tunnel syndrome). J Hand Surg Br. 1985;10(2):202-204. [DOI] [PubMed] [Google Scholar]
- 19. Tomlinson D, Gardiner N. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36-45. [DOI] [PubMed] [Google Scholar]
- 20. Ludvigson M, Sorenson R. Immunohistochemical localization of aldose reductase I. Enzyme purification and antibody preparation localization in peripheral nerve, artery and testis. Diabetes. 1980;29(6):438-449. [DOI] [PubMed] [Google Scholar]
- 21. Sharma A, Thomas P. Peripheral nerve structure and function in experimental diabetes. J Neurol Sci. 1974;23:1-15. [DOI] [PubMed] [Google Scholar]
- 22. Sima A. Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil. New Engl J Med.1988;319:548-555. [DOI] [PubMed] [Google Scholar]
- 23. Obrosova I. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figueroa-Romero C, Sadidi M, Feldman EL. Mechanism of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song Z. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol Cell Neurosci. 2003;23:638-647. [DOI] [PubMed] [Google Scholar]
- 26. Cameron N, Cotter M, Maxfield E. Antioxidant treatment prevents the development of peripheral nerve dysfunction in streptozotocin-diabetic rats. Diabetologia. 1993;36:299-304. [DOI] [PubMed] [Google Scholar]
- 27. Shimizu F, Sano Y, Haruki H, Kanda T. Advanced glycation end-products induce basement membrane hypertrophy in endoneurial microvessels and disrupt the blood-nerve barrier by stimulating the release of TGF-beta and vascular endothelial growth factor (VEGF) by pericytes. Diabetologia. 2011;54:1517-1526. [DOI] [PubMed] [Google Scholar]
- 28. Rosenbloom AL, Silverstein JH. Connective tissue and joint disease in diabetes mellitus. Endocrinol Metab Clin North Am. 1996;25(2):473-483. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe T, Ito H, Morita A, et al. Sonographic evaluation of the median nerve in diabetic patients: comparison with nerve conduction studies. J Ultrasound Med. 2009;28:727-734. [DOI] [PubMed] [Google Scholar]
- 30. Watanabe T, Ito H, Sekine A, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med. 2010;29:697-708. [DOI] [PubMed] [Google Scholar]
- 31. Liu F, Zhu J, Wei M, Bao Y, Hu B. Preliminary evaluation of the sural nerve using 22-MHz ultrasound: a new approach for evaluation of diabetic cutaneous neuropathy. PLOS ONE. 2012;7(4):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riazi S, Bril V, Perkins BA, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? Diabetes Care. 2012;35:2575-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee D, Dauphinee DM. morphological and functional changes in the diabetic peripheral nerve. J Am Podiatr Med Assoc. 2005;95(5):433-437. [DOI] [PubMed] [Google Scholar]
- 34. Lee D, Dauphinee DM, Bastawros D. Ultrasound evaluation of the tarsal tunnel in diabetic foot neuropathy. Radiol Soc North Am. 2004;90:1. [Google Scholar]
- 35. Zhang W, Li S, Zheng X. Evaluation of the clinical efficacy of multiple lower extremity nerve decompression in diabetic peripheral neuropathy. Br J Neurol Surg. 2013;74(2):96-100. [DOI] [PubMed] [Google Scholar]
- 36. Hobson-Webb LD, Massey JM, Juel VC. Nerve ultrasound in diabetic polyneuropathy: correlation with clinical characteristics and electrodiagnostic testing. Muscle Nerve. 2013;47(3):379-384. [DOI] [PubMed] [Google Scholar]
- 37. Mosconi T, Kruger L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructral morphometric analysis of axonal alterations. Pain. 1996;64(1):37-57. [DOI] [PubMed] [Google Scholar]
- 38. Barac S, Jiga LP, Barac B, Hoinoiu T, Dellon AL, Ionac M. Hindpaw withdrawal from a painful thermal stimulus after sciatic nerve compression and decompression in the diabetic rat. J Reconstr Microsurg. 2013;29:63-66. [DOI] [PubMed] [Google Scholar]
- 39. Dahlin LB, Meiri KF, McLean WG, Rydevik B, Sjostrand J. Effects of nerve compression on fast axonal transport in streptozotocin-induced diabetes mellitus. An experimental study in the sciatic nerve of rats. Diabetologia. 1986;29(3):181-185. [DOI] [PubMed] [Google Scholar]
- 40. Rosson GD, Larson AR, Williams EH, Dellon AL. Tibial nerve decompression in patients with tarsal tunnel syndrome: pressures in the tarsal, medial plantar, and lateral plantar tunnels. Plast Reconstr Surg. 2009;124(4):1202-1210. [DOI] [PubMed] [Google Scholar]
- 41. Dahlin LB, McLean WG. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurol Sci. 1986;72(1):19-30. [DOI] [PubMed] [Google Scholar]
- 42. Kale B, Yuksel F, Celikoz B, Siranci S, Ergun O, Arbak S. Effect of various nerve decompression procedure on the functions of distal limbs in streptozotocin-induced diabetic rats: further optimism in diabetic neuropathy. Plast Reconstr Surg. 2003;111(7):2265-2272. [DOI] [PubMed] [Google Scholar]
- 43. Hansen GA, Looft C. Leprosy in Its Clinical and Pathological Aspects. Bristol, UK: John Wright; 1895. [Google Scholar]
- 44. Dellon ES, Dellon AL. Functional assessment of neurologic impairment: track analysis in diabetic and compression neuropathies. Plast Reconstr Surg. 1991;88:686-694. [DOI] [PubMed] [Google Scholar]
- 45. Dellon ES, Dellon AL, Seiler WA., IV The effect of tarsal tunnel decompression in the streptozotocin-induced diabetic rat. Microsurg. 1994;15:265-268. [DOI] [PubMed] [Google Scholar]
- 46. Dellon AL. Treatment of symptomatic diabetic neuropathy by surgical decompression of multiple peripheral nerves. Plast Reconstr Surg. 1992;89(4):689-697. [PubMed] [Google Scholar]
- 47. Valdivia Valdivia JM, Weinand M, Maloney CT, Jr, Blount AL, Dellon AL. Surgical treatment of superimposed, lower extremity, peripheral nerve entrapments with diabetic and idiopathic neuropathy. Ann Plast Surg. 2013;70(6):675-679. [DOI] [PubMed] [Google Scholar]
- 48. Baltodano PA, Basdag B, Bailey CR, et al. The positive effect of neurolysis on patients with compressed nerves of the lower extremity: a systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2013;1(e24):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dellon AL. The Dellon approach to neurolysis in the neuropathy patient with chronic nerve compression. Handchir Mikrochir Plast Chir. 2008;40:1-10. [DOI] [PubMed] [Google Scholar]
- 50. Mondelli M, Padua L, Reale F, Signorini A, Romano C. Outcomes of surgical release among diabetics with carpal tunnel syndrome. Arch Phys Med Rehabil. 2004;85:7-13. [DOI] [PubMed] [Google Scholar]
- 51. Dellon AL. Neurosurgical prevention of ulceration and amputation by decompression of lower extremity peripheral nerves in diabetic neuropathy: update 2006. Acta Neurochir Suppl. 2007;100:149-151. [DOI] [PubMed] [Google Scholar]
- 52. Ducic I, Taylor NS, Dellon AL. Relationship between peripheral nerve decompression and gain of pedal sensibility and balance in patients with peripheral neuropathy. Ann Plast Surg. 2006;56(2):145-150. [DOI] [PubMed] [Google Scholar]
- 53. Dellon AL, Muse VL, Nickerson DS, et al. Prevention of ulceration, amputation, and reduction of hospitalization: outcomes of a prospective multicenter trial of tibial neurolysis in patients with diabetic neuropathy. J Reconstr Microsurg. 2012;28(4):241-246. [DOI] [PubMed] [Google Scholar]
- 54. Aszmann O, Tassler PL, Dellon AL. Changing the natural history of diabetic neuropathy: incidence of ulcer/amputation in the contralateral limb of patients with a unilateral nerve decompression procedure. Ann Plast Surg. 2004;53(6):517-522. [DOI] [PubMed] [Google Scholar]
- 55. Nickerson DS, Rader AJ. Low long-term risk of foot ulcer recurrence after nerve decompression in a diabetes neuropathy cohort. J Am Podiatr Med Assoc. 2013;103(5):380-386. [DOI] [PubMed] [Google Scholar]
- 56. Nickerson DS, Rader AJ. Nerve Decompression After Diabetic Foot Ulceration May Protect Against Recurrence: A 3-Year Controlled, Prospective Analysis. J. Am. Podiatr. Med. Assoc. 2014;104(1):66-70. [DOI] [PubMed] [Google Scholar]


