Abstract
In patients with diabetes mellitus (DM), early retinal microvascular alterations can be observed even before the clinical diagnosis of diabetic retinopathy. This study aimed to investigate morphological and functional changes in retinal microvascular blood flow in type 1 diabetic patients with and without peripheral neuropathy (PNP) as compared to nondiabetic controls. Retinal microvascular blood flow (RBF) was assessed using scanning laser Doppler flowmetry (Heidelberg Retina Flowmeter, Heidelberg Engineering, Germany) before and after stimulation with flicker light. PNP was assessed using the neuropathy disability score (NDS) and by the evaluation of the vibration perception threshold (VPT). A total of 41 subjects were recruited for study participation and were stratified to 3 different groups according to their metabolic and neurological status: 14 nondiabetic subjects without PNP, 14 diabetic patients without PNP, and 13 diabetic patients with PNP. All subjects were free from diabetic retinopathy as assessed by fundoscopy. In diabetic patients with PNP, baseline and stimulated RBF was higher compared with diabetic patients without PNP and the nondiabetic control group. No difference with regard to RBF could be observed between the nondiabetic control subjects and patients with type 1 DM without PNP. No difference in the arterial WLR could be observed between the 3 groups. A linear correlation was found for VPT and RBF (r = .38, P < .001) and for NDS and RBF (r = .44, P < .0001). In our study population of patients with type 1 diabetes, PNP was associated with functional but not morphological changes in RBF.
Keywords: diabetic polyneuropathy, retinal blood flow, wall to lumen ratio, type 1 diabetes mellitus
Microvascular disease such as retinopathy is a frequent complication in patients with DM. Several studies indicate that alterations in retinal blood flow develop early after the diagnosis of DM and that functional disturbances in retinal microvascular blood flow precede the occurrence of the morphological features of diabetic retinopathy.1-3 While in advanced diabetic retinopathy retinal microvascular blood flow is reduced, contradictory results have been observed in diabetic patients without or in early stages of diabetic retinopathy.4-7 Several studies have shown that in patients with DM, retinal microvascular regulation and the microvascular response to flicker light was found to be impaired.8-10 At least 2 mechanisms may be attributable to the blunted flicker response in patients with DM. Endothelial dysfunction and a diminished release of nitric oxide (NO) has been documented in patients with DM,11,12 which might cause a reduction in the flicker induced vasodilatation.13-15 In addition, the microvascular response to flicker light requires retinal neural integrity, and electroretinographic investigations confirmed an impaired flicker response caused by disturbed neurovascular coupling.16
Beside these functional disturbances in retinal microvascular blood flow, morphological alterations in retinal angioarchitecture are a common feature in developing diabetic retinopathy.17,18 In a recent study, an increase in retinal vascular caliber could be observed in association with peripheral diabetic neuropathy.19
Therefore, it seems conceivable that impaired neural function in patients with type 1 DM might interfere with functional or morphological changes in retinal microcirculation. The aim of this cross-sectional, exploratory study was to compare several retinal microvascular parameters as evaluated by scanning laser Doppler flowmetry (SLDF) in nondiabetic and type 1 diabetic subjects with and without diabetic neuropathy.
Research Design and Methods
This was a single-center, exploratory, cross-sectional study. The study was performed with a group of nondiabetic volunteers and patients with type 1 DM. A total of 14 nondiabetic controls (C), 14 type 1 diabetic patients without neuropathy (DNP-), and 13 type 1 diabetic patients with diabetic neuropathy (DNP+) were recruited for study participation. All patients underwent retinal assessment by nonmydriatic fundus photography with subsequent judgment by a clinical ophthalmologist. Only patients with no signs of clinical retinopathy (level 1 according to the Airlie House Classification) were included in the final analysis of the study. All study participants had to be free from vasoactive medications. The study adhered to the tenets of the Declaration of Helsinki, and was approved by the local ethics committee. All patients gave their written informed consent prior to any study procedure.
Assessment of Retinal Microcirculation
Retinal capillary blood flow was assessed using SLDF at 670 nm (Heidelberg Retina Flowmeter, Heidelberg Engineering, Germany). The reliability of the method is in the range of 0.96 – 0.99. For further details see Kreis et al.20 Briefly, a retinal sample of 2.56 × 0.64 × 0.30 mm was scanned within 2 seconds at a resolution of 256 points × 64 lines × 128 lines. The confocal technique of the device ensured that only the capillary blood flow of the superficial retinal layer of 300 µm was measured. Measurements were performed in the juxtapapillary area of both eyes 2 to 3 mm temporally to the optic nerve; the average from 3 singular measurements was taken for further analysis.
Analysis of perfusion images was performed offline with automatic full-field perfusion imaging analysis (AFFPIA). This led to a perfusion map excluding vessels with a diameter of > 30 µm, without lines with saccades, and without pixels with inadequate reflectivity. The mean retinal capillary blood flow was calculated in the area of interest and expressed as arbitrary units (AU). In our study, retinal capillary blood flow was measured before and after flicker-light stimulation (10 Hz over 3 minutes; Photo Stimulator 750, Siemens-Elema AB, Solna, Sweden).
Analysis of vessel diameters was performed offline with automatic full field perfusion imaging analysis (SLDF version 3.7).21,22 Outer arteriole diameter (AD) was measured in reflection images, and lumen diameter (LD) was measured in perfusion images. The wall to lumen ratio (WLR) was calculated as (AD-LD)/LD.
The laser scanning records were stored electronically and sent to a central reading center (Interdisciplinary Center for Ophthalmic Preventive Medicine and Imaging (IZPI) of the Friedrich-Alexander-University Erlangen-Nuernberg, Germany), for the assessment of retinal microvascular blood flow and the calculation of the retinal WLR. The ophthalmological reading center was blinded about the clinical characterization of the study participants.
Assessment of Peripheral Neuropathy
The neuropathy disability score (NDS) was used for the characterization of diabetic peripheral sensory neuropathy.23 Vibration perception thresholds were assessed at the proximal joint of the first toe in both feet within a range of 0.1 to 4.0 microns/sec using the vibratory sensory analyzer (Medoc, Ramat Yishai, Israel).24
Statistical Analysis
This trial was designed as an exploratory, cross-sectional study aimed to generate descriptive information about a potential link between peripheral sensory dysfunction and functional changes in retinal microvascular blood flow or retinal angioarchitecture. Therefore, no a priori confirmatory sample size estimation was performed. All study endpoints were analyzed with equal priority in a nonconfirmatory, exploratory sense. RBF and WLR were assessed in a central reading center blinded about the clinical characterization of the study volunteers. All other study endpoints were assessed in an open label approach. All study results were evaluated using primarily descriptive statistics. Differences in means of study endpoints were tested by Student’s t test. In case of not equal variances in the data, the results of the Welch approximation were taken into account as result of the unpaired comparison. Linear regression analysis was performed to search for potential associations between retinal microvascular parameters and other study parameters. Significance level was set at a P value less than .05.
Results
A total of 41 subjects were recruited for study participation and stratified to 3 different groups according to their metabolic and neurological status: 14 no-diabetic volunteers without neuropathy (C), 14 diabetic patients without neuropathy (DNP-), and 13 diabetic patients with peripheral sensory neuropathy (DNP+). The clinical characteristics of the different study groups are presented in Table 1. Both diabetic groups had higher HbA1c levels compared with the control group. Patients with diabetic neuropathy were slightly older compared with the control group and had a higher BMI compared with diabetic patients without neuropathy. No other significant differences in the clinical characteristics could be observed between the study groups.
Table 1.
Clinical Characteristics of the Investigated Groups.
| C | DNP- | DNP+ | |
|---|---|---|---|
| Gender (male/female) | 7/7 | 6/8 | 7/6 |
| Age (years) | 45.2 ± 7.6 | 48.6 ± 6.4 | 53.5 ± 7.5* |
| BMI (kg/m²) | 29.0 ± 5.6 | 25.7 ± 3.0 | 30.7 ± 6.2$ |
| Syst blood pressure (mmHg) | 122 ± 20 | 128 ± 11 | 129 ± 9 |
| Diast blood pressure (mmHg) | 79 ± 15 | 76 ± 7 | 79 ± 7 |
| HbA1c (%) | 5.6 ± 0.4 | 7.7 ± 0.7* | 7.6 ± 0.9* |
| Duration of diabetes (years) | 23.6 ± 7.7 | 26.3 ± 7.6 |
Values are mean ± SD. C, control; DNP-, type 1 diabetic patients without neuropathy; DNP+, type 1 diabetic patients with neuropathy.
P < .05 vs control group. $ P < .05 vs DNP-.
As shown in Figure 1, baseline and retinal blood flow after stimulation with flicker light were significantly higher in patients with diabetic polyneuropathy compared with nondiabetic volunteers and type 1 diabetic patients without polyneuropathy. No difference in basal retinal blood flow or in flicker stimulated retinal blood flow could be observed between the nondiabetic control group and the group of type 1 diabetic patients without polyneuropathy. The absolute increase in retinal blood flow from baseline after retinal stimulation with flicker light was comparable between all 3 groups. No difference could be observed with regard to the arterial WLR between the 3 different groups (Table 2).
Figure 1.
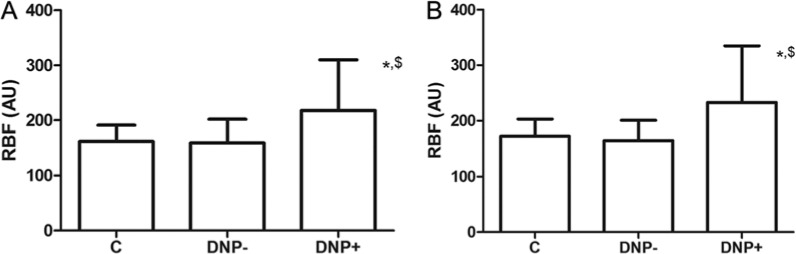
(A) Baseline retinal blood flow in nondiabetic volunteers and patients with type 1 diabetes mellitus with and without peripheral sensory polyneuropathy. (B) Flicker light stimulated retinal blood flow in nondiabetic volunteers and patients with type 1 diabetes mellitus with and without peripheral sensory polyneuropathy. Values are mean ± SD. AU, arbitrary units; C, nondiabetic control subjects; DNP-, diabetic patients without polyneuropathy; DPN+, diabetic patients with polyneuropathy; RBF, retinal blood flow. *P < .05 vs nondiabetic volunteers. $ P < .05 vs diabetic patients without polyneuropathy.
Table 2.
Retinal Blood Flow (RBF), Arterial Wall to Lumen Ration (WLR), Neuropathy Disability Score (NDS), and Vibration Perception Threshold (VPT) in the Investigated Groups.
| C | DNP- | DNP+ | |
|---|---|---|---|
| RBF baseline (arbitrary units) | 162 ± 29 | 159 ± 42 | 217 ± 112* ,$ |
| RBF stimulated (arbitrary units) | 173 ± 30 | 164 ± 36 | 232 ± 101* ,$ |
| Delta RBF after flicker (%) | 10.2 ± 10.9 | 10.4 ± 14.9 | 14.0 ± 13.1 |
| WLR | 0.42 ± 0.07 | 0.46 ± 0.09 | 0.43 ± 0.09 |
| NDS | 0.6 ± 1.2 | 0.4 ± 0.9 | 3.8 ± 1.2* ,$ |
| VPT | 4.6 ± 3.0 | 6.5 ± 7.0 | 11.4 ± 11.7* ,$ |
Values are mean ± SD. C, control; DNP-, type 1 diabetic patients without neuropathy; DNP+, type 1 diabetic patients with neuropathy.
P < .05 vs control group. $ P < .05 vs DNP-.
Patients with historically established polyneuropathy had a significant higher NDS and a higher vibration perception threshold compared with type 1 diabetic patients without polyneuropathy and the group of nondiabetic volunteers (Table 2). A significant linear correlation could be observed between the NDS and basal retinal blood flow (r = .44, P < .0001) as well as stimulated retinal blood flow (r = .44, P < .0001). As shown in Figure 2, vibration perception threshold at the feet was also related to basal retinal blood flow (r = .38, P < .001) as well as stimulated retinal blood flow (r = .43, P < .0001). No association could be observed between basal or stimulated retinal blood flow with HbA1c, diabetes duration, or systolic or diastolic blood pressure.
Figure 2.
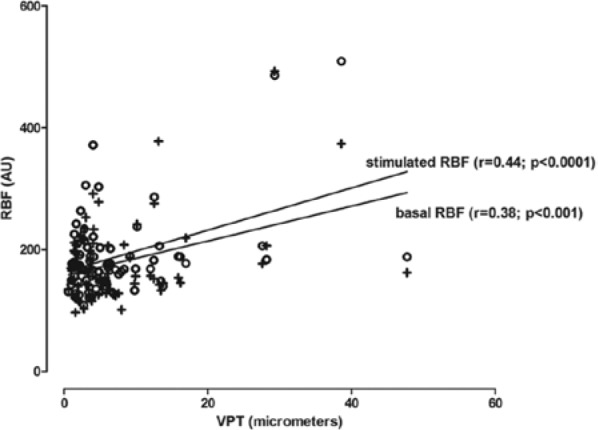
Linear regression between vibration perception threshold and basal or flicker stimulated retinal blood flow. AU, arbitrary units; VPT, vibration perception threshold; ○, baseline retinal blood flow; +, stimulated retinal blood flow.
Discussion
The main thesis generated by our study is that in type 1 diabetic patients suffering from peripheral sensory neuropathy retinal microvascular blood flow appears to be increased, while no difference in retinal blood flow was found between type 1 diabetic patients without neuropathy and the nondiabetic control group. A linear correlation could be observed between the increase in retinal blood flow and an increased NDS or the vibration perception threshold.
Recent studies on retinal blood flow in patients with DM revealed conflicting results. Several studies found an increased retinal blood flow and vasodilatation of retinal vessels in patients with DM.4,5,25,26 Others reported a reduction in retinal blood flow in diabetic patients even at early stages of diabetic retinopathy.6,7 It was assumed that elevated blood glucose levels might affect retinal microcirculation. Increased blood glucose levels up to 300 mg/dl were found with an augmentation in retinal blood flow,7,27 while other studies showed a negative correlation or no effects of blood glucose levels on retinal blood flow.4,5 In our study, no association could be observed between HbA1c and retinal blood flow. Arterial blood pressure was assumed as another confounder in RBF. While in nondiabetic subjects a weak association between blood pressure and retinal blood flow could be observed, this association was not confirmed in patients with DM.4,28 In our study, no association between systolic or diastolic blood pressure and retinal blood flow could be observed.
Neurovascular coupling is the process that enables the retina to regulate blood flow in response to neural activity. Neural cells of the retina are also affected in DM, resulting in dysfunction and degeneration,29 and diabetic retinopathy is a disease of both retinal neurons and microcirculation.30 In our study, type 1 diabetic patients with peripheral neuropathy were found with an increased retinal blood flow at baseline and after stimulation with flicker light. This finding is consistent with a study from Sabanayagam et al, where an association between peripheral neuropathy and an increase in retinal arterial and venous caliber was observed in a large Asian population with DM.19
The vascular response to luminance flicker light stimulation was shown to be reduced in patients with DM.8,10 The increase of neural activity induced by flicker stimulation leads to arterial and venous dilatation.31 Because retinal blood flow is coupled with neuronal activity, reduced flicker light induced vasodilatation might also reflect neurodegeneration. In a recent investigation by Lecleire-Collet et al, a linear association was found between flicker light induced retinal arterial vasodilatation and electroretinographic abnormalities,16 indicating that a reduced neural activity in the retina might cause a decreased flicker response in DM. We did not find a significant difference in the vascular response to flicker light between the patients with or without neuropathy. It needs to be considered that in the study by Lecleire-Collet et al vessel diameter was assessed, while our study addressed retinal microvascular blood flow as measured by laser Doppler flowmetry.
Several recent studies have shown that the retinal microvascular response to flicker light is impaired under certain pathological conditions like DM8,9 or essential hypertension.32,33 It is suggested that in case of DM or hypertension, endothelial dysfunction and the restricted capability of the endothelial cell to secrete NO might cause a disturbed microvascular blood flow in several tissues prone for the development of microvascular complications. Therefore, it seems conceivable that diabetic neuropathy is associated with an increase in overall retinal blood flow, while an attenuated hyperemic response to luminance flicker light might indicate impaired endothelial microvascular function.
In DM, the LD of retinal arterioles and small arteries is narrowed due to growth of smooth muscle cells and vascular fibrosis. These changes are described as arterial remodeling that is best characterized by an increase in the WLR. An increased arterial retinal WLR has been described in patients with a history of cerebrovascular events and in patients with an increased albumin excretion rate.34-36 It is well known that the thickening of basement membrane is a hallmark of diabetic retinopathy, and a main contributor to capillary narrowing. In our study population, no significant difference in the retinal arterial WLR could be observed between nondiabetic controls and type 1 diabetic patients with or without peripheral neuropathy. It needs to be considered that only patients without morphological findings of diabetic retinopathy were included in our study, which most probably could explain the normal WLR found in our group of type 1 diabetic patients. Further research is necessary to evaluate the retinal WLR in those patients with more advanced stages of diabetic retinopathy.
In conclusion, our study has shown an augmented retinal microvascular blood flow in type 1 diabetic patients with peripheral sensory polyneuropathy. No structural changes in retinal angioarchitecture could be observed between nondiabetic controls and type 1 diabetic patients with or without peripheral neuropathy. Our results suggest functional changes in retinal microvascular blood flow in association with peripheral sensory impairment even before the development of morphological alterations in angioarchitecture or the development of clinically evident diabetic retinopathy.
This study has some important limitations. First, this study was designed as an exploratory study without a priori sample size calculation. All results have to be interpreted with equal magnitude in a nonconfirmatory sense. Second, the cross-sectional design of the study does not allow justification of a causal relationship between diabetic neuropathy and changes in retinal microvascular blood flow. The association between peripheral sensory impairment and functional changes in retinal blood flow might reflect a causal relationship and/or suggest that they may share a common pathogenic mechanism. Prospective studies with well-powered sample sizes are now warranted to confirm our study results and to assess the clinical significance of our findings for the development of diabetic retinopathy.
Footnotes
Abbreviations: AD, arteriole diameter; AFFPIA, automatic full-field perfusion imaging analysis; BMI, body mass index; DM, diabetes mellitus; IZPI, Interdisciplinary Center for Ophthalmic Preventive Medicine and Imaging; LD, lumen diameter; NDS, neuropathy disability score; NO, nitric oxide; RBF, retinal blood flow; SLDF, scanning laser Doppler flowmetry; VPT, vibration perception threshold; WLR, wall to lumen ratio.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603-607. [DOI] [PubMed] [Google Scholar]
- 2. Schmetterer L, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Int J Obes Relat Metab Disord. 1999;42:387-405. [DOI] [PubMed] [Google Scholar]
- 3. Ludovico J, Bernardes R, Pires I, Figueira J, Lobo C, Cunha-Vaz J. Alterations of retinal capillary blood flow in preclinical retinopathy in subjects with type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2003;241:181-186. [DOI] [PubMed] [Google Scholar]
- 4. Burgansky-Eliash Z, Barak A, Barash H, et al. Increased retinal blood flow velocity in patients with early diabetes mellitus. Retina. 2012;32:112-119. [DOI] [PubMed] [Google Scholar]
- 5. Cuypers MH, Kasanardjo JS, Polak BC. Retinal blood flow changes in diabetic retinopathy measured with the Heidelberg scanning laser Doppler flowmeter. Graefes Arch Clin Exp Ophthalmol. 2000;238:935-941. [DOI] [PubMed] [Google Scholar]
- 6. Arend O, Wolf S, Harris A, Reim M. The relationship of macular microcirculation to visual acuity in diabetic patients. Arch Ophthalmol. 1995;113:610-614. [DOI] [PubMed] [Google Scholar]
- 7. Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37:886-897. [PubMed] [Google Scholar]
- 8. Mandecka A, Dawczynski J, Blum M, et al. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048-3052. [DOI] [PubMed] [Google Scholar]
- 9. Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen TT, Kawasaki R, Wang JJ, et al. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009;32:2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dogra G, Rich L, Stanton K, Watts GF. Endothelium-dependent and independent vasodilation studies at normoglycaemia in type I diabetes mellitus with and without microalbuminuria. Int J Obes Relat Metab Disord. 2001;44:593-601. [DOI] [PubMed] [Google Scholar]
- 13. Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc Res. 1996;52:13-26. [DOI] [PubMed] [Google Scholar]
- 14. Michelson G, Patzelt A, Harazny J. Flickering light increases retinal blood flow. Retina. 2002;22:336-343. [DOI] [PubMed] [Google Scholar]
- 15. Kondo M, Wang L, Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand. 1997;75:232-235. [DOI] [PubMed] [Google Scholar]
- 16. Lecleire-Collet A, Audo I, Aout M, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52:2861-2867. [DOI] [PubMed] [Google Scholar]
- 17. Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122:76-83. [DOI] [PubMed] [Google Scholar]
- 18. Rogers SL, Tikellis G, Cheung N, et al. Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008;31:761-763. [DOI] [PubMed] [Google Scholar]
- 19. Sabanayagam C, Tai ES, Lee J, Lim SC, Wong TY. Retinal vessel caliber and peripheral neuropathy in diabetic participants. Microcirculation. 2010;17:297-302. [DOI] [PubMed] [Google Scholar]
- 20. Kreis AJ, Nguyen T, Rogers S, et al. Reliability of different image analysis methods for scanning laser Doppler flowmetry. Curr Eye Res. 2008;33:493-499. [DOI] [PubMed] [Google Scholar]
- 21. Michelson G, Welzenbach J, Pal I, Harazny J. Functional imaging of the retinal microvasculature by scanning laser Doppler flowmetry. Int Ophthalmol. 2001;23:327-335. [DOI] [PubMed] [Google Scholar]
- 22. Michelson G, Welzenbach J, Pal I, Harazny J. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthalmol. 1998;82:1294-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dyck PJ, Kratz KM, Lehman KA, et al. The Rochester Diabetic Neuropathy Study: design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology. 1991;41:799-807. [DOI] [PubMed] [Google Scholar]
- 24. Yarnitsky D, Sprecher E. Thermal testing: normative data and repeatability for various test algorithms. J Neurol Sci. 1994;125:39-45. [DOI] [PubMed] [Google Scholar]
- 25. Falck A, Laatikainen L. Retinal vasodilation and hyperglycaemia in diabetic children and adolescents. Acta Ophthalmol Scand. 1995;73:119-124. [DOI] [PubMed] [Google Scholar]
- 26. Pemp B, Polska E, Garhofer G, Bayerle-Eder M, Kautzky-Willer A, Schmetterer L. Retinal blood flow in type 1 diabetic patients with no or mild diabetic retinopathy during euglycemic clamp. Diabetes Care. 2010;33:2038-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grunwald JE, Riva CE, Martin DB, Quint AR, Epstein PA. Effect of an insulin-induced decrease in blood glucose on the human diabetic retinal circulation. Ophthalmology. 1987;94:1614-1620. [DOI] [PubMed] [Google Scholar]
- 28. Fuchsjager-Mayrl G, Polak K, Luksch A, et al. Retinal blood flow and systemic blood pressure in healthy young subjects. Graefes Arch Clin Exp Ophthalmol. 2001;239:673-677. [DOI] [PubMed] [Google Scholar]
- 29. Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bloomgarden ZT. Diabetic retinopathy. Diabetes Care. 2008;31:1080-1083. [DOI] [PubMed] [Google Scholar]
- 31. Formaz F, Riva CE, Geiser M. Diffuse luminance flicker increases retinal vessel diameter in humans. Curr Eye Res. 1997;16:1252-1257. [DOI] [PubMed] [Google Scholar]
- 32. Nagel E, Vilser W, Lanzl I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Invest Ophthalmol Vis Sci. 2004;45:1486-1492. [DOI] [PubMed] [Google Scholar]
- 33. Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke. 2004;35:1289-1293. [DOI] [PubMed] [Google Scholar]
- 34. Ritt M, Harazny JM, Ott C, et al. Wall-to-lumen ratio of retinal arterioles is related with urinary albumin excretion and altered vascular reactivity to infusion of the nitric oxide synthase inhibitor N-monomethyl-L-arginine. J Hypertens. 2009;27:2201-2208. [DOI] [PubMed] [Google Scholar]
- 35. Harazny JM, Ritt M, Baleanu D, et al. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007;50:623-629. [DOI] [PubMed] [Google Scholar]
- 36. Baleanu D, Ritt M, Harazny J, Heckmann J, Schmieder RE, Michelson G. Wall-to-lumen ratio of retinal arterioles and arteriole-to-venule ratio of retinal vessels in patients with cerebrovascular damage. Invest Ophthalmol Vis Sci. 2009;50:4351-4359. [DOI] [PubMed] [Google Scholar]


