Abstract
The risks associated with gestational diabetes (GD) can be reduced with an active treatment able to improve glycemic control. Advances in mobile health can provide new patient-centric models for GD to create personalized health care services, increase patient independence and improve patients’ self-management capabilities, and potentially improve their treatment compliance. In these models, decision-support functions play an essential role. The telemedicine system MobiGuide provides personalized medical decision support for GD patients that is based on computerized clinical guidelines and adapted to a mobile environment. The patient’s access to the system is supported by a smartphone-based application that enhances the efficiency and ease of use of the system. We formalized the GD guideline into a computer-interpretable guideline (CIG). We identified several workflows that provide decision-support functionalities to patients and 4 types of personalized advice to be delivered through a mobile application at home, which is a preliminary step to providing decision-support tools in a telemedicine system: (1) therapy, to help patients to comply with medical prescriptions; (2) monitoring, to help patients to comply with monitoring instructions; (3) clinical assessment, to inform patients about their health conditions; and (4) upcoming events, to deal with patients’ personal context or special events. The whole process to specify patient-oriented decision support functionalities ensures that it is based on the knowledge contained in the GD clinical guideline and thus follows evidence-based recommendations but at the same time is patient-oriented, which could enhance clinical outcomes and patients’ acceptance of the whole system.
Keywords: clinical guidelines, decision support system, gestational diabetes, m-health, telemedicine
Gestational diabetes (GD) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1 It is a prevalent condition present in 7% (ranging from 1% to 14%) of pregnancies.2 Several adverse outcomes are associated with GD, as cesarean section, shoulder dystocia, fetal macrosomia, neonatal respiratory problems, or metabolic complications. The risks of perinatal complications can be reduced with an active treatment, according to recent randomized control trials.3,4
GD has long-term health impact, being the probability of developing GD in a subsequent pregnancy of the order of 30% to 50%.5 Women with GD are at an increased later risk of type 2 diabetes, as high as 50% within 5 years in some populations.6
Telemedicine systems have been widely applied to diabetes care, showing that they are able to improve clinical outcomes and self-care by registering electronically diabetes monitoring data.7-11 In the field of pregnancy complicated with diabetes, telemedicine systems have shown that they are able to reduce the need for outpatient clinical encounters,12 achieve better pregnancy outcomes,13 and enhance feelings of psychosocial self-efficacy.14 Telemedicine for GD can be complemented by knowledge management tools to aid doctors with therapy planning.15 This type of decision-support system (DSS) could potentially contribute to enhance the general adoption of telemedicine systems for diabetes.16
DSSs could support not only doctors, but also patients; advances in the area of mobile communication for health care (m-Health) allow the design and development of patient-centric models to create personalized telemedicine services, increase patient independence and improve patient’s self-management capabilities. Patients can benefit from the use of personal assistants based on portable devices and supported by a telemedicine platform.17 Mobile applications for people with diabetes allow using automatic processing tools to provide real-time advice based on monitoring data and expert feedback,18 have a motivational effect on the users,19 and show important benefits when providing feedback to patients about blood glucose (BG) monitoring.20 In addition, mobile technologies are well accepted by patients with diabetes.21
DSSs can base their knowledge on clinical practice guidelines (CPGs). CPGs provide recommendations on the appropriate treatment and care of people with a specific disease and conditions, and they are based on a systematic review of clinical evidence. Even though the use of mobile systems to support self-management of diabetes is widely extended, there are gaps between the evidence-based recommendations in CPGs and the functionality used in study interventions or found in online markets, which could limit the obtained benefits and final outcomes.22
This article describes the process based on CPGs to extract personalized decision support for patients with GD, which will be delivered anytime everywhere, supported by a telemedicine system for patient guidance (MobiGuide).23 The MobiGuide services are based on computer-interpretable guidelines (CIGs): formal representations of CPGs that can be executed to provide guideline-based decision support specific to patient’s data.
The next sections describe the main components of MobiGuide, the methodology to elicit decision-support functionalities addressed to patients based on the GD clinical guideline, the components of the smartphone application, the formalization of the guideline into workflows, and patient-oriented advice to be generated in MobiGuide.
Methods
The MobiGuide Patient Guidance System
The MobiGuide system aims to help patients to manage their illness by monitoring disease parameters and providing, in a mobile environment, the appropriate feedback generated on the basis of the patients’ preferences (eg, meal times) and context (eg, routine vs semiroutine meal schedule).
The main components of MobiGuide system are (1) a back-end DSS, devoted to the representation and execution of CIGs, which projects parts of the knowledge to a complementary mobile DSS (mDSS) that supports the distribution of decision support; (2) a body area network (BAN) that provides real-time monitoring of biosignals supported by a smartphone application that communicates with the back-end server and manages the communications between different components in the smartphone (eg, QoD, mDSS) and provides authentication policies; (3) a personal health record (PHR) that is ubiquitously and securely accessible and integrates patients’ personal data with data from hospital electronic medical records (EMRs) and the BAN data sources; and (4) a knowledge base containing CIGs. The DSS matches the CIG knowledge with PHR data to deliver CPG-based recommendations. GD is 1 of the target clinical domains to test the MobiGuide system and its clinical effectiveness in a clinical pilot.
The Smartphone User Interface (UI)
The goal of the smartphone UI, which can be run in Android devices from version 2.x, is to enhance the efficiency and ease of use for the underlying logical design of the system. The mobile application is the patient’s interface to the MobiGuide system, allowing reception of patient guidance services supported by the CIG-based DSS. The UI provides functions to configure guidance through patient’s personal context selection and to receive personalized recommendations based on CPGs. The UI for GD patients allows manual data insertion, automatic acquisition from sensors (Bluetooth-enabled glucometer and blood pressure monitor and smartphone’s accelerometer) and visualization of monitoring data including BG measurements, diet incompliance, ketonuria results, insulin administration (when required), and physical activity sessions.
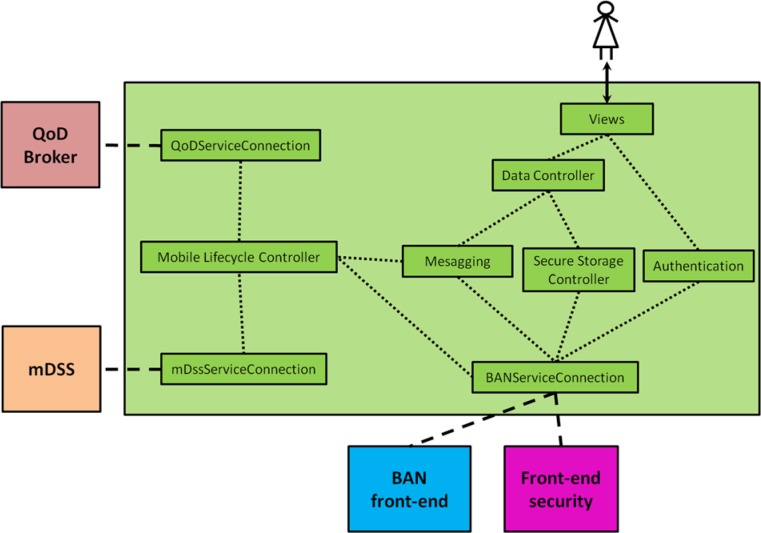
The smartphone UI has been designed following a modular approach based on the model-view-controller architecture. Figure 1 shows its internal structure, detailing the logic interactions amongst the functional modules of the smartphone UI, as well as the interactions between the UI and the rest of the mobile components of MobiGuide system (for clarity, Figure 1 does not reflect the interactions between the rest of components inside the smartphone).
Figure 1.
Internal structure of the smartphone user interface.
Views: The different graphical elements and screens of the UI.
Authentication: Manages BAN activation and user authentication and authorization information.
Data Controller: Manages application data (monitoring data inserted by the patient or to be visualized), context data, configuration data, and recommendations.
Secure Storage Controller: Deals with the secure storage of all configuration and personalization data.
Messaging: Manages the message exchange with other components of the system (the quality of data (QoD) service provided by the QoD broker, which impacts decision making) and the mDSS, which provides patient-tailored recommendations based on CIG knowledge projected from the back-end DSS.
BANServiceConnection: Interface between the smartphone UI and the BAN, facilitating all the BAN services that can be used by the GD application (including the methods to control its lifecycle).
QoDServiceConnection and mDssServiceConnection: The smartphone UI uses these interfaces to access mDSS and QoD broker lifecycle control methods.
Mobile Lifecycle Controller: Mobile components’ lifecycle choreographer.
Specification of Patient-oriented Decision Support From CPGs
The methodology to extract patient-oriented knowledge from CPGs has 3 steps. The first 2 are based on part of the knowledge acquisition and specification process, which has been previously developed and evaluated for the specification of CPGs by expert physicians and knowledge engineers.24,25
Selection of GD Guideline
Since 2005, several evidence-based guidelines have been published on diabetes in pregnancy1,26 and new diagnostic criteria and their implications needed to be considered.27 So, a local narrative consensus-guideline for GD was generated by expert endocrinologists of Hospital de Sabadell based on the previously published documents.
Specification of the Local Consensus
The GD local narrative consensus-guideline was analyzed and the main procedures involved in GD management were identified. We iteratively developed a semiformal graphical representation of the CPG to produce consensus workflows specifying just the parts of the CPG that would be within the scope of the telemedicine system, free from ambiguity and accepted by the knowledge engineers and medical experts, considering both explicit and implicit medical knowledge necessary to provide decision support.
Because the DSS provides advice to patients, in addition to care providers, we have formalized the GD guideline in 2 parallel workflows.28 Creating the patient’s workflow involved identifying those recommendations that would imply advice for the patient, typically to be carried out at home (eg, to measure BG). Another extension of the original knowledge acquisition method involved determining how decision-support functionalities should be distributed between the back-end DSS and mDSS.
A novel step added to the knowledge acquisition process included customization.
Customization
We elaborated the specification of the GD guideline with customization of the CIGs to address patient’s personal context (eg, reduce number of BG measurements during periods of semiroutine meal schedules) and the system’s technological context (eg, when the battery is low, delegate processing to different system components), as well as allow personalization based on local preferences (eg, sending reminders at patient-specific meal times).
Results
Formalization of Gestational Diabetes Guideline for the Telemedicine System
The analysis and formalization of the GD local guideline consensus, performed by knowledge engineers in consultation with experts of Hospital de Sabadell, was carried out in 10 iterations. The contents were formalized as a top-level workflow of the GD management process and more specific workflows for each of its main processes.
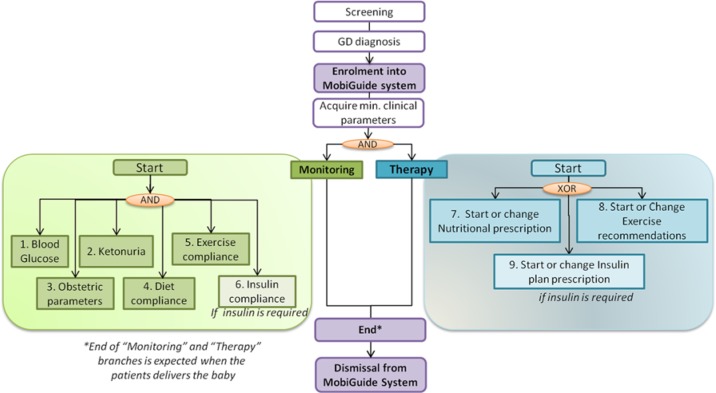
Figure 2 shows the main workflows identified in GD. We scoped the care process to be supported by the MobiGuide system to start after the diagnosis of GD. To initialize the system with preexisting conditions, it is required to acquire a set of minimum clinical parameters, such as clinical history, health state, current treatment, and so on. After GD is confirmed by the endocrinologist, the patient can be enrolled into the MobiGuide system, and 2 different workflows are activated: (1) Monitoring variables involved in the management of GD, including monitoring clinical parameters such as BG, ketonuria, or relevant obstetrical parameters and monitoring the patient’s compliance with the prescribed treatment; and (2) Therapy, which can be related to nutritional, exercise, or insulin prescription. The dismissal of the patient from the MobiGuide system will be done when GD is not present, which usually happens after delivery of the baby.
Figure 2.
Main workflows identified from the gestational diabetes guideline to be supported by the MobiGuide system.
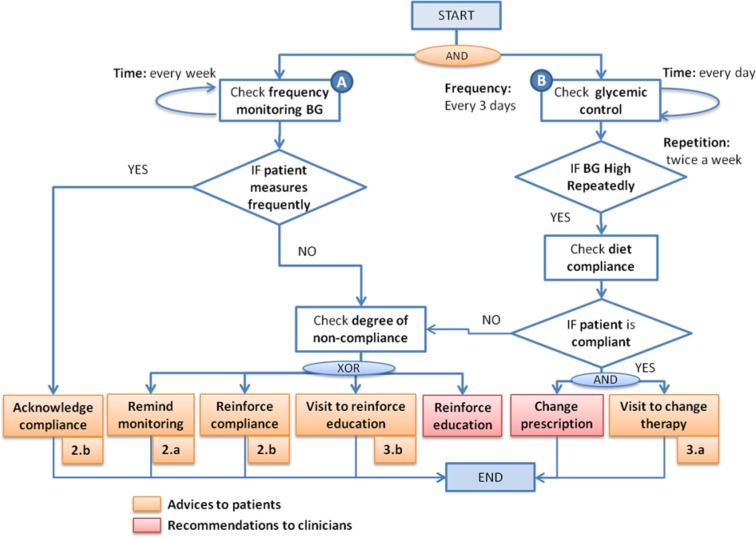
As an example of patient-tailored advice extracted from the GD guideline, Figure 3 shows a simplified version of the workflow for BG Monitoring. We consider 2 different workflows to establish the conditions that would generate patient advice, for a specific patient: (1) check frequency of monitoring BG and (2) check glycemic control (ie, BG level). It is important to assess the patient’s compliance to (1) monitoring instructions or to (2) nutritional prescription, as checking compliance is usually done by clinicians during clinical encounters while here it is done by the MobiGuide system.
Figure 3.
Example of patient advice associated with the workflow blood glucose (BG) monitoring. Monitoring advice: (2a) Reminder to monitor BG and (2b) messages to acknowledge compliance or to reinforce compliance. Clinical assessment advice: (3a) Inform about therapy change and (3b) ask to contact the hospital to reinforce education.
The formalization of each care process defines 2 parallel workflows: one to support patients and the other for providing decision support to clinicians. As in Figure 3, regarding BG monitoring, clinicians would receive recommendations to reinforce the patient’s education when the system detects that the patient is repeatedly not being compliant to nutritional prescription. Also, clinicians will get recommendations to change the current prescription regarding diet, exercise, and/or insulin when it is detected that glycemic control is not achieved.
Extraction of Patient-oriented Advice
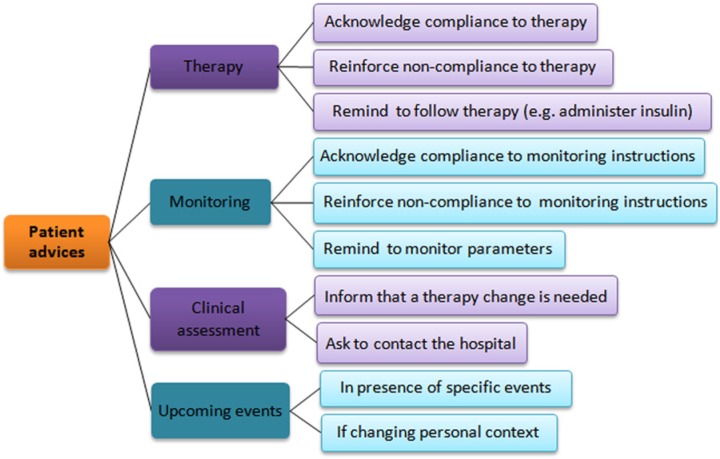
After creating the local consensus of the GD guideline as a set of parallel workflows, we identified 4 different types of advice addressed to patients (Figure 4):
Figure 4.
Classification of patient advice extracted from the gestational diabetes guideline formalization.
Therapy Advice
The aim of therapy advice is to help the patient to comply with medical prescription. Two different types of advice are identified:
Messages to acknowledge or to reinforce the patient’s compliance with therapy prescriptions
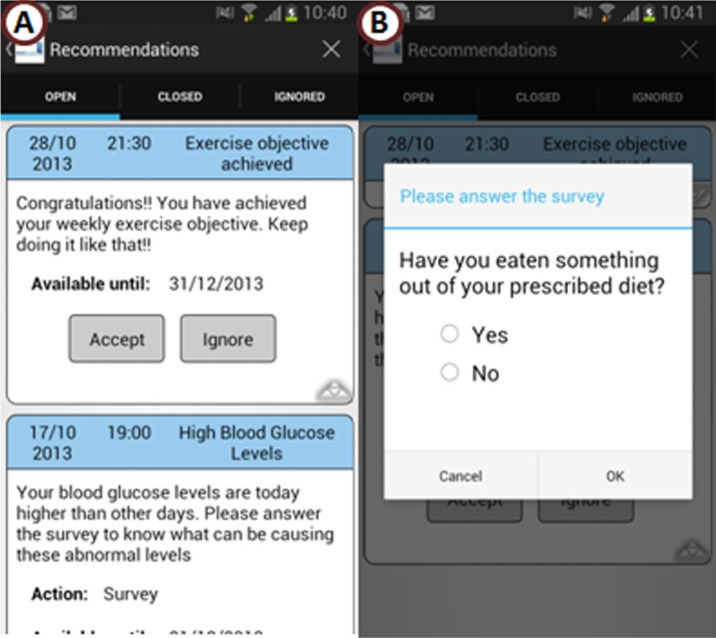
Patients with GD are responsible for following therapy prescriptions at home. The workflows related to “monitor diet compliance” and “monitor exercise compliance” will control the patient’s adherence to clinicians’ recommendations. In addition, if the patient needs to administer insulin, the workflow “monitor insulin compliance” will be used. Monitoring diet or insulin compliance will allow establishing the cause and effect relationship between prescribed therapy (diet or insulin) and BG level. As in Figure 5, the system will ask the patient about diet compliance when anomalous BG levels are detected. On the contrary, when the patient shows good compliance with therapy prescription, she or he will receive feedback from the system to reinforce her or his motivation while managing the disease (Figure 5).
Figure 5.
Example of therapy advice delivered to the patient: (A) Congratulations message to acknowledge good compliance and advice about the presence of high BG levels; (B) survey to check if the patient is following diet compliance.
Reminders to follow therapy prescription related to insulin administration
This type of advice will be generated according to a personalized schedule of the patient. The patient may choose to get reminders for all of her or his daily injections or just specific ones.
Monitoring Advice
The aim of monitoring advice is to help the patient comply with monitoring instructions. Two different types of advice are identified:
Reminders to monitor specific parameters such as BG, exercise, or ketonuria (Figure 3, Remind monitoring)
In MobiGuide, physical activity practicing and its intensity is detected automatically with a physical activity detector. Reminders will be generated for those patients who activate this functionality, according to the personalized schedule.
Messages to acknowledge or reinforce the patient’s compliance with monitoring parameters
When the patient does not perform measurements frequently enough or when she is not downloading data to the system so that data can be analyzed according to periodic requirements, the patient will be warned. If the situation persists, the patient could be asked to attend an on-site visit, after notifying the clinician (Figure 3, Reinforce compliance). On the contrary, if the patient is following monitoring instructions appropriately, she or he will receive periodic congratulations messages to keep her or him motivated (Figure 3, Acknowledge compliance).
Clinical Assessment Advice
The aim of clinical assessment advice is to inform patients about the assessment of GD conditions, generally reflected by monitoring parameters such as BG or ketonuria. We identified 2 types of advice:
Messages to inform that a therapy change is needed after the analysis of current monitoring parameter values (Figure 3, Visit to change therapy)
Some of these messages (such as diet or insulin therapy changes) should be confirmed by clinicians before they are delivered to patients.
Messages asking patients to contact the hospital
Some conditions detected by the telemedicine system could require a physical encounter at the hospital, such as a session to reinforce education when the patient is not being compliant repeatedly (Figure 3, Visit to reinforce education) or when it is required to start insulin treatment.
Upcoming event advice
The aim of these advice is to help modulating the patient’s monitoring instructions or therapy follow-up, according to personal context or sporadic events. The advice are related to personal context situations when the diet, physical activity, or monitoring schedule of the patient may not be routine, as in an upcoming holiday. These events would cause the generation of reminders or specific messages, once physicians relate patient-specific events to personal contexts in the customized CIGs. The context of the messages could be preconfigured by the physician.
Patients too have a role in personalizing advice. Patients can personalize their preferred time to perform physical activity and its intensity level or duration, depending on their personal context. For example, if the patients are traveling, they can activate their “semiroutine meal schedule” context and then will not receive alerts about low compliance to physical activity recommendations.
Discussion
Patients with GD need to acquire the appropriate knowledge about the disease in a relatively short period of time (in most cases from diagnosis to delivery lower than 6 months). And at the same time they need to manage complex procedures at home, including monitoring of BG, estimating proportions of carbohydrates for each meal, or, when required, administer insulin. On the other hand, these are generally very motivated patients as they are concerned with possible complications of the disease to the baby.
A telemedicine patient guidance system such as MobiGuide can accompany the patient wherever she goes, helping to manage GD at anytime, through personalized advice according to the knowledge contained in the clinical guideline. The intelligent DSS analyzes historical clinical data, as it is connected to the PHR that integrates all relevant patient data, whether arriving from the hospital EMRs, BAN sensors, or entered manually by the patient (on her or his own initiative or when requested by the DSS).
CIGs have been traditionally used to support clinicians’ decision making processes, by developing DSS able to automatically generate patient-specific recommendations at the point of care. So those recommendations related to “monitoring” and “therapy” processes are directed toward care professionals who can explain them to their patients, but they are not actually applied in a functional automatic manner by a DSS. In this work, we formalized the knowledge from the GD guideline to generate patient-oriented decision support in a patient-centric approach. The formalization included identifying those recommendations that could imply advice that is delivered by the system to the patient and in that way constitutes the starting point of a process of care that is parallel to the CPG and can be executed by the patient’s smartphone.
We have extracted 4 different types of advice from the GD guideline. Some of these recommendations will require approval from the clinician before being sent to the patient (eg, a therapy change). But other advice (eg, medication and measurement reminders and positive compliance feedback) can be applied independently of clinicians, which will contribute to minimizing the workload of clinicians because the system can assess the patients’ compliance to monitoring instructions or to therapy prescription instead of them, and present these results to the clinicians during routine visits.
Also, we extracted different types of reminders that will be available to each patient depending on her or his preferences. These reminders could be used to help the patient comply with monitoring instructions (eg, BG monitoring) or to remind her or him to administer insulin. This type of advice is considered optional to avoid excessive generation of advice that could overload the patient. However, balancing the right amount of reminders and feedback to the patient is a challenging issue that we still need to address.
Conclusions
This work presents a process, based on clinical guidelines, to extract the workflows that allow generating personalized decision support for patients with GD in the telemedicine patient guidance system. The formalization of the GD local consensus guideline as a CIG has allowed identifying 2 parallel patient-centric processes: one for the patient, and the other for the clinician. These processes are continuously applied by the DSS to deliver patient-oriented advice via the patient’s smartphone. Some of these advice consider the patient’s personal context, such as different schedules along workdays or holidays or upcoming events, which could trigger reminders or modify the advice that are generally configured.
The whole process followed to specify patient-oriented decision support functionalities assures that it follows evidence-based recommendations collected during care processes in GD, which could improve clinical outcomes and patient’s acceptance of the entire system.
Acknowledgments
This study was carried out as part of the MobiGuide project partially funded by the European Commission under the 7th Framework Program, grant 287811.
Footnotes
Abbreviations: BAN, body area network; BG, blood glucose; CIG, computer-interpretable guideline; CPG, clinical practice guidelines; DSS, decision-support system; EMR, electronic medical record; GD, gestational diabetes; PHR, personal health record; UI, user interface.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the European Commission under the 7th Framework Program, project MobiGuide: Guiding Patients Anytime Everywhere, grant 287811.
References
- 1. IDF Clinical Guidelines Task Force. Global Guideline on Pregnancy and Diabetes. Brussels, Belgium: International Diabetes Federation; 2009. [Google Scholar]
- 2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(suppl 1):S64-S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486. [DOI] [PubMed] [Google Scholar]
- 4. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30(5):1314-1319. [DOI] [PubMed] [Google Scholar]
- 6. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51(9):2796-2803. [DOI] [PubMed] [Google Scholar]
- 7. Gómez EJ, del Pozo F, Hernando ME. Telemedicine for diabetes care: the DIABTel approach towards diabetes telecare. Medical Inform. 1996;21(4):283-295. [DOI] [PubMed] [Google Scholar]
- 8. Bellazzi R, Larizza C, Montani S, et al. A telemedicine support for diabetes management: the T-IDDM project. Comput Methods Programs Biomed. 2002;69(2):147-161. [DOI] [PubMed] [Google Scholar]
- 9. Chase HP, Pearson JA, Wightman C, Roberts MD, Oderberg AD, Garg SK. Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care. 2003;26(5):1475-1479. [DOI] [PubMed] [Google Scholar]
- 10. Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: a systematic literature review. J Diabetes Sci Technol. 2010;4(3):666-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez-Ferre N, Galindo M, Fernandez MD, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol. 2010;2010:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalfra MG, Nicolucci A, Lapolla A, on behalf of the TISG. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. J Telemed Telecare. 2009;15(5):238-242. [DOI] [PubMed] [Google Scholar]
- 14. Homko CJ, Santamore WP, Whiteman V, et al. Use of an internet-based telemedicine system to manage underserved women with gestational diabetes mellitus. Diabetes Technol Ther. 2007;9(3):297-306. [DOI] [PubMed] [Google Scholar]
- 15. Hernando ME, Gómez EJ, Corcoy R, del Pozo F. Evaluation of DIABNET, a decision support system for therapy planning in gestational diabetes. Comput Methods Programs Biomed. 2000;62(3):235-248. [DOI] [PubMed] [Google Scholar]
- 16. Klonoff DC, True MW. The missing element of telemedicine for diabetes: decision support software. J Diabetes Sci Technol. 2009;3(5):996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García-Sáez G, Hernando ME, Martínez-Sarriegui I, et al. Architecture of a wireless personal assistant for telemedical diabetes care. Int J Med Inform. 2009;78:391-403. [DOI] [PubMed] [Google Scholar]
- 18. Hernando ME, García-Sáez G, Gómez EJ, del Pozo F. Intelligent alarms integrated in a multi-agent architecture for diabetes management. Trans Inst Meas Control. 2004;26(3):185-200. [Google Scholar]
- 19. Arsand E, Tatara N, Ostengen G, Hartvigsen G. Mobile phone-based self-management tools for type 2 diabetes: the few touch application. J Diabetes Sci Technol. 2010;4(2):328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farmer AJ, Gibson OJ, Dudley C, et al. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446). Diabetes Care. 2005;28(11):2697-2702. [DOI] [PubMed] [Google Scholar]
- 21. Martínez-Sarriegui I, García-Sáez G, Rigla M, et al. How continuous monitoring changes the interaction of patients with a mobile telemedicine system. J Diabetes Sci Technol. 2011;5(1):5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chomutare T, Fernandez-Luque L, Arsand E, Hartvigsen G. Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J Med Internet Res. 2011;13(3):e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalez-Ferrer A, Peleg M, Verhees B, Verlinden JM, Marcos C. Data integration for clinical decision support based on openEHR archetypes and HL7 virtual medical record. In: Lenz R, Miksch S, Peleg M, et al., eds. Process Support and Knowledge Representation in Health Care. 7738 ed Berlin, Germany: Springer; 2013:71-84. [Google Scholar]
- 24. Shalom E, Shahar Y, Taieb-Maimon M, et al. Ability of expert physicians to structure clinical guidelines: reality versus perception. J Eval Clin Pract. 2009;15(6):1043-1053. [DOI] [PubMed] [Google Scholar]
- 25. Shalom E, Shahar Y, Taieb-Maimon M, et al. A quantitative assessment of a methodology for collaborative specification and evaluation of clinical guidelines. J Biomed Inform. 2008;41(6):889-903. [DOI] [PubMed] [Google Scholar]
- 26. National Collaborating Centre for Women’s and Children’s Health. Diabetes in Pregnancy. London, UK: RCOG Press; 2008. Available at: http://www.nice.org.uk. Accessed October 30, 2013. [Google Scholar]
- 27. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
- 28. Sacchi L, Fux A, Napolitano C, et al. Patient-tailored workflow patterns from clinical practice guidelines recommendations. Paper presented at: Medinfo; August 19-23, 2013; Copenhagen, Denmark. [PubMed] [Google Scholar]