Abstract
The aim was to summarize research on telehealth remote patient monitoring interventions that incorporate key elements of structured self-monitoring of blood glucose (SMBG) identified as essential for improving A1C. A systematic review was conducted using the Medline, Cumulative Index to Nursing and Allied Health Literature, EMBASE, and OVID Medline databases with search terms “Telemedicine” AND “Monitoring, Physiologic” AND “Diabetes Mellitus, Type 2.” Study selection criteria included original randomized clinical trials evaluating the impact of telehealth remote patient monitoring on A1C among adults with type 2 diabetes and incorporated 1 or more essential elements of SMBG identified by the International Diabetes Federation (patient education, provider education, structured SMBG profile, SMBG goals, feedback, data used to modify treatment, interactive communication or shared decision making). Fifteen studies were included, with interventions ranging from 3 to 12 months (mean 8 months) with sample sizes from 30 to 1665. Key SMBG elements were grouped into 3 categories: education, SMBG protocols, and feedback. Research incorporating 5 of the 7 elements consistently achieved significant A1C improvements between study groups. Interventions using more SMBG elements are associated with an improvement in A1C. Studies with the largest A1C decrease incorporated 6 of the 7 elements and computer decision support. Two studies with 5 of the 7 elements and active medication management achieved significant A1C decreases. Telehealth remote patient monitoring interventions in type 2 diabetes have not included all structured monitoring elements recommended by the IDF. Incorporating more elements of structured SMBG is associated with improved A1C.
Keywords: A1C, diabetes, diabetes self-management education, remote patient monitoring, self-monitoring of blood glucose, telehealth
Approximately 26 million Americans have diabetes. When uncontrolled, diabetes is the seventh leading cause of death and the leading cause of kidney failure, blindness, and nontraumatic amputations in the United States.1 Researchers have found a decrease in complications and improved quality of life when persons with type 2 diabetes achieve national diabetes outcomes targets for blood glucose, blood pressure, and blood fats.2 Identifying evidence-based solutions to improve outcomes in this population is critical.
Patient self-management is an integral component of effective diabetes care and improves diabetes short-term outcomes.2-4 The American Association of Diabetes Educators (AADE) identifies healthy eating, being active, monitoring, taking medications, problem solving, healthy coping, and reducing risks as 7 essential self-management skills, the AADE7,5 required for successful behavior change to promote health for persons with diabetes.6 Clinical studies have demonstrated the importance of self-monitoring of blood glucose (SMBG) in improving hemoglobin A1C (A1C) for persons with type 2 diabetes who use insulin.7 However, critics cite meta-analyses that demonstrate small improvements in A1C in studies of type 2 diabetes not managed with insulin that, although significant, may not be clinically meaningful.8 Because the majority of individuals with type 2 diabetes are not treated with insulin, studying and understanding this population independently is important.9,10 In 2009 the International Diabetes Federation (IDF) published guidelines that focus on structured SMBG protocols to improve SMBG data quality and interpretation in type 2 diabetes.11 Structured SMBG profiles—patterns of SMBG data that vary in intensity and frequency—are essential to evaluate glycemic patterns and to identify lifestyle or therapy alterations in all persons with type 2 diabetes including those not treated with insulin.11-14 Recent research addressing current guidelines demonstrate that improved research design, with structured and purposeful use of SMBG data that includes feedback, improves A1C.13,15-19 A 2011 consensus report10 concluded that SMBG should be performed in a structured manner utilizing specific SMBG profiles and incorporate education for persons with type 2 diabetes and their providers. Guidelines recommend individualized analysis and behavioral treatment based on SMBG profiles. Interventions with structured SMBG for people with type 2 diabetes must include the following features:10-12
Educational intervention (including self-management, SMBG, and/or behavior change) described for participants (Education)
Educational intervention described for health care providers (Education)
Use of a specific structured SMBG profile (describing frequency and intensity of monitoring) identified and individualized to patient (SMBG profile)
SMBG goals identified using evidence based guidelines for both pre- and postmeal (SMBG profile)
SMBG data used to modify behavior or treatments (Feedback)
Evidence of feedback provided to the patient including documentation of communication methods, for example, telephone, email, in-person, and/or computer generated (Feedback)
Evidence of interactive, 2-way communication or shared decision making strategies between patient and provider to implement plan (Feedback)
Telehealth support for persons with diabetes is a burgeoning area of research and practice, with the goal of deploying enabling technology to improve communication, monitoring, and successful enactment of the plan of care. The ongoing debate surrounding the benefit of SMBG in people with type 2 diabetes not treated with insulin is highly relevant to telehealth interventions, suggesting the need to incorporate fundamental structured SMBG concepts in telehealth research. Telehealth includes a variety of telecommunications technologies including telephones, mobile phones, texting, email, videoconferencing, e-health patient portals, nurse call centers, and monitoring of vital signs to deliver health care remotely.20 In general, studies of telehealth interventions for people with type 2 diabetes reveal mixed outcomes and blood glucose improvement. Several systematic reviews and randomized clinical trials (RCTs) demonstrate participant satisfaction with telehealth technology,21-26 yet these studies do not demonstrate consistent improvement in A1C. Evidence indicates high acceptance of technology among individuals with diabetes, which may increase engagement in self-management and empower people with diabetes.27 A systematic review evaluating remote patient monitoring (RPM)—an automated process including the transmission of data directly from the person with diabetes to the health care provider—found a statistically significant decrease in A1C of 0.22%, (95% CI −0.35 to −0.08); however, there was variability in glycemic control and a modest clinical effect.28 A separate review found that telehealth RPM resulted in significant improvements in A1C and reduced complications while empowering participants to engage in their own care.29 Telehealth systems with RPM that incorporate a complete feedback loop—data collection and interpretation combined with prompt feedback to the person with type 2 diabetes to modify treatment plans—between participant and provider are associated with improved outcomes.22
While the complete feedback loop has been identified as an essential component of both SMBG and telehealth RPM, researchers have found limited and inconsistent integration of feedback in diabetes management by both health care providers and people with diabetes.10,22,30 Structured SMBG incorporated within a telehealth RPM intervention is a method to foster a complete feedback loop. A recent paper focusing on personalized diabetes management presents a case for structured SMBG within an overall e-health model incorporating a feedback loop in diabetes and provides a framework to support the premise of this review.31 Telehealth RPM technology creates an opportunity to provide education while at the same time increasing access to frequent structured SMBG data to intervene in a timely manner with appropriate feedback and treatment plan modification, capitalizing on the benefits of telehealth RPM.
Objective
The purpose of this systematic review was to identify telehealth RPM interventions that incorporate key elements of structured SMBG identified as essential for decreasing A1C.
Methods
Data Sources and Review
The systematic literature search was conducted on December 11, 2012 using the Medline, Cumulative Index to Nursing and Allied Health Literature (CINHAL), EMBASE, and OVID Medline databases using the following search terms: “Telemedicine” (Mesh) AND “Monitoring, Physiologic” (Mesh) AND “Diabetes Mellitus, Type 2” (Mesh). Only studies published in peer-reviewed journals in English were considered, with no limit on the publication date.
Study selection criteria included original RCTs evaluating the impact of telehealth RPM (defined as remote blood glucose monitoring data transfer over a telephone [mobile or landline], computer or Internet-based platform) on A1C and incorporating 1 or more of the 7 features of structured SMBG. Study participant inclusion criteria were adults (≥ with type 2 diabetes.
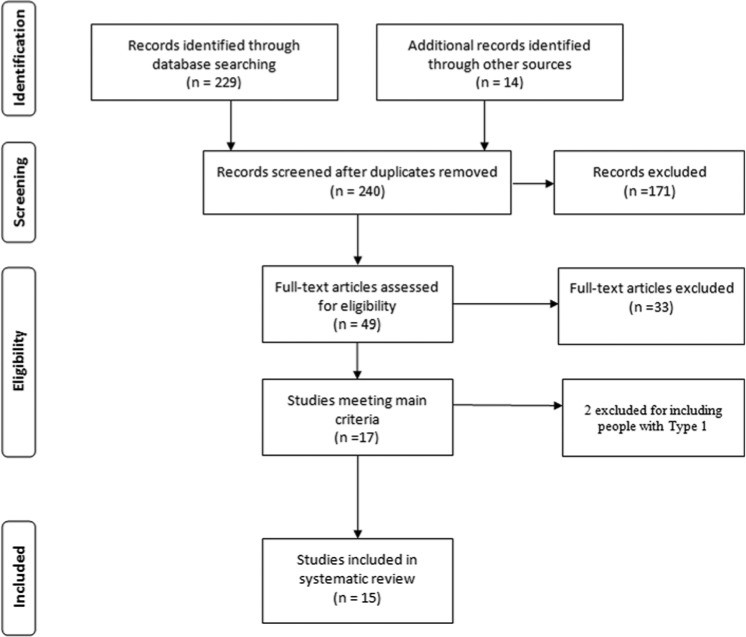
The initial search resulted in 229 abstracts evaluated against search criteria. Systematic reviews and reference lists of included studies were reviewed and 14 additional relevant studies were identified. One hundred seventy-one study abstracts were eliminated after review of title and abstract. The methods sections of the remaining 49 complete studies were evaluated for relevance to this systematic review. The selection process resulted in 15 published RCTs selected for review (Figure 1).
Figure 1.
Flow diagram for studies included in the systematic review.
The data were abstracted using the Matrix method32 and organized by the following headings: author/year published; sample, proportion of participants using insulin, country, time frame; description of intervention and control situations; outcome measures and instruments; and the intervention effect (IE). The IE on A1C was not reported in a consistent manner between studies. When it was not reported, the authors calculated the IE, the difference between the change in pre- and postprogram mean A1C from baseline to conclusion of the intervention (Table 1). The specific elements of structured SMBG described in the interventions were extracted by DAG and confirmed by CCQ and are defined in Table 2.
Table 1.
Summary of Study Characteristics Included in Final Review (n = 15).
| Author, year | Sample, insulin use, country, time frame | Intervention (I) and control (C) groups | Outcome measures | Results (only significant differences between intervention and control are reported) |
|---|---|---|---|---|
| Bujnowska-Fedak et al, 2011 | n = 100 2 groups (insulin and no insulin) Insulin: I: 51%, C: 50% Poland 6 m |
C: UC • Doctor visit every 2 months • I: • Weekly glucose results sent to doctor using wireless technology • Alerts to clinic • Texts to providers • Software for patients and provider to enter additional data, exercise, food, stress • Computer decision support system • Summary BG data sent to patients including graphs, charts and trend patterns |
Primary: A1C Secondary: FBG PPG SMBG QOL Adherence Hypo |
• A1C NS • QOL: • ↑ Sense of control over glucose levels P = .03 • Diabetes in general P = .045 |
| Carter et al, 2011 | n = 74 Insulin not reported USA All African American 9 m |
C: UC I: RN • 3 online education modules-self-management, health education, and social networking • Home laptop with wireless scale, BP cuff, and glucometer • Telehealth RN developed action plans with patient during biweekly 30-minute videoconference |
Primary: A1C Secondary: BMI BP DKT |
• A1C Δ I: –2.18%; C: –0.9% • IE: −1.28% • P < .05 at 9 months Significant association between participation in intervention and achieving: (p < .05) • A1C ≤ 7% < .05 • I group 4.58 times more likely to reach A1C target • BMI • DKT |
| Cho et al, 2009 | n = 75 Insulin not reported Korea 3 m |
C: Web-based reporting of SMBG I: Mobile phone automated SMBG |
Primary: A1C Secondary: BP FBG Height PPG Satisfaction Adherence Weight WC |
• A1C NS • PPG ↓ in both groups (P < .001) |
| Del Prato et al, 2012 | n = 241 All starting Insulin Italian 12 m |
C: Insulin titration with algorithm • SMBG log • Provider visits • I: • Telecare glucose transmission to provider • Feedback re: dose titration to patient via phone • Titration daily at meal with largest PPG |
Primary: A1C Secondary: SMBG (8 point profile) Insulin dose Weight |
• A1C NS |
| Faridi et al, 2008 | n = 30 Insulin not reported USA 3 m |
C: UC I: • Daily tailored messages via cell phone • Prompts to improve self-care in response to SMBG and pedometer |
Primary: A1C Secondary: SDSCA Self-efficacy |
• A1C NS • I: Self-efficacy ↑ P = .008 |
| Kim et al, 2006 | n = 60 Insulin: I: 32% C: 31% Korea 3 m |
C: UC • 1 or 2 endocrinologist visits • RN or RD PRN I: • SMBG and medication info transfer via text or Web entry • RN review |
Primary: A1C Secondary: FBG PPG |
• A1C Δ I: –1.15%; C: +0.07% • IE: −1.22% • P < .005 at 3 months • I: PPG ↓ P < .05 |
| Lim et al, 2011 | n = 144 Insulin not reported Korea 6 m |
C: UC • Clinic visit every 3 months • I: 2 groups (I, IU) • I: traditional SMBG 8 times per week • IU: mobile phone an wireless glucometer • Wireless transfer of SMBG to a hospital-server. • Computer decision support system rule engine • Automatic patient specific messages by text via mobile phone in 2 minutes • Guidelines based • Uses previous weekly and monthly glucose averages for lifestyle or medication changes |
Primary: A1C |
• A1C NS • ↑ SMBG frequency between groups • P = .01 • C: 31.2% • I: 68.5% • IU: 81.2% |
| McMahon et al, 2005 | n = 104 Insulin: I: 52% C: 50% USA Veterans 12 m |
C: UC and education I: UC and • Notebook computer • Glucometer and BP to upload data • Access to care management website with education modules • Secure messaging with care manager using internal system via website • Graphic data display for patient and care team • Recommendations to PCP based on algorithms • Telephone contact |
Primary: A1C Secondary: BP |
• A1C Δ I: –1.6%; C: –1.2% • IE: −0.4% • P < .05 at 12 months • High website users had greater ↓ A1C compared with intermittent users: (1.9% vs 1.2%; P = .051) • Frequent data uploads associated with greater ↓ A1C (highest tertile 2.1% vs lowest tertile1.0%; P = .02) • Systolic BP Δ I: –10; C: –7 mmHg • P < .01 |
| Quinn et al, 2008 | n = 30 Insulin: I: 31% C: 31% USA 3 m |
C: UC • SMBG every 2 weeks to provider via fax or cell • I: • Cell phone software with real time feedback on SMBG • Treatment based on algorithms • Computer requests additional data from patients for management • Computer generated log books with suggested treatment plans to providers • Electronic log book sent to providers every 4 weeks with analysis trends and patient’s behavior assessed by statistical models and diabetes team • Virtual coach for patients and virtual endocrinologist for provider |
Primary: A1C Secondary: SDSCA PCP prescribing practice |
• A1C Δ I: –2.03%; C: –0.68% • IE: −1.38% • P < .02 at 3 months • ↑ medication change or dose change by PCP I: 84%; C: 23%; p = .002 • PCPs received patient logbook more • I: 100%; C: 7.7% • P < .001 • I: 91% patient satisfaction • I: PCP satisfaction |
| Quinn et al, 2011 | n = 26 (cluster RCT by PCP practice) Insulin not reported USA 12 m |
C: UC I: 3 groups • Diabetes educator virtual case managers • Mobile phone to enter glucose, carbohydrate, medications, etc • Automated real time feedback: educational, behavioral, and motivational messaging specific to entered data • Virtual coaching • Secure patient web portal for messages, PHR, library, logbook review • Provider web access different based on group Group 2: • No PCP web access to patient data • Patient must forward electronic log book data to PCP • Log book data are not analyzed Group 3 : • PCP training on portal they can access from Personal computer at office • Access to raw data in logbook format • No reminders or prompts to review data Group 4: • PCP training on portal they can access from personal computer at office • Fax or email to review an analyzed report of all patient entered data • Summary of glycemic control and medication adherence, self-management skills and health maintenance • Automated support based on algorithms and Computer decision support system tailored to data • Education to patient • Computer/system driven guidance on when to test BG based on disease status, medications, time of poorest control • Creates a patient specific multipoint profile for data analysis and future self-management coaching |
Primary: A1C Secondary: DDS17 DStoC Health care utilization PHQ9 Satisfaction Self-efficacy Symptoms Hypo |
• A1C Δ I : –1.9%; C: –0.7% • IE: −1.2 % • P < .001 at 12 months • Significant ↓ in A1C with stratified baseline A1C ≥ 9.0% • IE: −1.3%, P = .01 |
| Rodríguez-Idígoras et al, 2009 | n = 328 Insulin: I: 38.5% C: 37.2% Spain 12 m |
C:UC I: • Mobile phone to patient and PCP • Real time SMBG via mobile phone • Feedback sent to a call center based on alerts and protocols • Web access to patient data by PCP • Software recorded frequency of SMBG and results • Patient had access to RN specialist at call center |
Primary: A1C Secondary: BP BMI Lipids |
• A1C NS • A1C > 8% ↓ 35% to 22.5% P < .001 • Users adherent to protocol greater ↓ A1C at 12 months, P = .033 |
| Shea et al, 2006, 2009 | n = 1665 Insulin: I: 25.3% USA 12 m 5 years |
C:UC I: 2 groups of patients (rural and inner city) • RN care managers trained in diabetes • Home telemonitoring unit • Transmit SMBG and BP to RN care manager • Access educational materials • Synchronous videoconference • Web access • Secure messaging, portal to access data by patient and provider • Empower and influence health behavior change • Feedback by videoconference every 2 weeks by RN • Phone at 3 month intervals for follow-up data |
Primary: A1C Secondary: QOL Self-efficacy PHQ9 Symptoms DDS17 Health care utilization Satisfaction |
• A1C Δ I : –0.38%; C: –0.25% • IE: −0.18% • P = .006 at 12 months • When baseline A1C ≥ 7%, • IE: −0.32%, P = .002 • Significant ↓ : • Systolic BP, P = .003 • Diastolic BP, P < .001 • Total cholesterol, P < .001 • LDL, P < .001 • Patients satisfied with intervention • 5 year outcomes: • A1C Δ I : –0.34%; C: –0.07% • IE: −0.29% • P = .001 • Significant ↓ : • Systolic BP, P = .024 • Diastolic BP, P < .001 • LDL, P < .001 |
| Stone et al, 2010 | n = 150 Insulin 50% USA Veterans 6 m |
C: UC • Monthly phone calls for care coordination • I: • Home telemonitoring unit for SMBG upload • Active medication management by nurse practitioner based on guidelines • SMBG, BP, and weight remote transmission • ADA SMBG targets |
Primary: A1C Secondary: BP Lipids |
• A1C Δ I : –1.2% C: –0.3% • IE: −0.7% • P < .001 at 6 months |
| Tang et al, 2013 | n = 415 Insulin not reported USA 12 m |
C: UC • Phone RN care management • I: • Wireless upload SMBG • Graphic feedback • Patient summary reports • Nutrition and exercise log • Insulin record • Online secure messaging with team • RN care management with behavior advice and medication management • Personalized text and video educational “nuggets” |
Primary: A1C Secondary: BP Health care utilization LDL Framingham risk Satisfaction well-being DKT PAID DTSQ CAHPS |
• A1C Δ I : –1.32%; C: –0.66% • IE: −0.66% • P < .001 at 6 months • No IE at 12 months • At 12 months: • I ↓ A1C by ≥ –0.5%, P = .006 • ↑ frequently and upload of SMBG data associated with ↓ A1C • ↓ LDL, P = .001 • ↑ online messages to RN, P < .001 |
| Wakefield et al, 2011 | N = 302 Insulin not reported USA Veterans 12 m |
C: UC I: 2 groups Low-I: • RN care management, home telemonitoring unit for SMBG and BP upload • RN responded to patient via phone, letter or home telemonitoring unit • SMBG per PCP • Subset of DSME and questions via HTU • “Have you taken medication?” and 1 additional question High-I: • RN care management, HTU for SMBG and BP upload • RN responded to patient via phone, letter or HTU • SMBG per PCP (no changes suggested) • DSME and daily questions • Branching algorithm based on ADA and Veterans Administration • DSME and questions via telehealth |
Primary: A1C BP Secondary: Adherence |
• A1C Δ High-I: –0.44; Low-I: –0.40; • C: –0.07 • High-I IE: −0.37, P = .02 at 6 months • Low-I IE: −0.33, P = .03 at 6 months • No IE at 12 months • BP ↓ in High-I group vs C P = .001 |
Abbreviations: ADA, American Diabetes Association; A1C, hemoglobin A1C; BG, blood glucose; BMI, body mass index; BP, blood pressure; C, control ; CAHPS, computer assessment of health care providers and systems; DDS17, Diabetes Distress Scale–17; DKT, Diabetes Knowledge Test; DSME, diabetes self-management education; DTSQ, Diabetes Treatment Satisfaction Questionnaire ; FBG, fasting blood glucose; I, intervention; IE, intervention effect; NS, not significant; PAID, problem areas in diabetes; PCP, primary care provider; PHQ9, Patient Health Questionnaire–9; PHR, personal health record; PPG, postprandial glucose; QOL, quality of life; RCT, randomized clinical trial; RD, registered dietitian; RN, registered nurse; SDSCA, summary of diabetes self-care activities; SMBG, self-monitoring of blood glucose; UC, usual care; WC, waist circumference.
Table 2.
Incorporation of Key Elements of Structured Self-Monitoring of Blood Glucose (SMBG) and Change in A1C Between Intervention and Control Groups.
| Author, Year | Elements of Structured SMBG Incorporated10,11,a | Baseline A1C % | Intervention Effect (IE), Significance of Difference in A1C Between Groups (I, C)b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Faridi et al, 2008 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 6.4 | No IE |
| X | C: 6.5 | ||||||||
| Bujnowska-Fedak et al, 2011 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 7.63 | No IE |
| X | X | C: 7.61 | |||||||
| Lim et al, 2011 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | U:7.86 | No IE |
| X | X | X | I: 7.96 | ||||||
| C:7.96 | |||||||||
| Rodríguez-Idígoras et al, 2009 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 7.62 | No IE |
| X | X | X | C: 7.44 | ||||||
| Wakefield et al, 2011 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | High I: 7.1 | IE: −0.37 High-I |
| X | X | X | Low I: 7.2 | IE: −0.33 Low-I | |||||
| C: 7.2 | No IE at 12 months | ||||||||
| Carter et al, 2011 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 9.0 | IE: −1.28% |
| X | X | X | X | C: 8.8 | |||||
| Cho et al, 2009 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 8.3 | No IE |
| X | X | X | X | C: 7.6 | |||||
| Del Prato et al, 2012 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 8.83 | No IE |
| X | X | X | X | C: 8.89 | |||||
| Kim et al, 2006 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 8.09 | IE: −1.22% |
| X | X | X | X | C: 7.59 | |||||
| McMahon et al, 2005 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 10.0 | IE: −0.4% |
| X | X | X | X | C: 9.9 | |||||
| Stone et al, 2010 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 9.6 | IE: −0.7% |
| X | X | X | X | X | C: 9.4 | ||||
| Tang et al, 2013 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 9.24% | IE: −0.66% at 6 months |
| X | X | X | X | X | C: 9.28% | No IE at 12 months | |||
| Shea et al, 2006, 2009 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 7.36 | IE: −0.18%b at 12 months |
| X | X | X | X | X | X | C: 7.40 | IE: −0.29%b at 5 years | ||
| Quinn et al, 2008 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 9.51 | IE: −1.38% |
| X | X | X | X | X | X | C: 9.05 | |||
| Quinn et al, 2011 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | I: 9.9% | IE: −1.2 % |
| X | X | X | X | X | X | C: 9.2% | |||
Abbreviations: C, control group; I, intervention group; IE = intervention effect—difference between intervention and control over time; m, months; Δ, change.
Elements of structured SMBG: (1) educational intervention described for person with diabetes; (2) educational intervention described for health care providers; (3) use of specific structured SMBG profiles identified and individualized; (4) SMBG goals identified for pre- and postmeal; (5) SMBG data used to modify behavior or treatment; (6) evidence of feedback to the patient including documentation of communication methods; (7) evidence of an interactive, 2-way communication or shared decision strategies between patient and provider.
Adjusted difference as reported by authors.
Boldface type is used in the table to highlight the elements of structured SMBG.
Results
Study Design and Subjects
Studies represented multiple countries including the United States (n = 9), Korea (n = 3), Italy (n = 1), Poland (n = 1), and Spain (n = 1). Most studies included participants on insulin, 2 studies included individuals on oral antihyperglycemic medications and lifestyle therapy alone,33,34 and 2 did not indicate the medication status.35,36 One study focused on insulin titration.37 Study sample sizes ranged from 30 to 1665 with a mean of 259 participants and a median of 144 participants. Age ranged from 47 to 71 years. A1C was the primary outcome in all studies. Secondary outcomes are identified in Table 1 and include weight loss, body mass index, quality of life, and blood pressure, among others. The length of the intervention varied from 3 to 60 months with median of 7.5 months with 1 study reporting a 5-year follow-up.24 Eight studies utilized registered nurses (RN), nurse practitioners (NP), or certified diabetes educators (CDE) to provide the intervention.23,24,33,36,38-42 Two studies provided automatic feedback as the main intervention, generated from computer algorithms, without provider input.37,43
Elements of Structured SMBG
Table 2 summarizes elements of structured SMBG incorporated in each study, mean baseline A1C and IE. On average, 4 of the 7 elements of structured SMBG were included in telehealth RPM interventions. Studies that incorporated at least 5 of the 7 elements achieved significant decreases in A1C between intervention groups, possibly indicting that more key SMBG elements are associated with greater A1C changes in an intervention.23,24,39-42 Studies with a greater A1C decrease incorporated 6 of the 7 elements and enrolled individuals with A1C greater than 7.5% at baseline.41,42 In the study implementing the computer decision support algorithm, although a specific SMBG profile was not utilized, patterns were analyzed, and prompts provided to participants for additional SMBG data based on existing patterns. Two studies39,40 incorporated 5 of the 7 elements and achieved significant decrease in A1C by implementing active medication management utilizing protocols. Of the 6 studies which used 4 of the 7 elements, only 4 achieved a significant percentage decrease in A1C.33,36,44,45 These studies emphasized feedback and interactive communication. One study incorporating 4 of the 7 elements achieved significant A1C reduction at 6 months but not 12 months.36 To further synthesize these data, the 7 categories were grouped into 3 sections: education, SMBG profiles, and feedback.
Education
Education for both participants and providers is necessary in interventions evaluating SMBG data, and 11 studies described an initial education intervention for the study participants.23,24,33,35,36,38-44 Education ranged from a half-day educational program based on the American Diabetes Association (ADA) guidelines44 to ongoing access to online educational content through an Internet portal,23,24,33,44 personal health records,40 text messaging,34,38,43 or a home telehealth system.36,39,41,42 Some studies only referenced education to implement the telehealth RPM intervention technology.34,37,46,47 While many studies stated the health care intervention team consisted of specialized staff with experience in diabetes management,35,36,39,40,43,44,47 only 3 studies specifically described an educational intervention addressing SMBG for health care providers.23,24,41,42
SMBG Profiles
Structured SMBG profiles were minimal in these studies. The Evaluation of Lantus Effect on Optimization of Use of Single Dose Rapid Insulin (ELEONOR) study protocol, targeting insulin titration, implemented a 6-point profile (before and 2 hours after meals) and focused on achieving specific SMBG goals.37 One study advised participants to measure their blood glucose level at least 8 times a week43 while 4 studies gave recommendations on frequency per day but did not specify a pattern to follow (eg, before and after specific meals).33-35,46 Frequency of SMBG was either based on primary care provider (PCP) order, not changed for the study,36 or stated as individualized for the participants.41,42,44 The most complex system analyzed data, including medications and time of poor SMBG control, to generate a patient specific multipoint profile to enhance data analysis and self-management support.42
Goals for SMBG target ranges were not clearly defined in most studies. Only 1 study titrating insulin doses defined specific fasting and postprandial glucose goals.37 In some studies SMBG goals were individualized for the participant41,42,46 and followed standard guidelines from the ADA39,40 or both ADA and Veterans Administration.23,24 Some studies did not specify SMBG goals but focused on A1C outcomes, to achieve an A1C of less than 7%40,43 or less than 6.5%41,42 or simply suggested that SMBG values be in the “normal range.”38
Feedback
All studies incorporated feedback between patients and providers or the telehealth RPM system. Feedback methods included telephone calls,23,24,36,39,47 videoconferencing,23,24,33 cellular phones using short message service (SMS) or text messaging,34,35,37,38,43 or secure messages via a patient portal23,24,33,38,40,44 or through the telehealth system.36,39,41,42 Almost all studies indicated that SMBG data were used to provide feedback, modify behavior, or change treatment, however the depth and detail varied. Five studies specifically described interventions focused on adjusting medications and behavior change by the telehealth provider based on SMBG data.37-40,43 Two studies described active medication management in response to daily SMBG data transmitted through telehealth39 or patient portal.40 The most descriptive use of SMBG data for behavior change was presented by Kim et al,38 where the RN adjusted medication and encouraged behavior change based on SMBG via SMS message. Although 7 studies described complex systems to analyze data,36,40-44,46 outcomes varied. Significant change in A1C between groups was associated with feedback that was individualized to the participant, whether through automated algorithms or by skilled health care providers, and when the participant’s individual preferences were incorporated.
Some studies describe feedback provided to the patient’s PCP through Internet,23,24,38,44,46 while others provided feedback to both patients and providers implementing multiple methods of contact including social media (eg, online communities for communication between members) with peer support.23,33 One study described the practice of shared decision making between the patients and RN,33 wherein the provider transmitted plans via an Internet portal so the RN and patient could then review and use data to guide behavior, exchange coping strategies, pose questions, and share preferred educational resources for adopting a healthy lifestyle.
Many studies describe a method for communication between patients and the health care team.23,24,33,35,36,38-42,44,46,47 Ten studies describe an interactive communication process that may include problem solving and shared decision-making strategies involving both patients and providers.23,24,33,35,36,38-42,44,47
Baseline A1C is an important consideration in telehealth research. In this review 3 RTCs had baseline A1C less than 7.5% in all groups. Of those studies 1 did not have an IE34 and 2 did, but the effect was small in 1 study,23,24 and significant only at 6 months in the second study.36 Of the 8 RCTs with an A1C ≥ 8%, 7 had an IE33,37-39,41,42,44 with 1 study having significant results at 6 months but not 12 months.40 Studies with at least 4 of the 7 key elements of structured SMBG and an A1C ≥ 8 showed an A1C decrease of 0.7% or greater.
Discussion
We believe this is the first review to evaluate the elements of structured SMBG incorporated in telehealth RPM interventions. Recent research in structured SMBG emphasizes that implementing purposeful SMBG profiles results in improved A1C.15 Interestingly, the only telehealth study that incorporated a structured SMBG profile did not achieve a significant difference in A1C between groups. While both groups implemented structured SMBG profiles, only 1 group utilized telehealth RPM. The researchers suggest these outcomes may be because their system did not allow for real time communication from the provider to the patient. They also suggest that their prandial dose adjustment algorithm was developed at a basic level that participants were able to use on their own with their SMBG profile values. The authors of this review agree with these statements and hypothesize the outcomes may indicate that the structured SMBG profile combined with educational tools not the telehealth RPM intervention, improved A1C.37
Baseline A1C is an important consideration in planning telehealth RCTs. When study participants mean A1C is at or close to goal, it is not unrealistic to see a small IE. In this review, most studies had baseline A1Cs ≥ 8%; however, clinically significant results were achieved only when there were also at least 4 of the 7 key elements of structured SMBG which resulted in an A1C decrease of ≥ 0.7%.
Telehealth RPM RCTs have shown mixed results. Based on this systematic review, full deployment of all 7 key elements of structured SMBG was not achieved in any of the reviewed studies. Increased benefit was identified in studies that incorporated more features of the 7 key elements with significant A1C improvement between groups. Future research may benefit from incorporating and describing all key elements in the research methodology. In addition, comparing key elements of telehealth with key elements of structured SMBG will provide further information for research design.
Implications of Research
The systematic review informs intervention design for future studies. To continue to address the controversy surrounding the use of SMBG in persons with type 2 diabetes not using insulin, incorporating structured SMBG with feedback and behavior change is extremely important in future telehealth research. Future studies also should recruit individuals with baseline A1C ≥ 7.5% and account for differences in baseline A1C in the conclusions. In addition, the studies in this review included minimal description of how feedback from SMBG was used to facilitate behavior change. More telehealth RPM research in persons with type 2 not using insulin is required to evaluate clinical outcomes in this population. In addition, it would be useful for future studies to target primary clinical outcomes other than A1C, especially in the older adult population where individualized A1C may be less important than, for example, hypertension and depression. Patient-centered outcomes, including quality of life, are important and may increase patient engagement in SMBG. Studies in type 2 diabetes should also include an evaluation of hypoglycemic events. Only 2 studies in this review report hypoglycemic events, not sufficient to draw conclusions in this review. In addition outcomes that evaluate the potential cost savings of telehealth, reduction in hospital admissions, and costs of medication are among several essential factors to evaluate to support the case for telehealth care systems. Future research would benefit from addressing the approach described by Ceriello and colleagues that focus on individualized care that incorporates a feedback loop with both structured SMBG data and processes within an e-health model.31
Limitations of Review and Research
A strength of our study was to identify and synthesize structured SMBG elements into major categories. The key elements were collated from multiple documents published by expert consensus and modified into categories defined by the authors. Classification was dependent on descriptions provided in the methodological sections. Other individuals may identify, interpret, and synthesize content differently and result in different conclusions. This systematic review incorporated limited search terms and may have missed relevant studies. This review included studies of individuals with type 2 diabetes using insulin. However, use of SMBG for titration of insulin adjustment is different than use of SMBG for behavior change. Guidelines for SMBG in type 2 diabetes stress the importance of using SMBG data to change treatment and behavior. Studies that address individuals who do not use insulin provide an opportunity to target the analysis on behavior change in response to SMBG data. Limiting telehealth RPM studies to persons with type 2 not using insulin may also provide a better opportunity to examine the use and impact of feedback to change behavior. Ideally these populations need to be evaluated separately; however, there were insufficient studies to evaluate in a review at this time. Future telehealth RPM research focused on non–insulin users can provide important knowledge for the science and clinical practice of diabetes management.
Conclusion
Telehealth RPM clinical trials implementing SMBG should incorporate key elements of structured SMBG to consistently achieve a clinically significant decrease in A1C. To promote generalizability and subsequent meta-analyses, these elements should be carefully documented in published studies. Education must be provided to both patients and health care providers, specifically surrounding SMBG profiles and the importance of engaging in a complete feedback loop including shared decision making. Although education regarding use of technology is important, it is not sufficient to engage participants in effective SMBG. Clear SMBG goals could be specified for both before meal and postmeal values so participants can understand the relationship between behavior change and SMBG values and to identify potential actions and treatment choices that affect SMBG and eventually A1C.48,49 In addition, clear SMBG goals could encourage providers to base medication changes on glucose values and excursions not simply the A1C value (which is a blood glucose average). Finally, a shared decision-making process is essential for persons with diabetes to be fully engaged with treatment decisions and to ensure successful clinical outcomes. Providing SMBG feedback in formats that both health care providers and people with diabetes can read in a graphical presentation that synthesizes data will be important as more data are generated by telehealth RPM technology.
This review also identified that many telehealth RPM interventions were implemented by RNs, NPs, CDEs, or RNs board certified in advanced diabetes management (RN, BC-ADMs). These individuals specialize in assisting persons with diabetes with self-management, behavior change, and understanding of diabetes medication titration and are skilled at SMBG pattern management. CDEs and RN/NP, BC-ADMs incorporate structured SMBG principals in self-management education for persons with diabetes and can also be resources to clinicians and researchers to ensure that all key elements of structured SMBG are incorporated into RCT. Incorporating teams of clinicians, working at their full capacity,50 will help to manage the rapidly increasing diabetes population. When NPs have the ability to adjust treatments independently or RNs adjust treatments using predetermined titration protocols, outcomes are improved.51
Telehealth RPM interventions that incorporate more key elements of structured SMBG appear to have the greatest impact on A1C. It is critical to incorporate purposeful SMBG profiles that allow the individual to change behavior or the PCP to modify treatment. Engaging persons with diabetes in self-management requires education, an understanding of SMBG profiles and goals, and the opportunity for interactive feedback as they engage in behavior change.
Acknowledgments
The authors acknowledge Thomas Nesbitt, MD, MPH, and Shelley Blozis, PhD, for scientific insight in developing this paper.
Footnotes
Abbreviations: AADE, American Association of Diabetes Educators; A1C, hemoglobin A1C; BC-ADM, board certified in advanced diabetes management; CDE, certified diabetes educator; IE, intervention effect; NP, nurse practitioner; PCP, primary care provider; RCT, randomized clinical trial; RN, registered nurse; RPM, remote patient monitoring; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DAG has received research support from the Investigator Initiated Studies program of LifeScan Inc. and product support from Intel-GE Care Innovations. CCQ has been funded through a contract between the University of Maryland Baltimore and WellDoc, and through contributions from CareFirst Blue Cross/Blue Shield of Maryland, Sprint, LifeScan; grants from the University of Maryland’s Maryland Industrial Partnerships Program, an initiative of the A. James Clark School of Engineering’s Maryland Technology Enterprise Institute.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by scholarship support from the Betty Irene Moore School of Nursing and Sutter Institute for Medical Research.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. 2011. ed. Atlanta GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2. American Diabetes Association. Clinical Practice Recommendations. Diabetes Care. January 2013;36(suppl 1):S1-S110. [PubMed] [Google Scholar]
- 3. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. [DOI] [PubMed] [Google Scholar]
- 4. Davies MJ, Heller S, Skinner TC, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ. 2008;336(7642):491-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Association of Diabetes Educators. AADE7 self-care behaviors. Diabetes Educ. 2008;34(3):445-449. [DOI] [PubMed] [Google Scholar]
- 6. Zgibor JC, Peyrot M, Ruppert K, et al. Using the American Association of Diabetes Educators Outcomes System to identify patient behavior change goals and diabetes educator responses. Diabetes Educ. 2007;33(5):839-842. [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 8. Malanda UL, Welschen LM, Riphagen I, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Systematic Review. 2012;1:CD005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klonoff DC, Bergenstal R, Blonde L, et al. Consensus report of the coalition for clinical research-self-monitoring of blood glucose. J Diabetes Sci Technol. 2008;2(6):1030-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klonoff DC, Blonde L, Cembrowski G, et al. Consensus report: the current role of self-monitoring of blood glucose in non-insulin-treated type 2 diabetes. J Diabetes Sci Technol. 2011;5(6):1529-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International Diabetes Federation. Self-Monitoring of Blood Glucose in Non-Insulin-Treated Type 2 Diabetes. Available at: www.idf.org;2009.
- 12. Schnell O, Alawi H, Battelino T, et al. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Therapeut. 2011;13(9):959-965. [DOI] [PubMed] [Google Scholar]
- 13. Schnell O, Alawi H, Battelino T, et al. Self-monitoring of blood glucose in type 2 diabetes: recent studies. J Diabetes Sci Technol. 2013;7(2):478-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabetes Med. 2008;25(10):1151-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2(3):203-211. [DOI] [PubMed] [Google Scholar]
- 17. Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with Type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabetes Med. 2011;28(7):789-796. [DOI] [PubMed] [Google Scholar]
- 18. Bosi E, Scavini M, Ceriello A, et al. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care. 2013;36:2887-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lalic N, Tankova T, Nourredine M, Parkin C, Schweppe U, Amann-Zalan I. Value and utility of structured self-monitoring of blood glucose in real world clinical practice: findings from a multinational observational study. Diabetes Technol Ther. 2012;14(4):338-343. [DOI] [PubMed] [Google Scholar]
- 20. American Telemedicine Association. What is Telemedicine and Telehealth? 2012. Available at: http://www.americantelemed.org/files/public/abouttelemedicine/What_Is_Telemedicine.pdf. Accessed April 11, 2012.
- 21. Darkins A, Ryan P, Kobb R, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health. 2008;14(10):1118-1126. [DOI] [PubMed] [Google Scholar]
- 22. Jimison H, Gorman P, Woods S, et al. Barriers and drivers of health information technology use for the elderly, chronically ill, and underserved. Evidence Reports/Technology Assessment. 2008;175:1-1422. [PMC free article] [PubMed] [Google Scholar]
- 23. Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shea S, Weinstock RS, Teresi JA, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc. 2009;16(4):446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: a systematic literature review. J Diabetes Sci Technol. 2010;4(3):666-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verhoeven F, van Gemert-Pijnen L, Dijkstra K, Nijland N, Seydel E, Steehouder M. The contribution of teleconsultation and videoconferencing to diabetes care: a systematic literature review. J Med Internet Res. 2007;9(5):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pare G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc. 2007;14(3):269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K. Home telehealth for diabetes management: a systematic review and meta-analysis. Diabetes Obes Metab. 2009;11(10):913-930. [DOI] [PubMed] [Google Scholar]
- 29. Jaana M, Pare G. Home telemonitoring of patients with diabetes: a systematic assessment of observed effects. J Eval Clin Pract. 2007;13(2):242-253. [DOI] [PubMed] [Google Scholar]
- 30. Boren SA, Puchbauer AM, Williams F. Computerized prompting and feedback of diabetes care: a review of the literature. J Diabetes Sci Technol. 2009;3(4):944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ceriello A, Barkai L, Christiansen JS, et al. Diabetes as a case study of chronic disease management with a personalized approach: the role of a structured feedback loop. Diabetes Res Clin Pract. 2012;98(1):5-10. [DOI] [PubMed] [Google Scholar]
- 32. Garrard J. Health Sciences Literature Review Made Easy: The Matrix Method. 3rd ed. Ontario, Canada: Jones and Bartlett; 2011. [Google Scholar]
- 33. Carter EL, Nunlee-Bland G, Callender C. A patient-centric, provider-assisted diabetes telehealth self-management intervention for urban minorities. Perspect Health Inf Manag. 2011;8:1b. [PMC free article] [PubMed] [Google Scholar]
- 34. Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients’ self-management: the NICHE pilot study. J Eval Clin Pract. 2008;14(3):465-469. [DOI] [PubMed] [Google Scholar]
- 35. Cho JH, Lee HC, Lim DJ, Kwon HS, Yoon KH. Mobile communication using a mobile phone with a glucometer for glucose control in Type 2 patients with diabetes: as effective as an Internet-based glucose monitoring system. J Telemed Telecare. 2009;15(2):77-82. [DOI] [PubMed] [Google Scholar]
- 36. Wakefield BJ, Holman JE, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health. May 2011;17(4):254-261. [DOI] [PubMed] [Google Scholar]
- 37. Del Prato S, Nicolucci A, Lovagnini-Scher L, Turco S, Leotta S, Vespasiani G. Telecare provides comparable efficacy to conventional self-monitored blood glucose in patients with type 2 diabetes titrating one injection of insulin glulisine—the ELEONOR Study. Diabetes Technol Therapeut. 2012;14(2):175-182. [DOI] [PubMed] [Google Scholar]
- 38. Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual. 2006;21(3):266-271. [DOI] [PubMed] [Google Scholar]
- 39. Stone RA, Rao RH, Sevick MA, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care. 2010;33(3):478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang PC, Overhage JM, Chan AS, et al. Online disease management of diabetes: Engaging and Motivating Patients Online With Enhanced Resources-Diabetes (EMPOWER-D), a randomized controlled trial. J Am Med Inform Assoc. 2013;20:526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160-168. [DOI] [PubMed] [Google Scholar]
- 42. Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934-1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim S, Kang SM, Shin H, et al. Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system. Diabetes Care. 2011;34(2):308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McMahon GT, Gomes HE, Hickson Hohne S, Hu TM, Levine BA, Conlin PR. Web-based care management in patients with poorly controlled diabetes. Diabetes Care. 2005;28(7):1624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HS. A randomized controlled trial of a nurse short-message service by cellular phone for people with diabetes. Int J Nurs Stud. 2007;44(5):687-692. [DOI] [PubMed] [Google Scholar]
- 46. Bujnowska-Fedak MM, Puchala E, Steciwko A. The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemed J E Health. 2011;17(3):153-163. [DOI] [PubMed] [Google Scholar]
- 47. Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sánchez-Garrido-Escudero R, et al. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Ther. 2009;11(7):431-437. [DOI] [PubMed] [Google Scholar]
- 48. Schwedes U, Siebolds M, Mertes G. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25(11):1928-1932. [DOI] [PubMed] [Google Scholar]
- 49. Parkin CG, Hinnen D, Campbell RK, Geil P, Tetrick DL, Polonsky WH. Effective use of paired testing in type 2 diabetes practical applications in clinical practice. Diabetes Educ. 2009;35(6):915-927. [DOI] [PubMed] [Google Scholar]
- 50. Institute of Medicine. The Future of Nursing: The Institute of Medicine (IOM) Issues Report. The Future of Nursing: Leading Change, Advancing Health. January 2010-November 2011. [PubMed] [Google Scholar]
- 51. Ralston JD, Hirsch IB, Hoath J, Mullen M, Cheadle A, Goldberg HI. Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care. 2009;32(2):234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]