Abstract
Animal research suggests that vagus nerve stimulation (VNS) is associated with weight loss and decreased appetite. Results from human studies are mixed; some suggest that VNS affects weight whereas others do not, and it is unclear how VNS affects eating behaviors. Baseline body mass index (BMI) and VNS device settings may moderate the effects of VNS on caloric intake. This study investigates the association among BMI, VNS device settings, and caloric intake of highly palatable foods during VNS on versus VNS off sessions in 16 adult patients (62.5% female; BMI mean = 29.11 ± 6.65) using VNS therapy for either epilepsy or depression. Participants attended 2 experimental sessions (VNS on versus off) where they were presented with 4 preferred snack foods totaling 1600 calories. At the start of the session, they either had their VNS devices turned off or left on. Caloric intake was calculated by weighing foods before and after each session. BMI category (overweight/obese and lean) was the between group factor in the analysis. After controlling for covariates, an interaction of condition and BMI category (P = .03) was found. There was an interaction of condition and device output current (P = .05) and a trend toward an interaction of condition and device on time (P = .07). Excess weight may impact how neurobiological signals from the vagus nerve affect appetite and eating. Future research is needed to further elucidate this relationship.
Keywords: caloric intake, eating, obesity, overweight, vagus nerve, vagus nerve stimulation
Stimulation of the vagus nerve has been associated with weight loss and decreased appetite in animals.1-3 The use of chronic intermittent vagus nerve stimulation (VNS) resulted in substantial weight loss with normal weight mongrel dogs. The dogs ate more slowly and terminated eating sooner than usual.2 Furthermore, Val-Laillet et al suggested that adult obese minipigs who received VNS ate 18% less than they did before surgery, while control animals exhibited no decreased food consumption.3 Among obese rats, decreased food consumption and overall weight loss was observed following stimulation of the vagus nerve in comparison to their control group, who experienced no vagal stimulation but had a sham device implanted.4 Banni and colleagues found that rats exposed to VNS for 4 weeks reduced food intake, body weight, and adipose tissue compared to rats in the control group.5 These findings are not entirely surprising given that the vagus nerve has long been linked to neurobiological systems associated with hunger and satiety, including those in humans.6-9
The effects of VNS on appetite and weight change have been inconsistent in humans using VNS therapy for either treatment-resistant epilepsy or depression. Burneo et al found that 62% of participants experienced significant weight loss (with a quarter losing more than 5% of their body weight) during VNS treatment for epilepsy. A tendency toward greater weight loss was observed when the output current of the stimulator was increased.9 The effect of participants’ baseline body mass indexes (BMIs) on VNS-related weight loss was not examined in this study. Pardo and colleagues found that among patients receiving VNS therapy for treatment-resistant depression, weight loss was proportional to initial BMI, with the heaviest participants experiencing the greatest weight loss over a 2-year time period.8 In other studies, a decrease in weight was not observed in patients using VNS therapy.10-11 For instance, in a recent study examining VNS therapy in children with treatment-resistant epilepsy, no significant changes in BMI occurred over 1 year after implantation of the device.11 An important caveat to this study is the use of children who were on average normal weight. Koren and Holmes did not find significant weight change over a 2-year period in adults using VNS therapy for epilepsy; however, baseline BMI was not examined as a moderator in their findings.10 Baseline BMI may be an important moderator in the relationship of VNS and weight change, however more research is needed to further understand this relationship.
In addition to examining how VNS affects weight, previous research suggests that VNS may affect food cravings. Individuals who have difficulty regulating their weight often report frequent and intense food cravings, especially for palatable foods, which are widely believed to influence overall food consumption.10,12-14 Participants using VNS therapy for treatment-resistant depression were shown pictures of different types of foods twice: once when their VNS device was on and the other when it was turned off. Participants with lower device on-time, lower VNS output current, and lower BMI were more likely to exhibit increased food craving for palatable foods when the VNS device was on.13 That VNS influences cravings for highly palatable foods suggests that it may also affect the consumption of those foods, however this has yet to be examined.
The aim of our study is to examine whether participants consume fewer calories from highly palatable foods when their VNS device is on versus off and if BMI and VNS device settings moderate this relationship. We hypothesize that participants who are overweight or obese will consume less calories during the VNS on session than lean participants. Currently, there are no published studies that have explored the effects of left cervical VNS on food intake in humans. Understanding the relationship between VNS and eating behaviors in humans may elucidate the role of the vagus nerve in weight control.
Methods
Participants
Adults who currently had the left cervical VNS device implanted for treatment of treatment-resistant epilepsy (TRE) or treatment-resistant depression (TRD) were eligible for the study. The majority of participants were female (n = 10), Caucasian (n = 15), and average age was 43.5 years. Average BMI was 29.11 (SD = 6.65). Seven participants were obese, 5 were overweight, and 4 were normal weight. VNS participants were recruited through the epilepsy clinic and the Treatment Resistant Depression Registry at the University of Massachusetts Medical School (UMMS) as well as hospitals and communities in the New England region. Flyers were brought to VNS clinics in New England and distributed to VNS patients and posted in waiting areas. Interested participants contacted the office to inquire about the study and completed an initial telephone screening. Inclusion criteria for the study were that participants had to be between the ages of 18 and 65, have a BMI between 19 and 40, and be treated with a VNS device for either depression or epilepsy.
Procedure
Participants attended 3 different sessions as part of the study. The first session was a 1-hour screening visit where written informed consent was obtained. Participants were screened for inclusion and exclusion criteria through a demographic questionnaire and the Structured Clinical Interview for DSM-III-R. During this session, baseline data were obtained on history of VNS device, weight and height were measured, and depression was assessed via the Beck Depression Inventory (BDI). After BMI was calculated using the formula of weight in kilograms divided by height in meters squared, the following ranges were used to determine BMI category: 18.5-24.9 was normal; 25-29.9 was overweight, and 30 or higher was obese. During this visit, participants rated palatability of different snack foods to determine which foods would be presented at laboratory visits. A release form was signed by participants so that their physicians could be contacted to obtain their VNS device settings. Settings on VNS device include output current, the amount of electrical current delivered in a single pulse of stimulation (measured in mA), and signal on time, which is the length of the programmed output current that is delivered (measured in seconds). After completing this visit, participants attended two 1-hour laboratory visits that were scheduled at least 1 week apart in which their VNS device was either left on or turned off in counterbalanced order. On testing days, participants were asked to eat their normal amount of food and drink their usual amount of caffeine up until 2 hours before the experimental session to minimize caffeine withdrawal and also to avoid acute effects of caffeine intake on behavioral measures. At the beginning of each session, a dietary recall was performed to assess caloric intake on the testing day and to verify that the participant did not consume any food or drink 2 hours prior to the testing session. Then, baseline mood and hunger were assessed via self-report measures. Devices were turned off using the Cyberonics magnet and remained off until the end of the session. The Cyberonics magnet was taped to the participants’ chest. Following VNS manipulation, participants rested for 15 minutes and were given nature magazines to read during this time. This time allowed participants to get accustomed to the VNS manipulation. At the end of the 15 minutes, participants were presented with a tray of 4 servings of their preferred foods. Each of the 4 preferred foods were available to the participant in 400 kcal portions for a total of 1600 kcal. The participant was instructed to taste each food so that they could later answer questions regarding flavor. Participants were told that they may eat as much as they like, the remainder will be thrown away, and that they may not take food home with them. Participants were left alone for 20 minutes to eat as much as they wanted. When 20 minutes expired, a research assistant reentered the room and removed the food tray, and asked the participant to complete the tastiness scale. The study session was then concluded. Weights of all food presession and postsession were compared. Caloric intake was calculated for each session. Devices were reactivated at the end of the session and a psychiatrist was available to ensure that the settings were accurate. All participants received $75 reimbursement after completion of both sessions. This study was approved by the Institutional Review Board at the UMMS.
Data Analyses
Means and standard deviations of demographic variables were calculated. Independent sample t-tests and Mann–Whitney U tests were conducted to examine differences on demographic variables and VNS settings between the epilepsy and depression groups. Covariates that may theoretically impact food intake or BMI were included in the analyses: gender, sequence of sessions (VNS on versus VNS off), length of using VNS device (in months), VNS device output current setting, VNS device on-time (seconds), and BDI score. The Shapiro–Wilk test of normality was conducted prior to running the main analyses to assess whether caloric intake in each of the sessions was normally distributed. Given the small sample, we collapsed overweight and obese BMI categories together and compared this category to the lean BMI category. A mixed ANOVA was conducted to assess whether there were differences in calories consumed between VNS on versus off sessions (within subjects factor) and whether there was an interaction between BMI category and VNS session (on versus off). Follow-up repeated measures ANOVAs and independent sample t-tests were conducted to examine simple effects between the BMI categories for each of the sessions (VNS on and VNS off). Since we had a small sample size, “statistically significant” results refer to those with an alpha < .05 and trends refer to those with an alpha level < .10.
Results
No significant differences were observed between TRE and TRD participants on demographic variables (see Table 1 for demographic characteristics by group). Differences in age trended toward significance between the TRE (mean = 38.71 ± 8.62) and TRD groups (mean = 47.22 ± 8.06), t(14) = −2.03, P = .06. No significant differences were observed on BDI score between TRE (mean = 9.14 ± 10.22) and TRD (mean = 18.53 ± 15.26), t(14) = −1.40, P = .18. Furthermore, BMI was not different between TRE (mean = 27.29 ± 5.81) and TRD groups (mean = 30.53 ± 7.23), t(14) = −0.97, P = .35. Length of time with the VNS device did not differ between patients with TRD (mean = 17.9 ± 6.13 months) or TRE (mean = 21.2 ± 11.41 months, P = .50), t(11) = 0.71, P = .50. Groups did not differ on any of the VNS settings (data not shown). The Shapiro-Wilk test was not significant for VNS off session (P = .35) or VNS on session (P = .38) suggesting that the data were normally distributed.
Table 1.
Demographics by Treatment Group.
| Variable | TRD (n = 9) | TRE (n = 7) | Significance |
|---|---|---|---|
| Gender (%) | P = .17 | ||
| Male | 22.2 | 57.1 | |
| Female | 77.8 | 42.9 | |
| Marital status (%) | P = .53 | ||
| Single | 33.3 | 42.9 | |
| Married | 44.4 | 57.1 | |
| Divorced/widowed | 22.3 | 0 | |
| Education (%) | P = .71 | ||
| High school | 22.2 | 28.6 | |
| Some college | 55.6 | 42.8 | |
| Bachelor’s degree | 22.2 | 28.6 | |
| Employment status (%) | P = .18 | ||
| Employed (full- or part-time) | 33.3 | 14.3 | |
| Unemployed | 11.1 | 14.3 | |
| Disabled | 55.6 | 71.4 | |
| Annual household income (%) | P = .45 | ||
| <$15 000 | 11.1 | 28.6 | |
| $15 000-35 000 | 11.1 | 28.6 | |
| $35 000-60 000 | 55.5 | 28.6 | |
| >$60 000 | 22.2 | 14.3 | |
| VNS therapy settings (mean ± SE) | |||
| Output current | 1.45 ± 0.40 | 1.38 ± 0.14 | P = .36 |
| Frequency | 25.56 ± 1.76 | 20.00 ± 0.00 | P = .38 |
| Pulse width (micro-seconds) | 333.33 ± 41.6 | 250.00 ± 0.00 | P = .82 |
| On-time (seconds) | 33.33 ± 3.33 | 13.0 ± 4.46 | P = .43 |
| Off-time (minutes) | 2.53 ± 0.63 | 1.78 ± 0.083 | P = .37 |
| Mean ± SE | |||
| Age | 47.22 ± 2.68 | 38.71 ± 3.26 | P = .06 |
| BMI | 30.53 ± 2.41 | 27.28 ± 2.19 | P = .35 |
| Beck Depression Inventory | 18.5 ± 5.10 | 9.1 ± 3.86 | P = .18 |
| Education level | 10.99 ± 2.56 | 13.29 ± 2.48 | P = .72 |
| Monthly income ($) | 772.18 ± 577.93 | 1694.04 ± 1350.84 | P = .38 |
BMI, body mass index; TRD, treatment-resistant depression; TRE, treatment-resistant epilepsy; VNS, vagus nerve stimulation.
Correlations Among Study Variables
Table 2 presents the bivariate correlations among the study variables. Total caloric consumption during VNS on sessions was positively correlated with total calorie consumption during VNS off sessions. VNS output current was positively correlated with length of VNS use (measured in months), as well as with VNS device on time (measured in seconds). VNS device on time was also positively correlated to length of VNS use.
Table 2.
Correlations among Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Total calories consumed VNS off | — | |||||||
| 2. Total calories consumed VNS on | .794* | — | ||||||
| 3. Length of VNS use (in month) | −.074 | −.094 | — | |||||
| 4. Output current (mA) | .292 | .353 | .572* | — | ||||
| 5. “On” time (seconds) | .254 | .238 | .655* | .980* | — | |||
| 6. BMI | .046 | .376 | −.001 | .259 | .126 | — | ||
| 7. Age | −.190 | −.070 | −.524 | −.459 | −.459 | −.005 | — | |
| 8. BDI score | .007 | .046 | .328 | .330 | .330 | .349 | .296 | — |
BDI, Beck Depression Inventory; BMI, body mass index; VNS, vagus nerve stimulation.
P < .05.
Caloric Intake by Condition (VNS On Versus VNS Off) and BMI Category
No main effect was observed for condition on calories consumed between VNS on (mean = 723.34 ± 327.45) and off (mean = 753.61 ± 238.91) sessions (P = .96). The range in calories consumed during the VNS on session was 314.20 to 1292.92 calories. During the VNS off session, consumption ranged from 211.59 to 1037.97 calories. When looking at the effects of covariates on caloric intake by session, there was an interaction of VNS device output current and VNS session (P = .05, η2 = .56), such that higher current levels were associated with more calories consumed during the on session versus the off session. There was a trend toward significance in the interaction of VNS device on time and VNS session (P = .07, η2 = .51), such that longer on-time was associated with less calories consumed when the VNS device was on versus off. When controlling for covariates, there was a significant interaction between BMI and VNS session (P = .03, η2 = .67; see Figure 1). Independent sample t tests did not reveal significant differences between lean (mean = 878.03 ± 194.43) and overweight/obese groups (mean = 716.29 ± 249.97) on calories consumed during the VNS off session (P = .33, d = 0.72). Significant differences between lean (mean = 525.84 ± 163.54) and overweight/obese groups (mean = 782.59 ± 346.58) were not found during the VNS on session (P = .25, d = 0.95).
Figure 1.
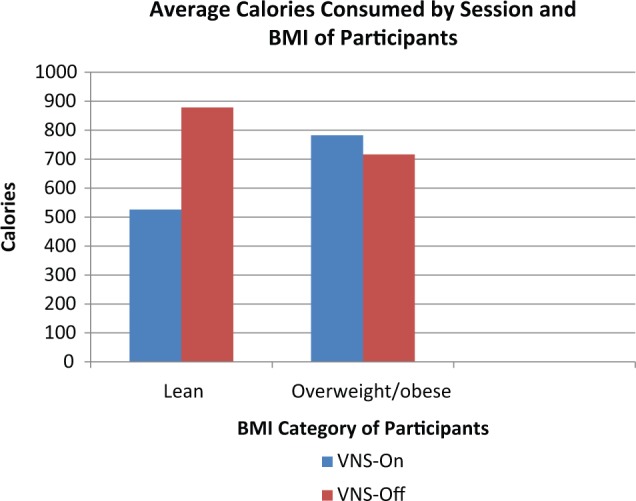
Average calories consumed by vagus nerve stimulation (VNS) on versus VNS off session by BMI category.
Discussion
Our results suggest that BMI is related to the effect that VNS has on caloric intake among individuals with epilepsy or depression. However, our findings did not support our hypothesis. Lean individuals consumed fewer calories when the device was on than when the device was off. On the other hand, participants who were overweight/obese consumed the same amount of calories when the device was on compared to when the device was off. These findings would suggest that an activated VNS may suppress intake in lean people.
Our findings may be explained by changes in the vagal afferent pathways in overweight and obese participants. Overweight and obese participants may be more resistant to satiety signals that are being received from vagal afferents. Obese patients are often resistant to the satiating effects of leptin.15 It has been found that the sensitivity of vagal afferent neurons to CCK is reduced in rats who are leptin resistant.16 It may be that the vagal afferent pathways are altered in patients with higher BMIs based on a previous history of overeating higher fat and caloric foods and therefore they are less likely to have food intake altered after a short period of VNS.
In our previous work,13 we found that in response to acute VNS, individuals with higher BMIs had decreased palatable food cravings and those with lower BMIs had increased cravings. Results from the current study are opposite of what would be expected given our findings with cravings. However, this may be a function of the differences in the task: responses to pictures of foods and consumption of real food. Although individuals with lower BMI report increased cravings for food during VNS, this may not translate to actual consumption. One limitation of comparing these 2 studies is that fewer than half of our participants (43%) in the current study were given sweet foods in the ad libitum eating session. Without data on sweet foods, we are limited in the conclusions we can draw from these differences.
It may also be that the location of stimulation on the vagal nerve makes a difference in how eating and weight are affected.17 Much of the current animal research that links VNS and eating behavior examines the role of the vagal afferents in the celiac branches.4,6,18 Our study focuses exclusively on the cervical branch of the vagus nerve. In treatments such as vagal blocking therapy, electrical currents sent through electrodes positioned near vagal trunks in the esophagogastric junction, result in 14% weight loss after 6 months in obese patients.19 In a recent clinical trial looking at vagal blocking therapy, hours of use of the treatment was linearly related to weight loss among morbidly obese patients.20 It is likely that such direct access to the vagus nerve near the gastric area has an impact on eating behavior that is more pronounced in obese patients and is not diluted as much as stimulation of the vagus nerve in the cervical area.
Similar to previous research, we found an effect of VNS output current on the VNS-related effects on caloric intake. Higher output current, amount of electrical impulse delivered by the device, was associated with consumption of more calories when the device was on versus off. This conflicts with previous literature that proposes a negative relationship between output current and weight.9 In the VNS and pain literature, it’s been suggested that during device activation, patients are acutely hypersensitive to painful stimuli, however in the long term, pain tolerance is increased.21 This may be the result of down-regulation of central pain mechanisms over time as a result of repeated hypersensitivity to stimuli when the device is on (or active). Similarly, the same process could explain this relationship between output current and caloric intake. In the short term, higher output current is associated with increased caloric intake but over time a system wide down regulation of eating occurs and these higher levels over time result in weight loss. This theory is speculative and research is needed to further test this relationship.
There are limitations of our study that should be noted. First, our sample was small, which limited our statistical power to examine differences by each BMI category and therefore led to the combined overweight/obese category. Although we found a significant interaction, because of small sample sizes, our simple effect analyses were not significant. With each subgroup analysis that we conducted, we lost power to detect differences as our cell sizes were small. Low power limits our ability to understand what is causing this interaction and how to make sense of our findings. Although it is difficult to recruit this population, studies are needed with larger sample sizes to tease out these effects. There was also a lot of variability in our sample in that half of our patients were epilepsy patients and the other half were using VNS therapy for TRD. If our sample were larger, it would have been ideal to examine disease status (epilepsy versus depression) as a moderator. One of the symptoms of depression is a change in eating (i.e., overeating or undereating) so it is difficult to understand the impact that these symptoms may have had on weight or eating behaviors.
Despite these limitations, this study is unique in that it is the first to explore the acute effects of VNS on caloric intake in adults who have a VNS device implanted. We add to the literature by suggesting that BMI is an important factor to consider when examining the role of the vagus nerve on appetite. Future research should explore appetite, consumption, hormone levels, and weight before and after VNS implantation, as changes might be more apparent. As we were using a clinical population, ethically, we could not manipulate the settings on the device. However, future research is needed to explore how higher levels of output current impact eating and weight. Research regarding the effect of acute VNS on eating and weight among individuals with the vagus nerve stimulated in closer proximity to the stomach is also needed.22
Footnotes
Abbreviations: BMI, body mass index; TRD, treatment-resistant depression; TRE, treatment-resistant epilepsy; UMMS, University of Massachusetts Medical School; VNS, vagus nerve stimulation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Boston Obesity Nutrition Center Grant; Worcester Foundation for Biomedical Research; Clinical and Translational Science Pilot Project Program at UMass Medical School. Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, and St Jude Medical, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, and Pfizer Inc. Dr. Rothschild has received royalties for the Rothschild Scale for Antidepressant Tachyphylaxis (RSAT)®; Clinical Manual for the Diagnosis and Treatment of Psychotic Depression, American Psychiatric Press, 2009; The Evidence-Based Guide to Antipsychotic Medications, American Psychiatric Press, 2010; and The Evidence-Based Guide to Antidepressant Medications, American Psychiatric Press, 2012.
References
- 1. Sobocki J, Krolczyk G, Herman RM, Matyja A, Thor PJ. Influence of vagal nerve stimulation on food intake and body weight—results of experimental studies. J Physiol Pharmacol. 2005;56(suppl 6):27-33. [PubMed] [Google Scholar]
- 2. Roslin M, Kurian M. The use of electrical stimulation of the vagus nerve to treat morbid obesity. Epilepsy Behav. 2001;2(suppl):S11-S16. [Google Scholar]
- 3. Val-Laillet D, Biraben A, Randuineau G, Malbert CH. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite. 2010;55(2):245-252. [DOI] [PubMed] [Google Scholar]
- 4. Bugajski AJ, Gil K, Ziomber A, Zurowski D, Zaraska W, Thor PJ. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J Physiol Pharmacol. 2007;58:5-12. [PubMed] [Google Scholar]
- 5. Banni S, Carta G, Murru E, et al. Vagus nerve stimulation reduces body weight and fat mass in rats. PLOS ONE. 2012;7(9):e44813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effects of cholecystokinin in the rat. Science. 1981;213:1036-1037. [DOI] [PubMed] [Google Scholar]
- 7. Ritter RC. A tale of two endings: modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. 2011;105(1):94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pardo JV, Sheikh SA, Kuskowski MA, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes. 2007;31(11):1756-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurol. 2002;59(3):463-464. [DOI] [PubMed] [Google Scholar]
- 10. Koren MS, Holmes MD. Vagus nerve stimulation does not lead to significant changes in body weight in patients with epilepsy. Epilepsy Behav. 2006;8(1):246-249. [DOI] [PubMed] [Google Scholar]
- 11. Chatrchyan S, Khachatryan V, Sirunyan AM, et al. Search for heavy neutrinos and W(R) bosons with right-handed couplings in a left-right symmetric model in pp collisions at sqrt[s] = 7 TeV. Physical Rev Lett. 2012;109(26):261802. [DOI] [PubMed] [Google Scholar]
- 12. Bodenlos JS, Kose S, Borckardt JJ, et al. Vagus nerve stimulation and emotional responses to food among depressed patients. J Diabetes Sci Technol. 2007;1(5):771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bodenlos JS, Kose S, Borckardt JJ, et al. Vagus nerve stimulation acutely alters food craving in adults with depression. Appetite. 2007;48(2):145-153. [DOI] [PubMed] [Google Scholar]
- 14. Basdevant A, Craplet C, Guy-Grand B. Snacking patterns in obese French women. Appetite. 1993;21(1):17-23. [DOI] [PubMed] [Google Scholar]
- 15. Myers MGJ, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibit cholecystokinin signaling and satiation in diet induced obese rats. PLOS ONE. 2012;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizrahi M, Ben Ya'acov A, Ilan Y. Gastric stimulation for weight loss. World J Gastroenterol. 2012;18(19):2309-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sobocki J, Fourtanier G, Estany J, Otal P. Does vagal nerve stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery. 2006;139(2):209-216. [DOI] [PubMed] [Google Scholar]
- 19. Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): Clinical results with a new implantable medical device. Surgery. 2008;143(g):723-731. [DOI] [PubMed] [Google Scholar]
- 20. Sarr MG, Billington CJ, Brancatisano R, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obesity Surg. 2012;22(11):1771-1782. [DOI] [PubMed] [Google Scholar]
- 21. Borckardt JJ, Kozel FA, Anderson B, Walker A, George MS. Vagus nerve stimulation affects pain perception in depressed adults. Pain Res Manag. 2005;10(1):9-14. [DOI] [PubMed] [Google Scholar]
- 22. Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: Evolving trends. J Nat Sci Biol Med. 2013;4(1):8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


