Abstract
Glycemic control remains suboptimal in youth with type 1 diabetes. Retrospective continuous glucose monitoring (CGM) has demonstrated utility in fine-tuning diabetes management by detecting postprandial hyperglycemia and hypoglycemia. In this study, we explored the process of 3-day masked CGM use, subsequent treatment recommendations, and impact on A1c in a clinic-based sample of youth with type 1 diabetes. Over 2 years, 122 youth were referred for masked CGM. Patients/families completed a diary of blood glucose levels, insulin doses, food intake, and exercise during CGM use. A1c was assessed pre- and 2-3 months post-CGM. Treatment recommendations were formulated using data from CGM reports and diaries. Mean age was 14.3 ± 3.9 years, diabetes duration was 7.5 ± 4.7 years, and A1c was 8.5 ± 1.1% (69 ± 12 mmol/mol); 61% were pump-treated. Patients received an average of 3.1 ± 1.1 treatment recommendations following review of the CGM report. Most (80%) received reinforcement of the importance of preprandial bolusing; 37% received a recommendation regarding advanced insulin management (use of combination boluses/attend to active insulin). Receipt of the latter recommendation was related to A1c improvement ≥0.5% (OR: 4.0, P < .001). Masked CGM offers opportunities to guide advanced insulin management (by injection or pump), which may yield A1c improvements in youth with type 1 diabetes.
Keywords: type 1 diabetes, continuous glucose monitoring, A1c, pediatrics
Glycemic control remains suboptimal in youth with type 1 diabetes.1,2 Modern treatment tools, such as continuous glucose monitoring (CGM), can reduce hypoglycemia and A1c;3,4 however, youth are often reluctant to use CGM continuously.5 A1c improvement has been associated with real-time CGM (RT-CGM) use ≥6 days/week, a challenge for pediatric patients.5
Masked or retrospective CGM provides an alternative to RT-CGM for patients.6 Studies have demonstrated the utility of masked CGM to identify postprandial hyperglycemia7 and hypoglycemia8 in pediatric patients. In this study, we explored the process of masked CGM use, subsequent treatment recommendations, and impact on A1c in youth with type 1 diabetes.
Methods
Over 2 years, we identified young patients with type 1 diabetes referred for masked CGM within a pediatric, adolescent, and young adult diabetes clinic. The Institutional Review Board granted waivers of informed consent and authorization for use/disclosure of protected health information from the electronic medical record. Following clinician referral, pediatric nurses implemented masked CGM (Medtronic iPro™) according to the manufacturer’s recommendations. Numbing cream/spray and/or child life support facilitated device insertion as needed.
Patients/families received a diabetes diary to record details of daily blood glucose levels, insulin doses, food intake, and physical activity during CGM use. (Copies of the diary are available from the authors upon request.) The research team determined completeness of diaries based on the 3 aspects of management (diet, insulin, and exercise) used to inform clinical decision making. A lack of recorded data was deemed as incomplete and given the score of 1; some data but lacking significant detail was described as partial and given the score of 2; comprehensive data recorded for diet, insulin, and exercise was considered complete and given the score of 3. Three research team members established interrater reliability of this scoring method, which was subsequently used to describe the completeness of the diaries. Patients/families received training related to completing the diabetes diary, calibrating the CGM 1 and 2 hours after insertion, synchronizing CGM and meter times, checking blood glucose values preprandially 4+ times/day, removing sensor after 3 days, and returning CGM and diary to the clinic.
Following device return, staff downloaded CGM data according to manufacturer’s guidelines. Download included the number of interstitial glucose values, mean sensor glucose, and standard deviation (SD) of sensor glucose values. A nurse practitioner reviewed CGM reports for safety to assess unrecognized hypoglycemia or sustained hyperglycemia with immediate contact to family when needed. At a subsequent phone or in-person visit, a nurse practitioner reviewed reports with the patient/family and made appropriate treatment recommendations (Figure 1). Patients/families were sent copies of the CGM reports prior to visits that occurred by phone. Treatment data, demographics, and A1c (obtained pre- and ~2-3 months post-CGM use) were extracted from medical records.
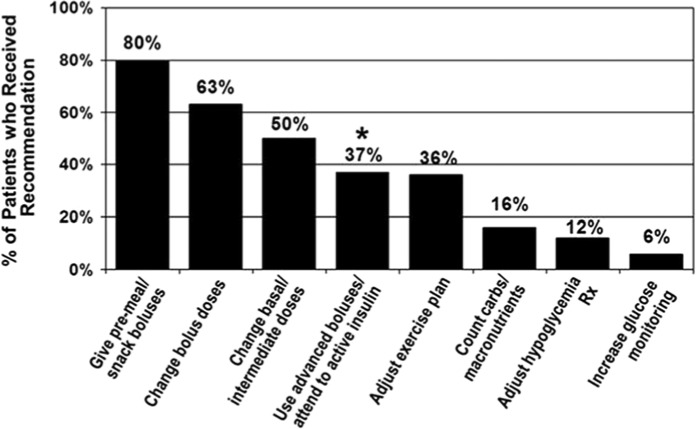
Figure 1.
Percentage of patients who received each treatment recommendation following CGM.
*Those who received the recommendation to use advanced boluses/attend to active insulin were 4.0 times more likely to improve A1c ≥0.5% than those who did not receive this recommendation (P < .001).
Data Analysis
Data are reported as mean ± SD (range), median (interquartile range, IQR), and proportions. We defined improvement as a decrement in A1c ≥0.5%. Analyses, performed using SAS (version 9.2; SAS Institute Inc, Cary, NC, USA), included paired and unpaired t tests and chi-square tests; P ≤ .05 defined significance.
Results
Sample
Patients referred for masked CGM (N = 122, 53% female) were 14.3 ± 3.9 years old (range 7-28) with type 1 diabetes for 7.5 ± 4.7 years (range 1-23). All received intensive therapy: 61% pump, 39% multiple injections (34% basal-bolus, 5% basal analog with AM NPH). Mean baseline A1c was 8.5 ± 1.1% (range 5.8-12.6%) (69 ± 12 mmol/mol, range 40-114).
Reasons for Masked CGM
Patients were often referred for multiple reasons. The most common reasons were assessment of hyperglycemia (39%) or hypoglycemia (37%), patient/family interest in RT-CGM (37%), and insulin dosing adjustments (27%). Other reasons included evaluation of impact of food and exercise and follow-up after diabetic ketoacidosis.
CGM Data
Most patients successfully wore CGM following a single insertion; 3 required reinsertions. Mean number of sensor glucose readings/patient was 894 ± 136 (range 435-1151), capturing 1.5-4 days of CGM, with an average of 3.1 days. Mean sensor glucose was 181 ± 34 mg/dL (range 103-265 mg/dL), and mean SD of sensor glucose was 75 ± 16 mg/dL (range 34-114 mg/dL).
Treatment Recommendations
Almost all patients (n = 116, 95%) received multiple recommendations following CGM, 5 (4%) received a single recommendation, and 1 received none. The mean number of recommendations/patient was 3.1 ± 1.1 (range 0-6). Most (80%) received the reminder to give insulin preprandially as reinforcement of standard care. Other common recommendations included specific dose adjustments (bolus and/or basal) and review of insulin action (advanced boluses/attention to active insulin) (Figure 1).
Treatment Outcomes
To assess impact of recommendations following CGM, we compared patients’ A1c levels a median of 2.6 months (IQR 1.8-4.3) post-CGM. Mean follow-up A1c was 8.4 ± 1.1% (range 5.9-12.3%) (68 ± 12 mmol/mol, range 41-111); mean A1c change was −0.1 ± 0.7% (range −1.9-2.1%) (1 ± 8 mmol/mol, range −21-23).
About a third of patients (n = 39, 32%) improved A1c by ≥0.5%. These patients, compared to those without improvement, were older (15.5 ± 4.4 vs 14.0 ± 3.6 years, P = .04), had longer diabetes duration (8.7 ± 4.9 vs 6.9 ± 4.5 years, P = .05), had higher initial A1c (8.9 ± 1.0 vs 8.2 ± 1.1%, P < .001), and received more treatment recommendations (3.5 ± 1.1 vs 3.0 ± 1.1, P = .01). A1c improvement ≥0.5% was associated with the recommendation to use advanced boluses/attend to active insulin); receiving this recommendation increased the odds of improving A1c ≥0.5% by 4-fold (P < .001). Of those who improved, 60% received this recommendation. Over 95% of participants provided complete or partially complete records with respect to diet intake, insulin administration, and exercise over the period of masked CGM use that offered adequate information to inform interpretation of the CGM data and direct treatment recommendations.
Discussion
Among intensively treated young patients with type 1 diabetes, 32% improved their A1c by ≥0.5% following masked CGM use. Specifically, those who improved were significantly older, had longer diabetes duration, had higher baseline A1c, and received more treatment recommendations following CGM than those without improvement (all P ≤ .05). As those with higher baseline A1cs were more likely to improve, this could represent regression to the mean. In addition, it is important to note that although the majority did not experience an improvement in A1c, this improvement was significant for older youth in worse glycemic control who need lower A1c levels. It is possible that more patients could experience A1c improvement if CGM use were repeated to confirm patterns to inform management decisions. Future research is needed to determine the optimal frequency of diagnostic CGM use to improve glycemic outcomes. Nonetheless, patients/families who received guidance about insulin action, that is, advanced boluses/attention to active insulin, were 4.0 times (P < .001) more likely to improve A1c by ≥0.5%. Notably, this recommendation applied to those treated by injections or pumps, and insulin treatment modality was not related to A1c improvement.
The study’s aim was to explore use of masked CGM and identify treatment recommendations associated with fine-tuning diabetes management and improving glycemic control. Our findings suggest that 3-day masked CGM may offer opportunities to improve A1c in pediatric patients with type 1 diabetes. In addition, while a need for reinforcement of fundamentals, like premeal bolusing, remains, focusing on advanced skills, like complex boluses/attention to active insulin, may yield improved glycemic control. This improvement may be attributable to an increased awareness of and adherence to the intricacies of the balance between insulin delivery and food intake that is essential to diabetes self-management. Review of CGM reports with patients/families helps to reinforce insulin kinetics/pharmacodynamics in efforts to explain the delayed peak in insulin action for rapid-acting analogs9 and the impact of different foods on postprandial glycemic excursions.10,11 CGM provided opportunities for patient self-management and family education around complex areas pertaining to diabetes management, particularly insulin action and use of advanced boluses.
This study also assessed the role of the pediatric team in clinical use of masked CGM, similar to its use in adult patients.12 Successful masked CGM requires qualified and trained nurses for multiple tasks including: CGM insertion and instruction/training regarding proper calibration and completion of the diabetes diary, CGM data downloads, safety assessment of CGM reports, and careful reviews of CGM reports alongside the detailed family-completed glucose, insulin, diet, and physical activity diary.
There are caveats to this analysis. We were unable to assess use of complex bolus doses (given by pump or prandial insulin separated into 2 doses for those treated by injections) or avoidance of insulin stacking. In addition, this study only included 1-time masked CGM use. While all patients received a review of the masked CGM data by phone or in-person, we are unable to evaluate whether the method of review or diary completeness directly impacted A1c change, future research can assess such details. Another limitation in our report is that we are unable to compare rates of hypoglycemia pre- and post-CGM use. Finally, as this was an observational, descriptive study, we were not able to assess if patients implemented the treatment recommendations and the relationship between adherence to treatment recommendations and A1c. Nonetheless, it is encouraging to see opportunities with CGM to improve A1c in young patients. Additional research could also explore the mode of masked CGM review to determine the optimal approach for relaying the data to the family, especially in the current era of telehealth and m-health. The need and timing of repeat masked CGM use and its potential long-term benefits related to implementation of treatment recommendations require additional study. Future studies can also evaluate whether patients are more likely to transition to RT-CGM after masked CGM use and whether such patients are more likely to sustain use of RT-CGM.
Conclusions
In summary, this study demonstrated that masked CGM offers opportunities to guide advanced insulin management (by pump or injection) and may yield A1c improvements in young people with type 1 diabetes.
Acknowledgments
We are grateful to all of the clinical staff involved in Joslin’s pediatric masked CGM program. Portions of this article were presented at the 2012 Scientific Sessions of the American Diabetes Association and the 2012 Annual Meeting & Exhibition of the American Association of Diabetes Educators.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; IQR, interquartile range; RT-CGM, real-time CGM; SD, standard deviation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KMM and JK were consultants for Medtronic at the time of the study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH grants R01DK089349 and P30DK036836, the Eleanor Chesterman Beatson Fund, the Katherine Adler Astrove Youth Education Fund, and the Maria Griffin Drury Pediatric Fund.
References
- 1. de Beaufort CE, Swift PG, Skinner CT, et al. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30:2245-2250. [DOI] [PubMed] [Google Scholar]
- 2. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730-2732. [DOI] [PubMed] [Google Scholar]
- 4. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 5. Chase HP, Beck RW, Xing D, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010;12:507-515. [DOI] [PubMed] [Google Scholar]
- 6. Pepper GM, Steinsapir J, Reynolds K. Effect of short-term iPRO continuous glucose monitoring on hemoglobin A1c levels in clinical practice. Diabetes Technol Ther. 2012;14:654-657. [DOI] [PubMed] [Google Scholar]
- 7. Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858-1862. [DOI] [PubMed] [Google Scholar]
- 8. Schiaffini R, Ciampalini P, Fierabracci A, et al. The Continuous Glucose Monitoring System (CGMS) in type 1 diabetic children is the way to reduce hypoglycemic risk. Diabetes Metab Res Rev. 2002;18:324-329. [DOI] [PubMed] [Google Scholar]
- 9. Swan KL, Weinzimer SA, Dziura JD, et al. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care. 2008;31:44-46. [DOI] [PubMed] [Google Scholar]
- 10. Ryan RL, King BR, Anderson DG, Attia JR, Collins CE, Smart CE. Influence of and optimal insulin therapy for a low-glycemic index meal in children with type 1 diabetes receiving intensive insulin therapy. Diabetes Care. 2008;31:1485-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maahs DM, Mayer-Davis E, Bishop FK, Wang L, Mangan M, McMurray RG. Outpatient assessment of determinants of glucose excursions in adolescents with type 1 diabetes: proof of concept. Diabetes Technol Ther. 2012;14:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miele A, Weiland K, Dungan KM. Clinical outcomes associated with referral-based continuous glucose monitoring using a central standardized interpretation strategy. Diabetes Technol Ther. 2012;14:765-771. [DOI] [PubMed] [Google Scholar]