Abstract
Uncertainty remains about effectiveness of continuous glucose monitoring (CGM) in pediatric type 1 diabetes (T1D). Success with CGM is related to CGM adherence, which may relate to readiness to make the behavior changes required for effective use. We hypothesize that readiness for change will be greater at initiation of insulin pump therapy than in established pump users, and that this will predict CGM adherence. Our objective was to evaluate the feasibility of a randomized controlled trial (RCT) in children with established T1D comparing simultaneous pump and CGM initiation to standard pump therapy with delayed CGM initiation. We randomized participants to simultaneous pump and CGM initiation or to standard pump therapy with the option of adding CGM 4 months later. CGM adherence was tracked via web-based download and readiness for change assessed with the SOCRATES questionnaire. Of 41 eligible children, 20 agreed to participate; 15 subjects completed the study (7 males; baseline age 11.8 ± 4.0 years; T1D duration 2.7 ± 2.7 years; mean A1C 8.2 ± 0.8%). Six of 8 simultaneous group subjects used CGM > 60% of the time for 4 months compared to 1 of 7 delayed group subjects (P = .02). Using SOCRATES, we could assign 87-100% of subjects to a single motivation stage at baseline and 4 months. This pilot study demonstrates the feasibility of randomizing pump naïve children and adolescents with established T1D to simultaneous pump and CGM initiation versus standard pump therapy with delayed CGM initiation. Lessons from this pilot study were used to inform development of a full-scale multicenter RCT.
Keywords: child, continuous glucose monitoring, diabetes mellitus, type 1, insulin pump, patient adherence
Continuous glucose monitoring (CGM) has been shown to be more effective than self-monitoring of blood glucose (SMBG) in reducing hemoglobin A1c (HbA1c) in adults with type 1 diabetes, with equivocal results in children and youth.1-6 A significant dose-response effect has been observed in all age groups with greater CGM use correlating with greater HbA1c reduction.2,7-9 Timing of CGM initiation may play a role in its successful use in the pediatric population.
Most CGM studies have focused on experienced pump or MDI users, believing that successful CGM use requires individuals to be knowledgeable and comfortable with their insulin delivery system.3,4,5,6,8 However, successful pump or MDI users, especially youth, may have less personal motivation for using CGM if they perceive that they are doing well without it. Alternatively, adding CGM to the regimen of less successful pump or MDI users (ie, those with suboptimal HbA1c) may fail because these individuals are already struggling with the demands of their insulin regimen. Several studies have suggested that CGM adherence, and its effect on HbA1c, may be greater if CGM is initiated at the same time or just before starting pump therapy.10-14 However, these studies have been unable to demonstrate whether sensor-augmented pump therapy (SAPT) is more effective than standard pump therapy in pump naïve individuals, nor whether adherence to CGM is greater if started at the same time as pump therapy rather than in experienced pump users.
We hypothesize that CGM will be more successful if introduced at the same time as pump therapy because it will be considered an integral part of pump therapy by individuals who have greater readiness for making and sustaining behavior change. The objectives of this pilot study were to (1) evaluate the feasibility of a randomized controlled trial (RCT) comparing simultaneous pump and CGM initiation to standard pump therapy with delayed CGM initiation in children and adolescents with established type 1 diabetes and (2) determine the ability to measure readiness for making and sustaining behavior changes in children and adolescents with type 1 diabetes.
Methods
This pilot RCT was conducted at 2 pediatric centers in Canada and in accordance with the Declaration of Helsinki and good clinical practice guidelines.15 The study was approved by the Institutional Review Boards at both centers. Written consent was obtained from parents or guardians with written assent from each participant.
Subjects
We invited subjects to participate if they met local criteria for starting pump therapy based on their diabetes team’s assessment, and the study’s inclusion criteria: age 5 to 18 years; type 1 diabetes duration ≥ 1 year; naïve to insulin pump therapy and about to start pump therapy with the Medtronic Paradigm® 522/722 pump (Medtronic Minimed Inc, Northridge, CA, USA); willing to use CGM; home computer with internet access; comprehension of written English or French by the primary caregiver; and considered capable of completing study requirements.
Study Design
Randomization was performed using a computer-generated randomization table with stratification by age (5-12 years, and 13-17 years), and allocation concealment. Subjects were assigned, in a 1:1 ratio, to either the “simultaneous group,” which initiated CGM at the same time as starting pump therapy, or the “delayed group,” which started standard pump therapy, with the option of adding CGM 4 months later. All subjects received standard pump education and were asked to perform SMBG at least 4 times daily. They all received training on Medtronic CareLink® Personal software and were asked to upload to CareLink weekly from home.
Two weeks before the pump start, simultaneous group subjects received training on the Medtronic REAL-Time glucose system, and were provided with the Minilink® REAL-Time Transmitter. Subjects used the Medtronic SofSensor® and were provided a 4-month supply of glucose sensors. CGM low- and high-glucose alarms were activated at <63 mg/dl and >306 mg/dl. Subjects were advised to use CGM on a continuous basis until their 4-month clinic visit.
Follow-up visits for both groups were at 1 and 4 months after pump initiation as per standard local practice. At 4 months (end of study), delayed group subjects were offered initiation of CGM including 4 months of CGM supplies and the same CGM training as the simultaneous group had received.
Measurements
HbA1c was measured locally (DCA 2000+ analyzer; Siemens Healthcare Diagnostics, Indianapolis, IN, USA [nondiabetic range, 4-6.2%]; Tosoh 2.2 analyzer, Somagen Diagnostics Inc, Foster City, CA, USA [nondiabetic range 4-6.0%]) at baseline and 4 months. Blood glucose meters and pumps were downloaded at each study visit. CGM adherence (hours per week expressed as the percentage of the maximum 168 hours per week) was obtained from Medtronic CareLink Professional (simultaneous group: baseline to 4 month study visit; delayed group: 4-month period following 4 month study visit). Parents, and subjects (older than 8 or 10 years of age depending on the questionnaire), completed self-report questionnaires at baseline and each study visit including (1) Illness Management Survey (IMS) (a measure of barriers to adherence in children with chronic diseases),16 (2) Diabetes Treatment Satisfaction Questionnaire (DTSQ),17 and (3) Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) (a measure of motivational stage modified for diabetes).18
Data Analysis
Given its pilot nature, this trial was not powered to detect a statistically significant difference in outcomes. No formal sample size calculation was performed. A convenience sample of 20 participants (10 in each group) was planned based on estimated recruitment at the 2 centers.
Descriptive statistical analyses were performed for baseline characteristics: frequencies and percentages were reported for categorical variables, means ± SD for normally distributed continuous variables, and medians and quartiles for non-normally distributed continuous variables. Statistical analyses of changes in HbA1c levels, mean number of BG tests per day, pump parameters (ie, total daily insulin dose, frequency of boluses and set changes), and questionnaire responses were performed between groups by use of means (and 95% confidence intervals) comparing baseline to 4 months. Analysis was by intention to treat.
Results
Study Recruitment and Baseline Characteristics
Between February 2009 and January 2011, 149 patients started insulin pump therapy in the 2 centers, with 41 (28%) meeting trial eligibility criteria; the remainder chose a pump other than Medtronic. Twenty of the 41 eligible subjects (48.7%) consented to participation. Ten subjects were randomized to the simultaneous group, and 10 to the delayed group. Five subjects (25%) withdrew from the study: 2 from the simultaneous group (one postrandomization because the child didn’t want to wear the sensor and 1 at 3 weeks due to difficulties with CGM use) and 3 from the delayed group (2 withdrew postrandomization to start simultaneous CGM). Fifteen subjects completed the study and provided data that could be included in the analyses, which was by intention to treat. Patient characteristics were similar at baseline except that the simultaneous group was performing blood glucose tests more frequently (difference 0.64/day, 95% CI: 0.14-3.09, P = .03) (Table 1).
Table 1.
Baseline Demographics.
| Simultaneous group (n = 8) | Delayed group (n = 7) | P value | |
|---|---|---|---|
| Male (n, %) | 3 (38) | 4 (57) | .45 |
| Age (years) | 12.2 ± 4.4 | 11.5 ± 3.8 | .78 |
| Duration of diabetes (years) | 3.2 ± 3.0 | 2.1 ± 2.5 | .05 |
| Body mass index (z score) | 0.52 ± 0.75 | 0.24 ± 0.60 | .69 |
| Mean HbA1c in the last year (%) | 8.0 ± 0.9 | 8.4 ± 0.8 | .54 |
| Total daily insulin dose (U/kg/d) | 0.92 ± 0.26 | 0.88 ± 0.40 | .87 |
Values are mean ± SD or number and %.
CGM Adherence
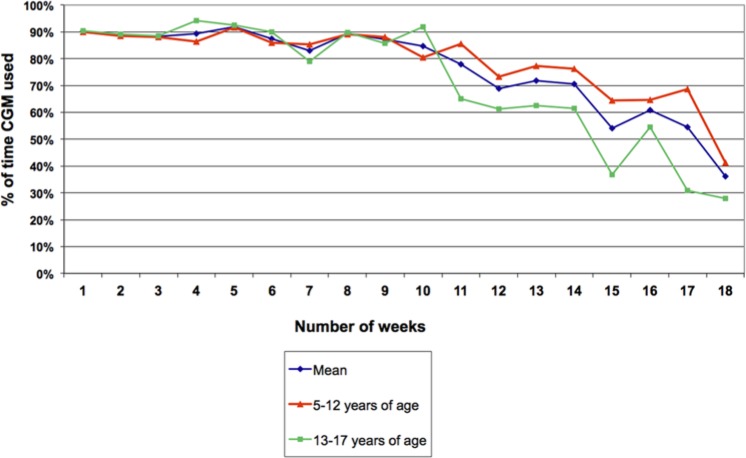
Amongst simultaneous group subjects, CGM adherence was highest over the first 10 weeks (individual subjects’ mean adherence during this period ranged from 69.7-91.5%) with mean CGM adherence > 65% until 14 weeks, then falling to 36.1% at the end of the study (Figure 1).
Figure 1.
Adherence to continuous glucose monitoring (CGM) in simultaneous group.
Six of the 8 simultaneous group subjects used CGM > 60% of the time (defined as wearing CGM> 100 out of a possible 168 hours per week) during the 4 month study period. Six of the 7 delayed group subjects agreed to try CGM 4 months after pump initiation. Five of these subjects discontinued CGM within 2 weeks of its initiation; only 1 delayed group subject used CGM > 60% of the time for 4 months (P = .02, compared to simultaneous group subjects).
Readiness for Change
The readiness for change model was first described by Prochaska and DiClemente19 who identified 5 stages that individuals go through when changing their habits and behavior—precontemplation, contemplation, determination, action and maintenance. We used the diabetes version of SOCRATES18 to measure readiness for change in parents and youth. Our results focused primarily on the parents’ responses as 5 of the subjects were too young to complete SOCRATES (ie, < 10 years of age). At baseline, 13/15 (87%) of the participants could be assigned to a single motivational stage according to parents’ questionnaires. Precontemplation was by far the largest group, including 7/15 subjects (47%); 0 were in contemplation or determination, 5 (33%) action, and 1 (7%) maintenance stage. Four months after pump initiation, 15/15 (100%) could be assigned to a single motivational stage according to parents’ questionnaires. There was no difference in distribution across stages compared to baseline (P = .89): 8 subjects (53%) were in precontemplation, 0 contemplation or determination, 5 (33%) action, and 2 (13%) maintenance stage. There was also no difference between parents’ and children’s distribution across stages, either at start (P = 1.0) or at 4 months (P = .76) (data not shown).
Metabolic Control
There was no difference in median HbA1c between the 2 study groups at 4 months (difference −0.35%, 95% CI: −1.5 to 1.3, P = .61). Change in HbA1c from baseline to 4 months was similar in both groups (between-group difference 0.05%, 95% CI: −1.2 to 1.3, P = .96). Four of 8 simultaneous group subjects achieved an HbA1c < 7.0% at 4 months compared to 1 of 7 delayed group subjects (P = .14).
Diabetes Treatment Satisfaction
There was no difference in the DTSQ score between the groups at 4 months. Change in DTSQ score from start to 4 months was significantly higher in the delayed group compared to the simultaneous group (difference −8, 95% CI: −16 to −1, P = .02), indicating greater improvement in treatment satisfaction in the delayed group.
Diabetes Adherence
CareLink uploads showed that compared to baseline, at 4 months the simultaneous group subjects were bolusing more often (difference 2.79/day, 95% CI: 1.93 to 3.88, P = .001) and subjects in the delayed group were performing more glucose tests (difference 3.29/day, 95% CI: 1.21 to 4.36, P = .03). Based on the Illness Management Scores at 4 months, simultaneous group subjects perceived more barriers to adherence than the delayed group subjects. They reported more cognitive difficulties (difference between groups 6, 95% CI 4 to 12, P = .01), felt less social support and self-efficacy (difference −4, 95% CI −5 to 0, P = .03) and had more peer-family issues (difference 3, 95% CI 1 to 5, P = .01).
Discussion
This pilot study is the first to compare initiation of simultaneous pump and CGM in pump naïve children and adolescents with established type 1 diabetes to standard pump therapy with delayed CGM initiation. Over our 4 month study, 75% of the simultaneous group subjects used CGM more than 60% of the time which is significantly greater than that observed in most,3,6,8,9 but not all4,5 studies which have added CGM to a pump or MDI regimen. The high CGM adherence rates observed in our simultaneous group are similar however to other studies of pump naïve individuals10-12 suggesting that uptake of CGM may be greater when integrated with pump therapy from the beginning. In contrast, only 1 of the 7 subjects in our delayed CGM initiation group wore CGM > 60% of time during the subsequent 4-month period. We postulate that the reduced uptake of CGM amongst the delayed group was because they perceived less benefit from adding CGM 4 months after pump initiation. Indeed, when participants in the delayed group were offered CGM initiation, they reported being more satisfied with their diabetes treatment according to DTSQ scores and fewer barriers to adherence than the simultaneous group.
This study was not powered to detect a difference in CGM adherence or A1C but rather to examine feasibility and inform the design of a full-scale trial. Recruitment to the pilot study was more difficult than expected for 2 reasons. First, 72% of potential participants were ineligible as they had chosen a pump that did not offer pump-integrated CGM (at the time, Medtronic had the only CGM approved in Canada). Second, 51% of eligible participants refused randomization, and 2 of 10 subjects in the delayed group withdrew immediately postrandomization due to dissatisfaction with their treatment assignment. This experience led us to modify the design of the full-scale trial such that recruitment efforts targeted potential subjects before they had made their pump selection, with greater emphasis in the consenting process on the meaning of randomization to ensure enrolled subjects were willing to accept their treatment assignment.
Sample size calculation for the definitive trial determined that 128 subjects were required for 80% power to detect a difference in CGM adherence of 30 hours per week allowing for a type 1 error rate of 0.05 and 10% dropouts. Thirty hours per week translates into an increase in CGM use from 49% to 69% of the time which is a clinically meaningful difference based on studies demonstrating a dose-response effect of CGM adherence on A1C reduction7,20 and that use of CGM > 60-70% of the time is associated with significant A1C reduction.2,5 We allowed for a 10% dropout rate for the definitive trial, rather than the 25% observed in the pilot study, because we believed that the lessons learned through this pilot study would enable us to keep the dropout rate below 10%. Specifically, we improved the consenting process and we added more frequent support to subjects between study visits. The planned sample size of 128 subjects for the definitive trial also provides 80% power for detection of a 0.5% difference in A1C between the groups. Our experience with the pilot study, and subsequent changes to the design of the full-scale trial, enabled us to predict that 5 sites with a combined total of 240 pump starts per year would be required to recruit 128 subjects over 26 months, assuming 50% met all inclusion criteria including choice of Medtronic pump, and a 50% consent rate.
The pilot study also identified 2 flaws in study design which may have contributed to the observed difference in CGM adherence between the groups. Delayed group subjects were told they had the option of adding CGM at 4 months whereas the simultaneous group were advised that they were expected to use CGM on a continuous basis for at least 4 months. Although both groups were offered 4 months of CGM supplies, the delayed group subjects may not have felt that they had committed to using CGM to the same extent as the simultaneous group. However, all subjects indicated they were prepared to start CGM if randomized to the simultaneous group. In fact, most delayed group subjects and parents expressed disappointment at their randomization assignment, leading 2 subjects to withdraw immediately postrandomization.
Another confounding factor identified through the pilot study was that following pump initiation, families had regular telephone contacts with the diabetes team for the next 1-2 weeks. This meant that subjects in the simultaneous group had more opportunities for discussion and problem-solving about CGM than the delayed group subjects for whom telephone contact after CGM initiation was left to the discretion of the family and local diabetes team. This difference in support following CGM initiation may have contributed to the lower CGM adherence in the delayed group. Similarly, lack of support post-CGM initiation in routine care may be 1 of the factors affecting CGM effectiveness when added to a pump or MDI regimen. Standardization of support after CGM initiation should be considered in the design of future CGM trials.
A primary objective of our pilot study was to determine whether SOCRATES could be used to categorize study participants and their parents by motivational stage given our hypothesis linking motivational stage with subsequent CGM adherence. The importance of readiness for change is often overlooked by health care professionals when altering the diabetes regimen. Readiness for making and sustaining behavior change by youth and parents has been shown to positively impact outcome in children with chronic illnesses such as obesity, substance abuse, and polycystic ovarian syndrome.21-23 This concept is especially applicable to the implementation of diabetes technologies such as CGM for which success is highly dependent on behavior change. The SOCRATES questionnaire was originally developed for use in the addiction field,24 and then adapted to assess readiness for making changes in diabetes behaviors.18 Using this version, Viner et al demonstrated an association between readiness for change in adolescents with type 1 diabetes, and their subsequent behavior in a clinical trial.25
In our pilot study, the parents’ SOCRATES questionnaires enabled us to assign 87% of the study participants to a single motivation stage at baseline and 100% at 4 months. These findings are similar to those of Trigwell et al’s adult study, which found that 86.7% of adults with diabetes could be assigned to a single motivational stage using SOCRATES.18 Surprisingly, at baseline 47% of our subjects/parents were in the precontemplation stage (ie, a stage which reflects having no interest in changing behaviors), even though they were about to start pump therapy, which is an important change in diabetes management. A similar proportion (53%) were in this stage at 4 months.
These preliminary observations about readiness to change are limited however, because our pilot trial’s sample was heterogeneous and underpowered. Therefore, these data do not allow us to examine whether readiness for change is higher when patients start pump therapy compared to 4 months later nor can the possible difference in CGM adherence between the 2 study groups be explained by this hypothesis. Our full-scale multicentre trial will examine these questions and help determine whether readiness for change can identify when new treatments and technologies should be introduced.
In conclusion, our pilot study is the first to demonstrate the feasibility of randomizing pump naïve children and adolescents with established type 1 diabetes to simultaneous pump and CGM initiation versus standard pump therapy with delayed CGM initiation 4 months later. Recruitment and operational challenges within the pilot trial and its preliminary results have been used to inform the design of a full-scale 5-site randomized controlled trial examining simultaneous versus delayed initiation of CGM in children and adolescents with established type 1 diabetes starting insulin pump therapy—the CGM TIME Trial (Timing of Initiation of Continuous Glucose Monitoring in Established Pediatric Diabetes)—funded by the JDRF Canadian Clinical Trial Network (www.clinicaltrials.gov NCT01295788).
Footnotes
Abbreviations: CGM, continuous glucose monitoring; DTSQ, Diabetes Treatment Satisfaction Questionnaire; HbA1c, Hemoglobin A1C; IMS, Illness Management Survey; MDI, multiple daily injections; RCT, randomized controlled trial; SAPT, sensor-augmented pump therapy; SMBG, self-monitoring of blood glucose; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MLL has been a speaker, without honorarium, at educational events sponsored by Medtronic and Animas with travel reimbursement for these events. CH has received consulting fees from Animas, Medtronic, Novo Nordisk, and Pfizer and lecture honoraria from Novo Nordisk, Eli Lilly, Roche, Aventis and Bayer and has received research funding from Boehringer, Merck and Novo Nordisk. No competing financial interests exist for the other authors.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This was an investigator-initiated trial. CGM devices and supplies were provided by Medtronic Canada.
References
- 1. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szypowska A, Ramotowska A, Dzygalo K, Golicki D. Management of endocrine disease: beneficial effect of real-time continuous glucose monitoring system on glycemic control in type 1 diabetic patients: systematic review and meta-analysis of randomized trials. Eur J Endocrinol. 2012;166:567-574. [DOI] [PubMed] [Google Scholar]
- 3. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 4. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mauras N, Beck R, Xing D, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2012;35:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slover RH, Welsh JB, Criego A, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 2012;13:6-11. [DOI] [PubMed] [Google Scholar]
- 8. Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10:377-383. [DOI] [PubMed] [Google Scholar]
- 9. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52:1250-1257. [DOI] [PubMed] [Google Scholar]
- 10. Moreno-Fernandez J, Gomez FV, Gazquez M, et al. Real-time continuous glucose monitoring or continuous subcutaneous insulin infusion, what goes first? Results of a pilot study. Diabetes Technol Ther. 2013;15:596-600. [DOI] [PubMed] [Google Scholar]
- 11. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311-320. [DOI] [PubMed] [Google Scholar]
- 12. Hermanides J, Norgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA1c in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28:1158-1167. [DOI] [PubMed] [Google Scholar]
- 13. Lee SW, Sweeney T, Clausen D, et al. Combined insulin pump therapy with real-time continuous glucose monitoringsignificantly improves glycemic control compared to multiple daily injection therapy in pump naïve patients with type 1 diabetes; single center pilot study experience. J Diabetes Sci Technol. 2007;1:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32:2245-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925-926. [PubMed] [Google Scholar]
- 16. Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383-392. [DOI] [PubMed] [Google Scholar]
- 17. Bradley C. The Diabetes Treatment Satisfaction Questionnaire: DTSQ. In: Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. New York, NY: Routledge; 1994:111-132. [Google Scholar]
- 18. Trigwell P, Grant PJ, House A. Motivation and glycemic control in diabetes mellitus. J Psychosom Res. 1997;43:307-315. [DOI] [PubMed] [Google Scholar]
- 19. Prochaska JO, DiClemente C. Transtheoretical therapy: towards a more integrative model of change. Psychother Theory Res Pract. 1982;19:276-288. [Google Scholar]
- 20. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maisto SA, Krenek M, Chung T, Martin CS, Clark D, Cornelius J. Comparison of the concurrent and predictive validity of three measures of readiness to change marijuana use in a clinical sample of adolescents. J Stud Alcohol Drugs. 2011;72:592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakubowski KP, Black JJ, El Nokali NE, et al. Parents’ readiness to change affects BMI reduction outcomes in adolescents with polycystic ovary syndrome. J Obes. 2012;2012:298067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhee KE, De Lago CW, Arscott-Mills T, Mehta SD, Davis RK. Factors associated with parental readiness to make changes for overweight children. Pediatrics. 2005;116:e94-101. [DOI] [PubMed] [Google Scholar]
- 24. Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: the stages of change readiness and treatment eagerness scale (SOCRATES). Psychother Theory Res Pract. 1996;20:81-89. [Google Scholar]
- 25. Viner RM, Christie D, Taylor V, Hey S. Motivational/solution-focused intervention improves HbA1c in adolescents with Type 1 diabetes: a pilot study. Diabet Med. 2003;20:739-742. [DOI] [PubMed] [Google Scholar]