Abstract
The development of accurate, minimally invasive continuous glucose monitoring (CGM) devices has been the subject of much work by several groups, as it is believed that a less invasive and more user-friendly device will result in greater adoption of CGM by persons with insulin-dependent diabetes. This article presents the results of preliminary clinical studies in subjects with diabetes of a novel prototype microneedle-based continuous glucose monitor. In this device, an array of tiny hollow microneedles is applied into the epidermis from where glucose in interstitial fluid (ISF) is transported via passive diffusion to an amperometric glucose sensor external to the body. Comparison of 1396 paired device glucose measurements and fingerstick blood glucose readings for up to 72-hour wear in 10 diabetic subjects shows the device to be accurate and well tolerated by the subjects. Overall mean absolute relative difference (MARD) is 15% with 98.4% of paired points in the A+B region of the Clarke error grid. The prototype device has demonstrated clinically accurate glucose readings over 72 hours, the first time a microneedle-based device has achieved such performance.
Keywords: continuous glucose, interstitial fluid, microneedle, minimally invasive
Continuous glucose monitoring (CGM) has been shown to be a useful tool in improving average blood glucose levels and reducing glycemic excursions in persons with diabetes.1 While usage of continuous glucose monitors has increased over the past decade, their adoption by the wider diabetic population has been limited, especially in patients with Type 2 diabetes. The pain associated with the implantation and wear of the commercially available devices has been shown to be 1 of the factors cited which hinders wider adoption,2 although other factors, such as cost/lack of reimbursement and complexity of using the CGM devices and interpreting data, are also barriers. That some patients do not tolerate the device is substantiated by the high drop-out rate in clinical trials.3
Much work has been done on the development of minimally invasive glucose sensors4 on the premise that a device that is less painful and less obtrusive to apply and wear would be more attractive to a greater fraction of the diabetic population. Sampling glucose through intact, or near-intact skin via reverse iontophoresis,5 microneedles,6 and ultrasound7 have all been studied and found wanting. Short measurement duration (24 hours or less) is a common problem with many minimally invasive technologies, due to the skin’s wound healing response. Key to obtaining accurate glucose measurement is reliable sampling of glucose from blood or interstitial fluid. While stable and reproducible sampling of glucose is somewhat more straightforward for needle-type sensors that are subcutaneously implanted (provided foreign body encapsulation is managed) and thus are directly in contact with interstitial fluid, it is more complex for many minimally invasive devices as the glucose in the interstitial fluid must be transported to a sensor located, for example, on the surface of the skin.8
For minimally invasive CGM systems utilizing microneedles, the effect of variables such as microneedle geometry, penetration depth, and stability of the implantation site on the interstitial fluid sampling process must be understood to obtain accurate glucose measurements for more than a short time.9 This article presents the results of a clinical study with diabetic subjects of a working prototype device utilizing an array of hollow microneedles implanted into the epidermal layer of the skin to allow glucose in the interstitial fluid to diffuse into an external glucose sensor.
Methods
Device Description
The clinical prototype device consists of 2 sections: a sensor pod that contains the microneedle array and glucose sensor (Figure 1) and an electronics module. The 2 sections are connected via flexible electric leads in the prototype device. The microneedle array fabrication method has been described previously.10 The microneedle array comprises approximately 200 hollow silicon microneedles, each approximately 300 µm in length with a 50 × 50 µm lumen, over a total area of 6 × 6 mm (Figure 2). The biosensor is situated on the opposite face of the glucose collection chamber, which is filled with a proprietary buffer solution containing phosphate ions to catalyze the mutarotation of glucose and citrate ion to slow the wound healing cascade at the tips of the microneedles.11 The sensor comprises a screen-printed, 3-electrode amperometric sensor with a Pt-C working electrode covered with a layer of cross-linked bovine serum albumin containing glucose oxidase. The sensor utilizes direct detection of the hydrogen peroxide product of the enzymatic oxidation of glucose at an applied potential of 0.46V versus an Ag/AgCl reference electrode.
Figure 1.
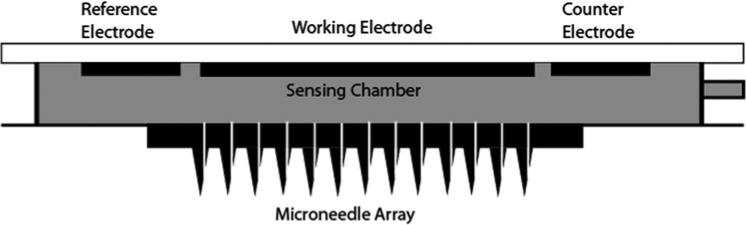
Schematic of the sensor pod portion of the prototype device.
Figure 2.
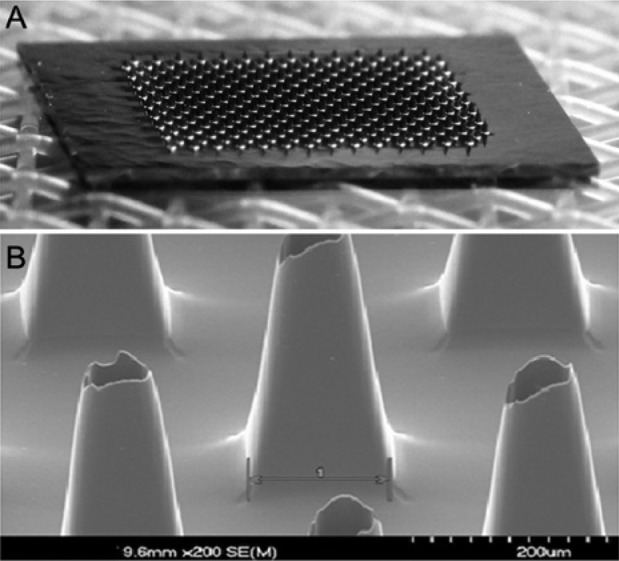
(A) Photograph of the microneedle array. (B) SEM photograph of several microneedles.
The sensor pod is applied to the skin using a spring-loaded applicator. This applicator accelerates the microneedle array into the skin so that the needles penetrate reproducibly. Because the needles are short, they penetrate only as deep as the epidermal layer (ie, shallower than the capillary bed and nerve endings in the underlying dermis).10 The sensor pod is affixed to the skin using skin adhesive on the area outside the microneedle array and protruding around the perimeter. In this prototype device, the buffer is introduced into the diffusion chamber by syringe after application of the sensor pod to the subject’s skin. While the device is operating, glucose passively diffuses from the epidermal layer of the skin through the microneedle array to the sensor via the buffer in the sensing chamber. There is no net fluid flow from the body into the device. Rather, because glucose is consumed at the sensor working electrode, there is a persistent concentration gradient for glucose from the ISF to the sensor, driving the diffusion of glucose through the microneedle lumens to the electrode. The electronics module contains a potentiostat circuit to operate the biosensor, a microprocessor to digitize and store data points, and a battery. Data were downloaded from the device after device removal for processing.
Clinical Study Design
Clinical trials took place at 2 external clinical sites in the United States. The study was performed under an institutional review board–approved nonsignificant risk protocol in accordance with the ethical guidelines of the Belmont Report. Informed consent was obtained from all subjects. Test devices were manufactured under Good Manufacturing Practice (GMP) and were sterilized via an e-beam process. Ten adult subjects with insulin-dependent diabetes were recruited. The subjects were equally split between males and females. Seven subjects were type 1, and 3 were type 2. The average age of the subjects was 61.1 years (range, 33-78 years) and the average duration on insulin was 16.1 years (range, 2-37 years). Subjects wore 4 devices simultaneously, applied to the upper arm or forearm. The trial was performed in 2 cohorts. In the first group, 6 subjects wore devices for 48 hours. The subsequent cohort consisted of 4 subjects who wore devices for 72 hours. Subjects reported to the study site daily for comparative fingerstick glucose measurements, taken every 20 minutes with an Abbott FreeStyle blood glucose meter (Abbott Diabetes Care, Alameda, CA, USA). Glucose excursions into both the hypo- and hyperglycemic ranges were created by manipulating insulin and diet based on the judgment of the investigator. Subject skin irritation was scored upon device removal using a Draize scale in which erythema and edema are scored on a 0 to 4 scale with higher numbers indicating more severe irritation.12
Data from the devices were downloaded to a personal computer for processing. Systems were calibrated using a reference blood glucose value after an initial 2-hour warm-up period and then once daily with the morning fingerstick glucose value. Prior to the clinical studies, an algorithm was trained using data from identical devices under a similar clinical protocol on nondiabetic volunteers. A lag time of 17 minutes between fingerstick measurements and device readings was determined using a process of shifting data points versus fingerstick readings in time to determine the lag which minimized error.13 This algorithm was locked before the clinical trial began and was applied prospectively to the 48- and 72-hour clinical data from the diabetic subjects. No accounting for error in the fingerstick BG measurements was undertaken; BG measurements were considered as truth, and all error was assumed to be assigned to the test devices. No higher level data processing, such as smoothing or temperature correction, was performed on the data from the test devices.
Results
A total of 1396 paired points were obtained from 37 devices. Data from 3 devices were excluded from the analysis. (One device had an electrical fault resulting in no sensor signal, and 2 devices leaked after application due to failure of the skin adhesive.) Figure 3 shows 2 example tracking plots over 72 hours. The calibration point is shown as a square box, the comparative blood glucose values are shown as discrete points, and the device readings are the solid lines. Good agreement is noted between the device readings and the fingerstick blood glucose values. The Clarke error grid for the entire data set is shown in Figure 4. A total of 74.6% of paired points were located in the A-region with 98.4% in the A+B region. The mean relative difference (MRD) was 5% with an overall mean absolute relative difference (MARD) of 15% (median ARD was 11%) indicating a low level of both systematic error (bias) and random error (scatter) in the data set.
Figure 3.
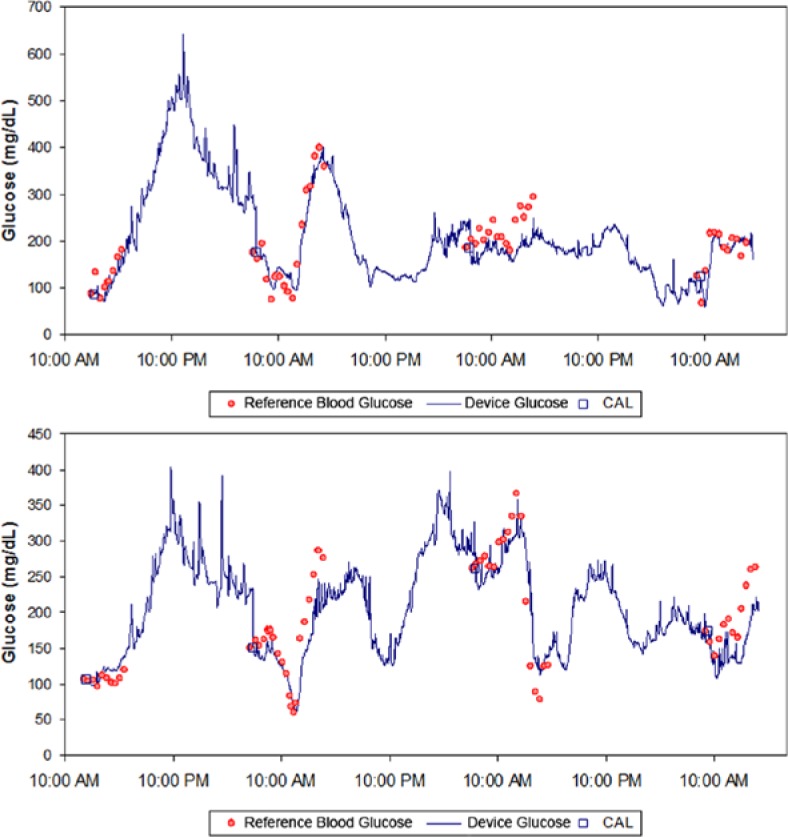
Sample plots of the device performance over 72 hours. Device signal is shown as a solid line, and the comparative blood glucose measurements are shown as discrete points. Comparative points used as calibrations are designated with a square symbol.
Figure 4.
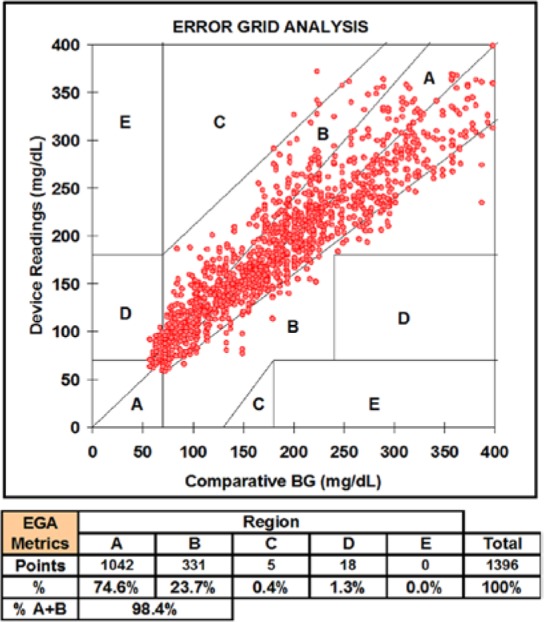
Clarke error grid analysis (EGA) for the 1396 paired points from 37 devices over up to 72 hours in this trial.
Figure 5 shows the glucose readings from 2 microneedle devices worn simultaneously on the same subject for 72 hours. A statistical analysis of the precision of the readings, defined as the difference between paired simultaneous readings of the 2 devices, was investigated using the entire data set from all 37 devices. The precision is characterized by an MARD of 10.4% with a mean percentage coefficient of variation of 7.3%.
Figure 5.
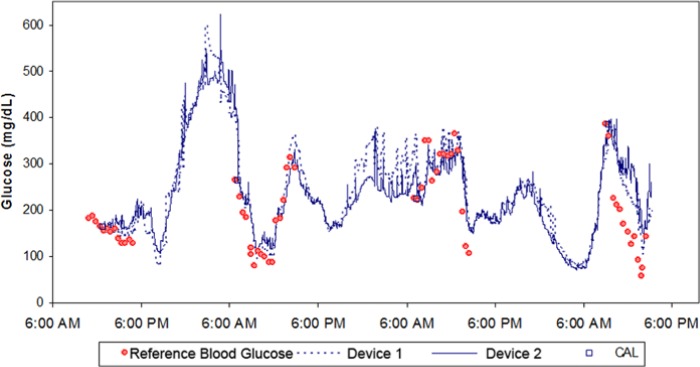
Example plots of 2 devices worn simultaneously by a subject to demonstrate interdevice precision.
Upon device removal, the skin at the sensor site was scored for erythema and edema according to a modified Draize scale (0-4 scale). Average erythema score immediately postremoval was 1.54; average edema score was 0.06. Irritation resolved completely without treatment in several days.
Discussion and Conclusions
Microneedle-based CGM systems have been the focus of much work over the past decades because of the presumed advantage of a less painful sampling means over subcutaneous needle sensors. However, much of this work has not progressed past in vitro studies, or in vivo studies of limited duration.9 The results shown in this article from a prototype device demonstrate for the first time that these microneedle-based devices can provide clinically accurate results over durations up to 72 hours, comparable to those published for implanted needle-type sensors. In addition, calibration with a finger-stick blood glucose measurement was performed only 1 time per day, a calibration frequency lower than most commercially available devices.
Microneedles, and specifically microneedle arrays, provide the ability to sample biological fluid with less pain provided that the needles do not penetrate the skin to the depth of the dermis where capillaries and most nerve endings reside.14 By not penetrating to this depth, the biological fluid sampled is not blood, but interstitial fluid residing in the viable epidermis. The 17-minute lag time observed in this study is similar to the lag observed with some implanted sensors. Shorter time lags have been reported in a commercially available implanted device, although it is impossible to determine whether the device’s algorithm partially offsets measurement lag.13 It has been shown that the glucose concentration in the epidermal interstitial fluid is in good equilibrium with the blood glucose as the dermal capillaries (which have high blood flow due to their role in thermal regulation) are in close communication with the sampling site.15
Devices utilizing minimally invasive transdermal interstitial fluid sampling to date have demonstrated limited operating lifetime. For examples, devices utilizing reverse iontophoresis, sonophoresis, or other skin permeabilization methods provide stable sampling for 24 hours or less. This limitation is likely due to skin reacting to the breach of the skin barrier and beginning the healing process. In the microneedle-based device shown in this article, operating lifetime has been extended by optimizing the microneedle geometry (length, width, tip characteristics, and pitch)10 and the proprietary collection buffer formulation containing citrate ions to slow the skin response to the intrusion of the microneedles into the epidermis.11
The demonstrated clinical performance of the prototype device described in this article has proven the feasibility and reliability of the approach of microneedle sampling of epidermal ISF. Converting this prototype into a more user-friendly integrated form factor, including automating the buffer fill step and miniaturizing the sensor electronics, can now commence.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; EGA, Clarke error grid analysis; MARD, mean absolute relative difference; MRD, mean relative difference.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AJ, MJT, JAT, SM, SD, and BC were full-time employees of ArKal Medical at the time this research was performed.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by ArKal Medical, Inc.
References
- 1. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. [DOI] [PubMed] [Google Scholar]
- 2. DirecNet Study Group. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes. 2009;10(2):91-96. [DOI] [PubMed] [Google Scholar]
- 3. Hermanides J, Phillip M, DeVries JH. Current application of continuous glucose monitoring in the treatment of diabetes: pros and cons. Diabetes Care. 2011;34(S2):S197-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hover CG, Stedeford T, Vigneulle RM. The development of minimally invasive continuous metabolic monitoring technologies in the U.S. Army TMM Research Program. Diabetes Tech Ther. 2005;7(1):213-224. [DOI] [PubMed] [Google Scholar]
- 5. Tierney MJ, Tamada JA, Potts RO, et al. The GlucoWatch® Biographer: A frequent, automatic and non-invasive glucose monitor. Ann Med. 2000;32:632-641. [DOI] [PubMed] [Google Scholar]
- 6. Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Tech Ther. 2005;7(1):131-141. [DOI] [PubMed] [Google Scholar]
- 7. Chuang H, Taylor E, Davison TW. Clinical evaluation of a continuous minimally invasive glucose flux sensor placed over ultrasonically permeated skin. Diabetes Tech Ther. 2004;6(1):21-30. [DOI] [PubMed] [Google Scholar]
- 8. Vadduraju S, Burgess DJ, Tomazos I, et al. Technologies for continuous glucose monitoring: current problems and future promises. J Diabetes Sci Tech. 2010;4(6):1540-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El-Laboudi A, Oliver NS, Cass A, Johnston D. Use of microneedle array devices for continuous glucose monitoring: a review. Diabetes Tech Ther. 2012;15(1):101-115. [DOI] [PubMed] [Google Scholar]
- 10. Chua B, Desai SP, Tierney MJ, Tamada JA, Jina AN. Effect of microneedles shape on skin penetration and minimally invasive glucose monitoring in vivo. Sens Act A. 2013;203:373-381. [Google Scholar]
- 11. Cormier M, Daddona P, Johnson J, et al. Methods for inhibiting decrease in transdermal drug flux by inhibition of pathway closure. US patent 7,438,926. October 21, 2008. [Google Scholar]
- 12. Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;83:377-390. [Google Scholar]
- 13. Garg SK, Voelmle M, Gottlieb PA. Time lag characterization of two continuous glucose monitoring systems. Diabetes Res Clin Pract. 2010;87:348-353. [DOI] [PubMed] [Google Scholar]
- 14. Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24(7):585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulco E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405-2409. [DOI] [PubMed] [Google Scholar]


