Abstract
How patients are benefitting from continuous glucose monitoring (CGM) remains poorly understood. The focus on numerical glucose values persists, even though access to the glucose waveform and rate of change may contribute more to improved control. This pilot study compared outcomes of patients using CGMs with or without access to the numerical values on their CGM. Ten persons with type 1 diabetes, naïve to CGM use, enrolled in a 12-week study. Subjects were randomly assigned to either unmodified CGM receivers, or to CGM receivers that had their numerical values obscured but otherwise functioned normally. HbA1c, quality of life (QLI-D), and fear of hypoglycemia (HFS) were assessed, at baseline and at week 12. Baseline HbA1c for the entire group was 7.46 ± 1.27%. At week 12 the experimental group HbA1c reduction was 1.5 ± 0.9% (p < .05), the control group’s reduction was 0.06 ± 0.61% (p > .05). Repeated measures testing revealed no significant difference in HbA1c reduction between groups. Both groups had reductions in HFS; these reductions were statistically significant within groups (p < .05), but not between groups. QLI-D indices demonstrated improvements (p < .05) in QLI-D total and the health and family subscales, but not between groups. The results of this pilot study suggest that benefits of CGM extend beyond reductions in HbA1c to reductions in fear of hypoglycemia and improvements in quality of life. The display of a numerical glucose value did not improve control when compared to numerically blinded units.
Keywords: diabetes, fear of hypoglycemia, continuous glucose monitor, glucose trending, quality of life, waveform
In the early 1980s home glucose monitoring became widely available to persons living with diabetes and was rapidly recognized as a valuable tool for improving glucose control.1,2 In the ensuing decades, self-monitoring of blood glucose (SMBG), along with improved understanding of insulin-carbohydrate-meal matching, have improved the ability of patients to self-manage their diabetes. Current standards of care recognize the value of patients actively self-managing their diabetes to improve glucose control.3,4
This ability to monitor and improve glucose awareness improved dramatically in 2006 with the release of modern “real-time” continuous glucose monitoring (CGM).5 Diabetes care today provides real-time CGM for clinical and personal use, which is increasingly accepted by both patients and clinicians as an important component in managing their disease process. CGM has had a significant impact on the care we provide for our insulin-dependent patients.6 For the first time patients have a degree of security that they will be alerted to high or low glucose levels as these changes occur, potentially before they become symptomatic, allowing corrective action to be taken promptly.6
The Problem With Hypoglycemia
Aggressive management of type 1 diabetes mellitus (DM) carry an inherent risk of hypoglycemia.7-10 Many patients will rapidly lose their hypoglycemic awareness due to autonomic nervous system changes.3,8,9 Recurring episodes of hypoglycemia appear to suppress physiologic awareness of future episodes, furthering impairing awareness.8,11 As hypoglycemia awareness decreases, many patients develop fear/avoidance behaviors to decrease the risk of these events.12
On average people with type 1 DM experience a symptomatic hypoglycemia 1 to 2 times a week; one-third of them have a severe hypoglycemic occurrence annually.12,13 The inherent risk of hypoglycemia associated with aggressive insulin therapy causes many patients to undertreat their diabetes, which negatively impacts control and is potentially 1 of the greatest barriers in adequate control of glucose levels.14
Fear of hypoglycemia (FOH) was recognized as a barrier to control and reported in the literature in 1922.15 Avoidance behaviors related to FOH are a documented phenomenon; patients undertreat glucose levels or decrease meal coverage to reduce the risk of a hypoglycemic event.10,16 FOH is underscored by the reality that nocturnal hypoglycemia has been reported to account for 6% of deaths in type 1 diabetics under the age of 40.17,18
Utilization of CGM
The benefits to patients using CGM to improve glucose control are well established.6,19-21 While personal use of CGM is increasingly accepted, there remains a limited understanding of how patients are using these devices. The general belief has been that patients are benefitting from seeing their numerical value of blood glucose, with some CGM manufacturers emphasizing numerical value over trending information.
Consistent use of CGM has been demonstrated as an effective tool in reducing HbA1c without increasing the incidence of hypoglycemia and concurrently reducing duration of hypoglycemia.6 This is noted despite repeated concerns expressed in the literature over the accuracy of CGM devices when compared to home glucometers.21
Many clinicians have questioned this focus on the numerical glucose value and accuracy, particularly given the inherent inaccuracies in SMBG.20 Depending on the device and glucose level at time of measurement, CGM can demonstrate a median absolute relative difference of 5-30%, when compared to laboratory standards for serum glucose.20,21 However, as CGM technologies continue to evolve they are demonstrating improved accuracy and reliability and increasing patient satisfaction with the devices.22
Accuracy issues associated with SMBG readings have a direct impact on CGM when they are used for the required calibrations. Real time CGM has demonstrated clinical accuracy superior to home finger stick testing when the potential for calibration errors is eliminated.23 The introduction of additional variance with home SMBG calibration creates a potential compounding of measurement errors when CGM is calibrated against SMBG.23 Recognizing this inherent risk for compounded inaccuracy, one can argue that patients benefit more from seeing their glucose pattern (direction and rate of change) rather than just a numerical value. Visualizing directions and rates of change allow patients to make better self-management decisions about the need for intervention as they strive to control their diabetes.24
Patients and CGM
Clinical experience with CGM remains limited. Although third-party acceptance and insurance coverage for these technologies are improving, many of the old arguments previously associated with SMBG have been revived. Should patients be aggressively trained to use the information they get from CGM? Is it too much information? Despite the concerns expressed in the diabetes care community, many patients and clinicians are embracing CGM.
From a clinical standpoint, however, adoption of these devices has been slow. Much of what has been learned is trial and error. As we integrate CGM into standards of diabetes care, we need a more formalized understanding of how patients use CGM data. Do patients benefit from an awareness of glucose trending (visualization of the glucose pattern and direction/rate of change) or from being able to see the numerical value of their glucose? This pilot study is the first to address this question.
Methods
A convenience sample of persons with type 1 diabetes (n = 10) was recruited from a suburban endocrine clinic into a 2-group quasi-experimental comparative design pilot study. Participants were randomized into either the control group (n = 5), which received unmodified commercially available CGM units, or the experimental group (n = 5), which received CGM units modified to obscure the numerical glucose value (Figure 1).
Figure 1.
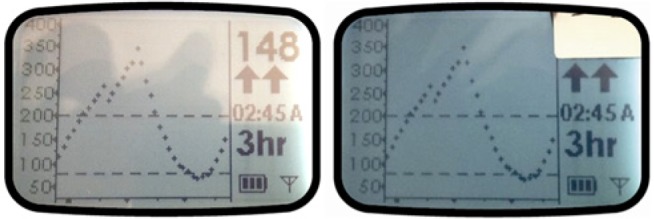
An example of the 2 devices provided to subjects (unmodified left). The tape visible in the right photo is a security/tamper proof sticker; there was another tamper proof sticker on the back on the device covering the access screws. The LCD screen (inside the case and not accessible) was altered to prevent visualization of the numerical glucose value. If the surface sticker was removed, the LCD numerical display was not visible. No effort was made to obscure the Y-axis scale.
Inclusion Criteria
Study participants were 18-60 years old, diagnosed with type 1 diabetes and on insulin therapy for at least 6 months prior to enrollment, and were either receiving multiple daily injection (MDI) therapy or continuous subcutaneous insulin infusion (CSII). Participants were naïve to CGM therapy but prior use of retrospective (blinded) CGM was allowed. Participants consented to a finger stick HbA1c testing at the start of the trial and at completion 12 weeks later, to a 1-week blinded sensor run-in to collect baseline data, and to clinical follow-up every 4 weeks to allow for data capture.
Instruments and Tools
Study data were collected using the Dexcom SEVEN PLUS™ CGM system (Dexcom Inc, San Diego, CA). The Dexcom CGM system consists of 3 parts: a subcutaneous biosensor that functions for 7 days, a wireless transmitter, and a rechargeable receiver unit. This device has a wireless range of 6 feet from the transmitter to the receiver.25
The Dexcom CGM provides the user with a numerical value of glucose and trending screens for the previous 1-, 3-, 6-, 12-, and 24-hour period. Acquired data can be downloaded via PC with Dexcom’s proprietary software. The Dexcom SEVEN PLUS can operate in a blinded mode, during which the subject is unable to see the glucose value or patterns but sensor data are recorded. The Dexcom software stores and analyzes data, providing trend graphs and statistical information including average glucose and standard deviation.
Finger stick HbA1c testing was conducted in the office with CLIA waived single use testing kits. Behavioral data were collected with 2 different tools. The Hypoglycemia Fear Survey (HFS) was utilized to evaluate the impact of hypoglycemia-related fear.16,26 Quality of life was assessed with the Quality of Life Index–Diabetes (QLI-D).27
Hypoglycemia Fear Survey
The HFS is a quantitative tool for assessing a patient’s concerns related to hypoglycemia. It has an established internal validity with a Cronbach’s alpha of .89-.96 and an established record of reliability.16,26 The 33-question Likert-type scale is divided into 2 subsections: Behavior (HFS-B 15 questions) and Worry (HFS-W 18 questions). Participants completed the HFS at baseline enrollment and again at completion of the study.
Quality of Life Index
Quality of life (QOL) was measured using the Ferrans and Powers’s QLI-D. The QLI-D has demonstrated consistency; Cronbach’s alpha has been validated at 0.94 and 0.97.28,29 In addition, the QLI-D is effective at detecting change in perceptions of QOL issues in pre- and posttest comparison.27 The QLI-D tool consists of 66 items divided into 2 subsections of 33 similar items, based on importance to the individual, and the individual’s satisfaction with each item. These 33 items cover 4 domains: Health/Function, Social/Economic, Psychological/Spiritual, and Family. Participants completed the QLI-D assessment at enrollment and at completion of the study. Scoring on the QLI-D survey was conducted using the tools available at http://www.uic.edu/orgs/qli/.
Procedure
Approval for this study was obtained from the University of Nevada, Las Vegas Institutional Review Board for the Protection of Human Participants (protocol 1106-3849M). Participants were seen for a total of 5 visits across a 12-week period.
At enrollment informed consent was obtained, a baseline HbA1c was performed, and participants completed the HFS and QLI-D. Participants were placed on a CGM operating in blinded (research) mode to establish baseline glucose patterns, allowing study participants to serve as their own controls.
At visit 2, participants were randomly assigned to either the control or the experimental group. The experimental units had the numerical glucose value mechanically obscured with an opaque label but otherwise functioned normally; all participants were given a refresher on CGM use and supplies for their CGM unit. Participants in both groups returned for data downloading on visits 3 and 4, at which time additional sensors were supplied. No review of CGM data or diabetes education was conducted during these follow-up visits. During the final visit, participants returned for final data download and HbA1c measurement and completed the HFS and QLI-D again.
Outcomes were measured at baseline and study completion: (1) HbA1c, (2) HFS, and (3) QLI-D. At the final visit, participants were asked an open-ended question, allowing expression of how they most benefitted from CGM use. Statistical analyses were conducted using SPSS version 20 (2011). Descriptive statistics (frequencies, mean, SD) were used to describe the sample’s demographics and characteristics. HbA1c, HFS, and QLI-D were analyzed by repeated measures ANOVA.
Results
Sample Description
The sample consisted of 4 male (40%) and 6 female (60%) participants, with an average age of 42.6 ± 9.6 years. Persons on MDI therapy composed the majority of the sample, representing 60% (6 participants); 40% (4 participants) used CSII. Duration of type 1 diabetes in study participants ranged from 2 to 40 years, with average duration of 20.0 ± 13.6 years.
Mean baseline HbA1c for the experimental group (1 male, 4 females) was 7.68 ± 1.56% and for the control group (3 males, 2 females) was 7.24 ± 1.05%. Study participants were requested to use the CGM on a full time basis, though in an effort to simulate real-life situations no value or restriction was placed on this request. There was no statistical difference in duration or continuity of use between the 2 groups (control: 78.2 ± 3.2 days, experimental: 75.2 ± 4.3 days, p = .251) across the trial period.
The study outcomes are shown in Table 1. The experimental group demonstrated a mean reduction in HbA1c of 1.5 ± 0.9%, which reached statistical significance (p < .05). The control participants’ mean HbA1c reduction was 0.06% ± 0.61%; this was not statistically significant. Repeated measures testing (RM ANOVA) revealed no significant difference in the HbA1c reduction between the experimental and control groups (p = .725). Post hoc power analysis demonstrated insufficient power to detect such a difference.
Table 1.
Study Outcomes by Experimental and Control Group.
| Experimental |
Control |
|||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| HbA1c % | 7.68 ± 1.56 | 6.18 ± 1.138* | 7.24 ± 1.05 | 7.18 ± 1.31 |
| HFS–Total | 41.6 ± 26.6 | 24.6 ± 16.4* | 47 ± 32.9 | 38.6 ± 23.5* |
| HFS–Worry | 25.4 ± 20.2 | 13.2 ± 12.5* | 29.2 ± 24.6 | 24.2 ± 20.2* |
| HFS–Behavior | 16.2 ± 7.7 | 11.5 ± 5.2* | 17.8 ± 8.7 | 14.2 ± 4.9* |
| QLI-D Total | 21.4 ± 5.6 | 24.6 ± 3.2* | 23.1 ± 2.7 | 25.6 ± 3.9* |
| QLI-D Health/Function | 20.4 ± 6.0 | 24.6 ± 3.9* | 22.2 ± 4.8 | 25.8 ± 6.9* |
| QLI-D Social/Economic | 20.8 ± 6.8 | 24.1 ± 2.9 | 23.8 ± 2.0 | 24.8 ± 3.9 |
| QLI-D Psychological/Spiritual | 22.5 ± 5.4 | 24.3 ± 4.4 | 23.4 ± 2.6 | 25.6 ± 3.9 |
| QLI-D Family | 24.1 ± 4.6 | 27.6 ± 2.4* | 27.2 ± 2.2 | 28.6 ± 1.8* |
All HbA1c testing was by CLIA waived kits. HFS, Hypoglycemia Fear Survey; QLI-D, Quality of Life Index–Diabetes. Ferrans and Powers’s scoring was calculated with tools available at http://www.uic.edu/orgs/qli/.
p < .05 within group over time.
RM ANOVA were used to assess the HFS Total, Behavior, and Worry scores from baseline to completion. A statistically significant drop (p < .05) was seen in both groups from baseline to completion for total scoring (p = .031), and the Worry (p = .034) and Behavior subscales (p = .044). These scores are shown in Table 1 and Figure 2, and total scores were statistically significant within the groups (p < .05), but not between groups (p = .547). Significance was not demonstrated between the groups for the Worry (p = .558) and Behavior (p = .595) subscales. While the trending was evident in favor of the experimental group, sample size limitations prevent us from establishing statistical significance between groups. Despite these limitations, data demonstrate a reduction in participants’ FOH scores, both overall and for the Worry and Behavior subscales.
Figure 2.
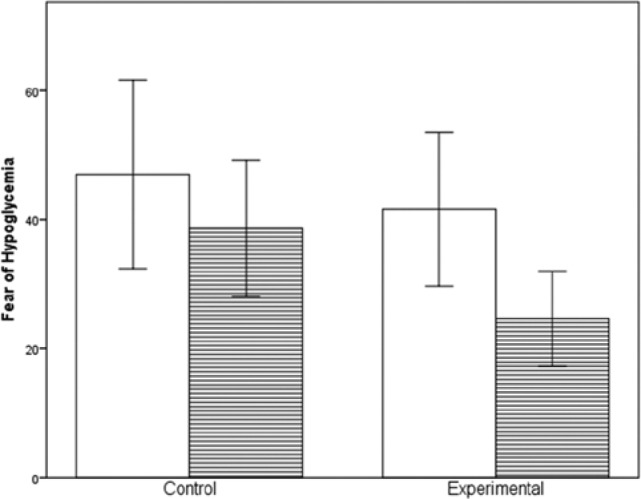
Hypoglycemia Fear Survey (Total) from baseline to completion. White bars represent baseline; lined bars represent week 12. Error bars are ±1 SD.
The QLI-D Total, Health, and Family scores demonstrated an improvement that reached significance (p < .05), as shown in Table 1. Improvements in the Social/Economic and Psychological/Spiritual measures did not achieve significance (p > .05). A larger sample may allow for these 2 subscales to reach statistical significance. The greatest improvement occurred in the category of perceived quality of Health. RM testing failed to demonstrate any significant difference between the groups on QOL total measures (p = .583). The QLI-D measurements were virtually parallel, suggesting that all of the patients using the CGM benefitted equally from improved awareness of their glucose patterns, whether or not they could see the numerical value.
At the completion of this study feedback was solicited regarding the impact of CGM on the subject, their family, and their disease management. There were concerns about inaccuracies of the CGM when compared to their home glucose meter, but interestingly, these were limited to the control group. While most participants valued the hypoglycemia alerts, 1 subject found them to be a drawback and unnecessarily intrusive. Participants in both arms recognized the limitations but still perceived the value of this technology and experienced improvement in their glucose control. The tone of the subjective responses was also clearly different: the control patients were concerned with numerical accuracy and alerts, which contrasted with the experimental patients, who noted an increase in awareness of patterns and direction of glucose change.
Discussion
We did not see a significant difference in HbA1c reduction in this pilot study. However, our sample size was very small and initial predictive analysis had suggested we would need a minimum of 24 participants to achieve a statistically significant threshold between groups.
In this pilot study, the participants in the experimental group demonstrated an average HbA1c reduction of 1.5 ± 0.9% compared to control group participants 0.06% ± 0.61%. This finding suggests that a numerical value may not be required for patients to benefit from CGM. We have been unable to find a similar comparison in the available literature, suggesting that this is the first report of this phenomenon. Average HbA1c reductions of 0.4% ± 0.5% have been reported when patients are given unrestricted access to CGM.21 As an aggregate group, our study participants (n = 10) demonstrated a mean HbA1c reduction of .78%. This is consistent with other trials reporting improvements with the use of CGM.
Findings from this pilot study suggest that participants who did not have access to the numerical value of their glucose demonstrated improved diabetes management and glucose control. This improvement could be related to several issues. First, participants in the control group may have possessed the propensity of many persons with type 1 diabetes to fixate on the “number” of their glucose. In contrast, the experimental group had only their trending lines and directional/rate of change arrows on which to rely. Second, the most significant improvements occurred in experimental participants 1 (MDI therapy) and 6 (CSII), who each reduced their HbA1c by more than 2.0% during the trial, without access to their numerical glucose values. These improvements are higher than has been commonly reported in the literature, but anecdotal reports of similar improvements exist.
While the FOH is recognized as being 1 of the major limitations in the effective treatment of diabetes, there is little support in the literature for CGMs as an effective tool in reducing this barrier. Efforts in addressing FOH have primarily focused on blood glucose awareness training and cognitive-behavioral therapies, though CGM is of increasing interest.30,31 This pilot study suggests that CGM may have the potential to significantly reduce the FOH, addressing this major treatment barrier.
Both groups demonstrated reductions in overall FOH as well as reduction in both the HFS-B and HFS-W subscales. Participants in both groups reported using their CGM alarms to alert them of potential hypoglycemic events and commented that this gave them a degree of security in their daily activities. The presence of the numerical glucose value did not have a demonstrable impact on that, but again we must acknowledge the small sample size.
There was no significant difference between the experimental group and the control group on the QLI-D. When comparing baseline and final data sets, both groups achieved a statistically significant reduction in total QOL score, and QOL Health and Family subscores. While CGM offered benefits in their health awareness and reductions in their FOH, and their QOL measures demonstrate this, there was little evidence of psychological/spiritual or socioeconomic impacts.
Again, we must emphasize that the results of this pilot study must be considered preliminary because of the small sample size. Randomization resulted in a gender imbalance between groups which might have affected the outcome. The small sample prevented us performing any subgroup analyses by gender, age, duration of diabetes, and type of therapy.
Improved awareness of glucose levels, with the ability to visualize the rate and direction of change, offers a distinct informational advantage to persons with diabetes. In a longer and larger trial we may have been able to elicit a better understanding of QOL impacts.
Patients continue to express concerns about disparities between their CGM and SMBG. While accuracy and reliability in CGM continues to improve, this remains a barrier we need to address as patients will better trust CGM devices they perceive as “accurate.” Recognizing the rate and direction of change may provide the needed insight to make appropriate therapy adjustments and improve control in diabetes management.
Conclusions
CGM is an evolving technology that is being rapidly adopted by patients even as clinicians continue to express their concerns over the best ways to utilize this tool. While failing to achieve statistical significance due to limited sample size, this study does suggest that the benefits of CGM are more complex than mere reductions in HbA1c. Notably, the QLI Total scores and subscore of Health both showed improvements during the 12-week trial, suggesting that the participants were happier with their health status when using a CGM.
Empowering patients to manage their own disease process is a cornerstone of the chronic care model and of particular interest in diabetes care.32 If future studies demonstrate the numerical glucose value is less important than glucose trending or rate of change, we may need to adapt our patient training curriculum. This may require guiding patients away from therapy decisions based on numerical values and point-based testing toward a decision process based on trends and rate of change of glucose levels.
Footnotes
Abbreviations: ANOVA, analysis of variance; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; FOH, fear of hypoglycemia; HbA1c, glycosylated hemoglobin; HFS, Hypoglycemia Fear Survey; HFS-B, Hypoglycemia Fear Survey Behavior Subset; HFS-W, Hypoglycemia Fear Survey Worry Subset; MDI, multiple daily injections; QLI-D, Quality of Life Index–Diabetes; QOL, quality of life; RM ANOVA, repeated measures analysis of variance; SMBG, self-monitoring of blood glucose; Type 1 DM, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was completed with the assistance of an unrestricted equipment grant from Dexcom Inc. TCW has worked as a consultant and clinical trials investigator for Dexcom Inc.
References
- 1. Tattersall R. Home blood glucose monitoring. Diabetologia.1979;16:71-74. [DOI] [PubMed] [Google Scholar]
- 2. Snoek F, Malanda U, deWit M. Self-monitoring of blood glucose: psychological barriers and benefits. Eur Diabetes Nurs. 2008;5(3):112-115. [Google Scholar]
- 3. American Association of Clinical Endocrinologists. AACE medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):S3-S68. [DOI] [PubMed] [Google Scholar]
- 4. American Association of Diabetes Educators. AADE guidelines for the practice of diabetes self-management education and training. Available at: http://www.diabeteseducator.org/export/sites/aade/_resources/pdf/research/Guidelines_Final_2_1_11.pdf. Accessed August 15, 2013.
- 5. Hirsch I. Realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94(7):2232-2238. [DOI] [PubMed] [Google Scholar]
- 6. Ghandi G, Kovalske M, Kudva Y, et al. Efficacy of continuous glucose monitoring in improving glycemic control and reducing hypoglycemia: a systematic review and meta-analysis of randomized trials. J Diabetes Sci Technol. 2011;5(4):952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 8. Graveling A, Frier B. Impaired awareness of hypoglycemia: a review. Diabetes Metab. 2010;36:S64-S74. [DOI] [PubMed] [Google Scholar]
- 9. Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15:42-46. [DOI] [PubMed] [Google Scholar]
- 10. Bakatselos S. Hypoglycemia unawareness. Diabetes Res Clin Pract. 2011;92:S93-S96. [DOI] [PubMed] [Google Scholar]
- 11. Carroll M., Burge M, Schade D. Severe hypoglycemia in adults. Rev Endocr Metab Disord. 2003;4:149-157. [DOI] [PubMed] [Google Scholar]
- 12. Andebro T, Amsberg S, Adamson U, et al. Fear of hypoglycaemia in adults with type-1 diabetes. Diabet Med. 2010;27(10):1151-1158. [DOI] [PubMed] [Google Scholar]
- 13. Strachan M. Frequency, causes and risk factors for hypoglycemia in Type-1 diabetes. In: Frier B, Fisher M, eds. Hypoglycaemia in Clinical Diabetes. Chichester, UK: John Wiley; 2007:49-82. [Google Scholar]
- 14. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joslin E, Gray H, Root H. Insulin in hospital and home. J Metab Res. 1922;2:651-699. [Google Scholar]
- 16. Irvine A, Cox D, Gonder-Frederick L. The fear of hypoglycemia scale. In: Bradley C, ed. Handbook of Psychology and Diabetes. New York, NY: Psychology Press; 1994:133-155. [Google Scholar]
- 17. Sovik O, Thordarson H. Dead-in-bed-syndrome in young diabetic patients. Diabetes Care. 1999;22(suppl 2):B40-B42. [PubMed] [Google Scholar]
- 18. Woodward A, Weston P, Casson I, Gill G. Nocturnal hypoglycaemia in type-1 diabetes: frequency and predictive factors. Q J Med. 2009;102:603-607. [DOI] [PubMed] [Google Scholar]
- 19. American Association of Clinical Endocrinologists. Statement by the American Association of Clinical Endocrinologists consensus panel on continuous glucose monitoring. Available at: https://www.aace.com/files/continuousglucosemonitoring.pdf. Accessed August 15, 2013. [DOI] [PubMed]
- 20. Kildegaard J, Christensen T, Hejlesen O. Sources of glycemic variability: what type of technology is needed? J Diabetes Sci Technol. 2009;3(4):986-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg S, Kelly W, Noelle M, et al. Continuous home monitoring of glucose. Diabetes Care. 2007;30(12):3023-3025. [DOI] [PubMed] [Google Scholar]
- 22. Christiansen M, Bailey T, Watkins E, et al. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther. 2013;15(10):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Apurv K, Mahalingam A, Brauker J. Analysis of time lags and other sources of error of the Dexcom SEVEN continuous glucose monitor. Diabetes Technol Ther. 2009;11(11):689-695. [DOI] [PubMed] [Google Scholar]
- 24. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitors in type 1 diabetes treated with insulin pump therapy: a randomized controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dexcom Inc. SEVEN PLUS Continuous Glucose Monitoring System: Users Guide. San Diego, CA: Dexcom; 2010. [Google Scholar]
- 26. Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation and utilization. Diabetes Care. 1987;10(5): 617-621. [DOI] [PubMed] [Google Scholar]
- 27. Ferrans C. Ferrans and Powers quality of life index. Available at: http://www.uic.edu/orgs/qli/index.htm. Accessed August 15, 2013.
- 28. DeSouza MS, Nairy KS. Nursing intervention for the quality of life of diabetic adults. Clin Eff Nurs. 2003;7:63-72. [Google Scholar]
- 29. Ozer Z, Efe E. Validity and reliability of the Turkish version of the Ferrans’ and Powers’ Quality of Life Index: diabetes version. Saudi Med J. 2006;27(1):123-125. [PubMed] [Google Scholar]
- 30. Cox D, Gonder-Frederick L, Ritterband L, et al. Blood glucose awareness training: what is it, where is it and where is it going? Diabetes Spectr. 2006;19(1):43-49. [Google Scholar]
- 31. Wild D, Maltzahn R, Brohan R, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns. 2007;68:10-15. [DOI] [PubMed] [Google Scholar]
- 32. Frei A, Chmiel C, Schlapfer H, et al. The chronic CARe for diAbeTes study (CARAT): a cluster randomized controlled trial. Cardiovasc Diabetol. 2010;9:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]


