Abstract
Self-monitoring of blood glucose (BG) by means of handheld BG systems is a cornerstone in diabetes therapy. The aim of this article is to describe a procedure with proven traceability for calibration and evaluation of BG systems to guarantee reliable BG measurements. Isotope dilution gas chromatography mass spectrometry (ID/GC/MS) is a method that fulfills all requirements to be used in a higher-order reference measurement procedure. However, this method is not applicable for routine measurements because of the time-consuming sample preparation. A hexokinase method with perchloric acid (PCA) sample pretreatment is used in a measurement procedure for such purposes. This method is directly linked to the ID/GC/MS method by calibration with a glucose solution that has an ID/GC/MS-determined target value. BG systems are calibrated with whole blood samples. The glucose levels in such samples are analyzed by this ID/GC/MS-linked hexokinase method to establish traceability to higher-order reference material. For method comparison, the glucose concentrations in 577 whole blood samples were measured using the PCA-hexokinase method and the ID/GC/MS method; this resulted in a mean deviation of 0.1%. The mean deviation between BG levels measured in >500 valid whole blood samples with BG systems and the ID/GC/MS was 1.1%. BG systems allow a reliable glucose measurement if a true reference measurement procedure, with a noninterrupted traceability chain using ID/GC/MS linked hexokinase method for calibration of BG systems, is implemented. Systems should be calibrated by means of a traceable and defined measurement procedure to avoid bias.
Keywords: blood glucose monitoring systems, blood glucose monitoring, accuracy, trueness, hexokinase, SMBG
Self-monitoring of blood glucose (SMBG) is most often performed by means of handheld blood glucose (BG) meters. The performance of BG systems (BG meter in combination with test strips) used for SMBG has improved considerably over the years. However, the accuracy of BG systems is dependent on the system-inherent precision and on calibration-dependent trueness (or bias to the “true” glucose value). The bias in turn is dependent on the method used for manufacturer’s measurement procedure during the calibration procedure of such BG systems. So the accuracy that is perceived during an evaluation is the combination of the accuracy of the BG system and the accuracy of the reference method.
For the calibration procedure and accuracy evaluations, glucose levels in the same blood samples are measured using 2 methods: the respective BG system and a laboratory analyzer, which is used as a comparison system. The comparison system itself has to meet 2 requirements: It needs to be specific to the analyte (in this case glucose) and the traceability to the true glucose value in the blood sample should be verified, including appropriate consideration of the contribution of the blood matrix (matrix effect). Clinical laboratory glucose analyzers are often used as a comparison system; however, this is only suitable if traceability to standards of higher order is verified. In 1 US FDA report the need of validating the accuracy of glucose laboratory methods by traceability to certified reference material is highlighted.1 The need for improving BG system calibration and standardization of the laboratory comparative methods was also mentioned in this and other reports.2 In addition, a linkage of BG system measurements to a reference standards (higher-order reference material) developed by NIST (National Institute of Standards and Technology) was recommended.1 The isotope dilution gas chromatography mass spectrometry (ID/GC/MS) technique is used in higher-order reference measurement procedure for many substrates, including glucose.3-6 By using a certified higher-order reference material as calibrator this reference measurement procedure, which is developed and used by NIST, allows metrological traceability.
It has to be taken into account that BG systems are calibrated by whole blood whereas the certified reference materials are usually aqueous or serum based. However, the ID/GC/MS method can be modified, that differences in matrices are accounted for by a reference system. Inaccuracy of clinical laboratory methods is often blamed on matrix effects and the contribution of the analytical method itself to inaccuracy is frequently overlooked. The term “matrix effect” is often used to describe apparent differences in trueness of glucose assays when moving from aqueous to plasma or whole blood. It may be reasonably argued, however, that the matrix effect is itself no more than a lack of specificity in the analytical technique.7 Methods used in clinical routine often set matrix separation aside in favor of the speed and convenience of direct measurements of serum specimens.8 The use of an aqueous-based standard for calibration and control of glucose analyzers without matrix adaptation may be the reason for a bias.9
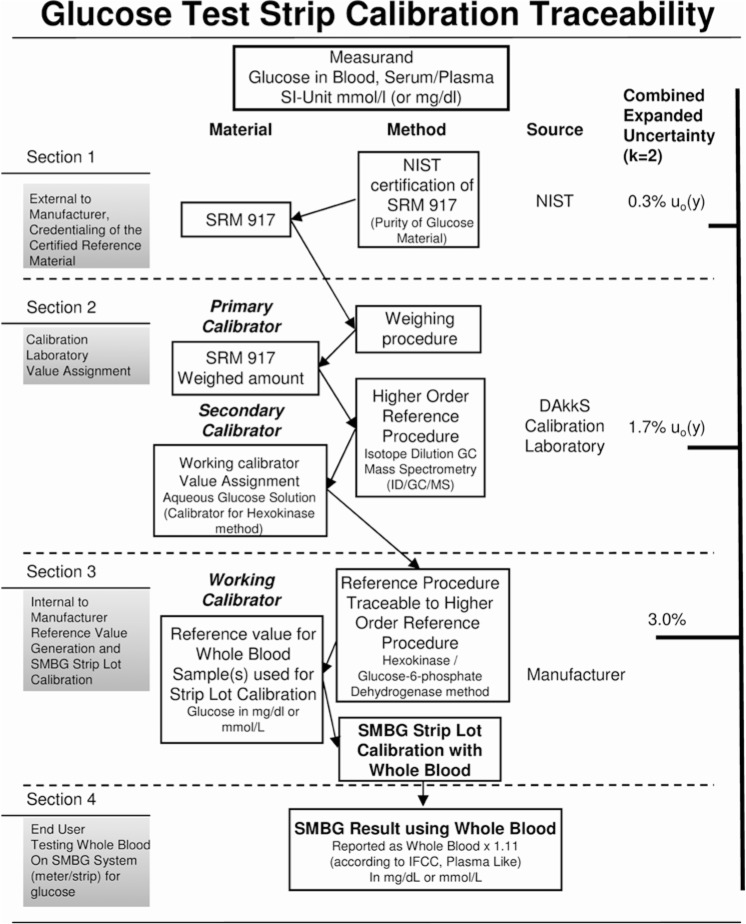
The European In-vitro Diagnostic Directive (IVDD) also requires calibration of quantitative diagnostic devices to existing higher-order reference methods or materials.10 Armbruster concludes that, primarily driven by the European IVDD, metrology is now being adapted by IVD manufacturers in the United States, as evidenced by calibrator traceability, measurement uncertainty estimates, and assay standardization/global harmonization initiatives.11 In consideration of the IVDD requirements, the Joint Committee for Traceability in Laboratory Medicine (JCTLM; http://www.bipm.org/en/committees/jc/jctlm/) pointed out that a suitable reference system ideally consists not only of accepted reference material and methods, but also of a suitable accredited reference measurement laboratory.12 Therefore, Roche Diagnostics has implemented a reference measurement procedure for glucose measurements consisting of a recognized higher-order reference measurement procedure using ID/GC/MS, certified higher-order reference materials (NIST) and an accredited reference laboratory. Such laboratories are defined as analytical centers of competence for quantifying well-defined analytes.5 Manufacturers have the option of building up their own reference lab according to ISO 17025 and ISO 15195 standards.13,14 Roche Diagnostics in Mannheim has established such a reference lab which is in accordance with the procedure suggested by others (Figure 1).15,16 Participation in several interlaboratory tests in regular intervals to ensure the appropriate analytical performance of the ID/GC/MS method is recommended. Maintaining such a laboratory is a complex task; however, having such a lab in-house accelerates the process of calibrating newly manufactured BG systems and ensuring reliable glucose measurement.
Figure 1.
Outline of the complete traceability chain of the ID/GC/MS reference method using a multistep calibration procedure.
As described above, BG systems have to be calibrated and controlled using whole blood samples because the test strips measure in this matrix and provide different results in cell-free solution. The highest-order reference material for glucose is called NIST SRM 917 and contains 99.7% ± 0.3% pure glucose (in crystalline form) while other higher-order materials like NIST SRM (standard reference material) 965 contains glucose in frozen human serum because laboratory analyzers measure glucose in plasma or serum.17 Therefore a multistep reference method was established that shows traceability from glucose measured in the matrix whole blood5 to NIST SRM 917. An unbroken metrological traceability chain according to ISO 15197 and ISO 1751118,19 can be configured using a laboratory analyzer (see below) and an ID/GC/MS method that is traceable to NIST SRM 917.
In the measurement procedure for routine calibration and accuracy evaluation of BG systems, a laboratory analyzer with a hexokinase method is used. A glucose solution as working calibrator is used for routine application of the hexokinase method. However, the determination of the glucose concentration of the working calibrator is carried out by the ID/GC/MS method to maintain a continuous traceability chain from SMBG measurements via hexokinase and ID/GC/MS to NIST SRM 917.
The aim of this article is to describe the performance of the ID/GC/MS method in the higher-order reference measurement procedure in combination with the hexokinase method in the measurement procedure for calibration and quality control of BG systems.
Materials and Methods
Characterization of ID-GC/MS
The ID-GC/MS is a special case of a well-established method, which has particular advantages in the quantification of analytes from complex matrices when multiple sample preparation steps are necessary. A chemical compound that is not identical to the analyte but does chemically and physically behave as identical as possible to the analyte is applied as internal standard in a known amount of the sample, so that analyte and internal standard join all sample preparation steps in the same way. Any losses during the working up of the analyte process step happen in the same manner also to the internal standard: the quantitative ratio of analyte to internal standard remained the same.
For quantification, a calibration is necessary. In this case, a precisely known quantity of pure analyte is added instead of the sample with an internal standard and subjected to a complete analysis step. If the resulting calibration is not linear, such a calibration standard is not sufficient. In this case, a calibration curve with several calibration standards must be created.
The requirements for an ideal internal standard are best met by an analyte synthetically made and marked with an isotope. This means that certain atoms of the analyte being substituted against their natural isotope distribution with heavier isotopes (eg, 12C is exchanged for 13C). The corresponding isotope-labeled compounds behave physically and chemically as the naturally occurring analyte coming, but are distinguishable by an GC/MS analysis in the mass spectrometer by the heavier molecular weight of this.
The advantages of an ID/GC/MS are
Compensation of variation in sample preparation by usage of an internal standard
Usage of a nearly ideal internal standard
Calibration across the whole measurement range
High selectivity by combination of gaschromatography and mass spectrometry
Apparatus ID/GC/MS
The instrumentation consists of a ThermoFisher Trace GC Ultra gas chromatograph (GC) (ThermoFisher, Dreieich, Germany) and a ThermoFisher DSQ II single quadrupole mass spectrometer (MS). The instruments are linked by a direct capillary GC/MS interface. The GC is equipped with a ThermoFisher TriPlus AS auto sampler and split/splitless injector. A fused silica capillary column Optima 1 (100% methylpolysiloxane, 15 m × 0.25 mm, coated with 0.25 μm film) is used for chromatographic separation. The carrier gas is helium (4.6) at a flow rate of 30 ± 5 mL/min. The injector is used in split mode and the temperature is kept at 200°C. The oven temperature is also kept at 200°C. The glucose derivatives are eluted after 4.0 minutes under these conditions. The capillary GC/MS interface temperature is kept at 250°C. The MS is calibrated with heptacosafluorobutylamine through the Tune program of the Xcalibur software (ThermoFisher, Dreieich, Germany).
The analyses are carried out by selected ion monitoring, with acquisition of ions at m/z 297 for unlabeled and 303 for 13C-labeled glucose. Acquisitions for the 2 ions are carried out during scan cycles of 0.1 s effecting about 60 scans during the elution of the glucose peak. The m/z 297 originates from the loss of a butyl group from the molecular ion. The corresponding ions of 13C glucose contain 6 atoms of 13C, respectively. The main purpose of NIST SRM 917 within the reference system is the value transfer to the secondary calibrators for the hexokinase method.
Standard Solutions and Control Material
Standard solutions are prepared from NIST SRM 917 (NIST, Gaithersburg, MD): This meets the strict criteria laid down in the international standard that defines requirements for reference materials (ISO 151943). A total of 500 ± 2 mg (as weighed with a semi micro balance (Model AX 205 DR/M; Mettler, Giessen, Germany) of the NIST SRM 917 is filled into a calibrated 50 mL volumetric flask. The flask is filled with exactly tempered (20°C) 1.5% sodium azide solution (Riedel-deHaen, 99.99% in deionized water). This stock solution is placed for 5 minutes in an ultrasonic bath to achieve complete dissolution. The stock solution (1000 mg/dL) is gravimetrically diluted with tempered sodium azide solution to glucose concentrations of 10, 20, 50, 100, 200, 300, 400, 500, 600, 700, 800 mg/dL. We use bracketing technique. Before and after the weighing of the standard solutions and the sodium azid solution the balance is checked with certified weights with target values below and above the diluted solutions. Stock solution and dilutions of the standard are stored at 4°C. For establishing an internal standard solution, 50 mg of [13C6]-D-Glucose (99%, CIL Cambridge Isotope Laboratories, Andover, MA) are weighed and mixed with 50 mL of sodium azide solution and stored at 4°C.
Preparation of the Specimens
There are 2 ways for the sample preparation: deproteinization with perchloric acid and deproteinization with ethanol. Both methods are equivalent and the approval is available for both methods. Sometimes the samples are from external sides (eg, clinics, research institutes) and here the deproteinization with perchloric acid is necessary because IDGCMS measurement may be later than the sample preparation.
Sample Preparation—Deproteinization With Perchloric Acid
Blood, serum, and working calibrator specimens are deproteinized prior to any further sample preparation (the same procedure is employed for the calibration and internal standard solutions): blood/serum samples 100 μL of each blood/serum specimen are pipetted in Eppendorf cups containing 1 mL 0.33 molar perchloric acid (KABE, Nümbrecht-Eisenroth, Germany). Every cup is vigorously shaken and centrifuged for 3 minutes at 14 000 r/min (= 16 100 “g”). Supernatants are pipetted into 2 mL Eppendorf cups. No significant change to these samples was observed during a storage period of 1 week at 4°C. In all, 50 μL of a given sample (or calibration standard) and 50 μL of internal standard solution are pipetted in a 7 mL screw-capped vial (SUPELCO, Bellefonte, PA, USA) and shaken afterward. Closed sample solutions are kept overnight at room temperature.
Sample Preparation—Deproteinization With Ethanol
In all, 50 μL of a given sample (Serum or calibration standard) and 50 μL of internal standard solution are pipetted in a 7 mL screw-capped vial and shaken afterward. Closed sample solutions are kept overnight at room temperature.
A total of 500 μL of ethanol (80% in water) is added to each vial for protein precipitation. After 30 minutes the vials are shaken vigorously. Samples are transferred to 2 mL Eppendorf cups and centrifuged for 3 minutes at 14 000 r/min (= 16 100 “g”) . Further sample preparation of the supernatant is done by a cation exchanger (100 mg SCX-2, Biotage, Uppsala, Sweden) run, followed by an anion exchanger (100 mg SAX, Biotage) run.
Sample Derivatization Because of Measurement Procedure in GC System
The sample and the calibration standard solution are lyophilized for 24 hours. The residue is diluted with 1.5 mL of derivatization solution (1.2% butyl boronic acid in pyridine) and vigorously shaken. The solution is kept for 35 minutes at 100°C to ensure complete derivatization. Afterward 150 μL of acetic anhydride is added to sample solution. The sample is kept at room temperature for 1 hour. The solution is then concentrated to dryness at 65°C and also lyophilized for 2 hours. The residue is diluted with 1 mL 1% acetic acid anhydride in hexane and shaken vigorously. The solution is filtrated (through a 0.2 μm filter) and transferred into a GC vial.
Analysis
One microliter solution from each GC vial is injected twice. Calibration curves are generated with 2 independent standard series. Independent means 2 separate weighed standards solutions. Standards are chosen in a range of ±100 mg/dL based on the expected glucose specimen content. In the case of blood/serum samples of each specimen as controls are prepared twice for the whole sample preparation procedure and an appropriate number of SRM 965 concentrations as control material are carried along every analysis series. Calibration curves are generated from the 12C-glucose/13C-glucose peak area ratio using a quadratic regression function to the known glucose content of the standards (Figure 2).
Figure 2.
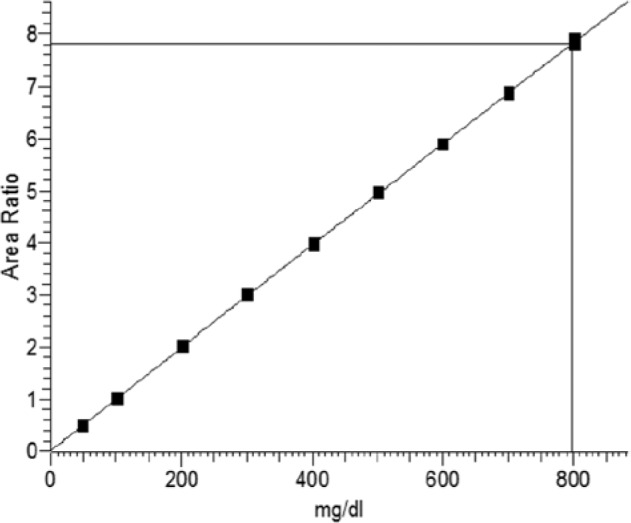
Calibration curve of the ID/GC/MS method for glucose: ratio of glucose to internal standard (area ratio) versus glucose concentration. The regression line has the formula: y = 0.0144625 + 0.0094011*X − 2.18869e−007*X2; r2 = 1.000.
The expanded uncertainty for the analytical procedures is calculated according to the guide to the expression of uncertainty in measurement (GUM).19
Calibration of BG Systems
In Figure 1 the used calibration procedure of BG systems is shown. NIST SRM 917 is used to calibrate the ID/GC/MS method, so NIST SRM 917 is the primary calibrator for the higher order reference procedure. ID/GC/MS is used for the value assignment for the secondary calibrator. The secondary calibrator is used to calibrate the hexokinase reference procedure. The hexokinase reference procedure is used to assign reference values for several whole blood samples (working calibrator) and the whole blood samples are applied to the test strips. With the readings (color) of the test strips and the assigned reference values of the hexokinase reference procedure, the mathematical correlation between color of the test strip and assigned reference values is calculated.
Results
ID/GC/MS Method
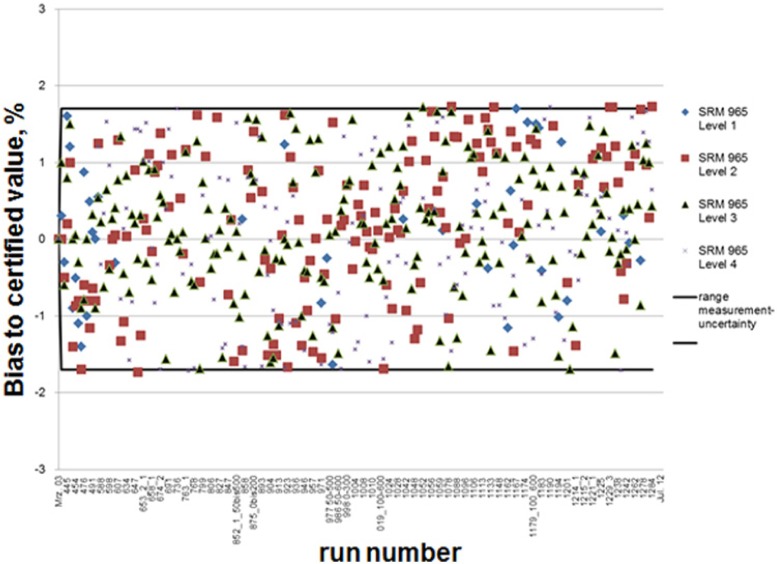
As an example of the chromatograms obtained with the ID/GC/MS method, in Figure 3 the profiles obtained with a calibrator specimen are shown. The chromatograms show sharp peaks for both traces with 12C and 13C with an m/z 297 and 303. The calibration curves constructed usually consist of 5 standards with an average correlation r2 of 0.9998. The expanded uncertainty value of the whole analytical procedure accumulates to 1.7% (95.5% confidence interval [CI]) according to the GUM. To characterize the accuracy of the ID/GC/MS measurement, data of routine series measured between the years 2003 and 2012 with glucose controls (SRM 965) are shown in Figure 4; 100% of all control specimens are within the 1.7% range. In this chart, the deviations of 4 different serum-based glucose controls with different glucose concentration to the target value provided by NIST are shown.
Figure 3.
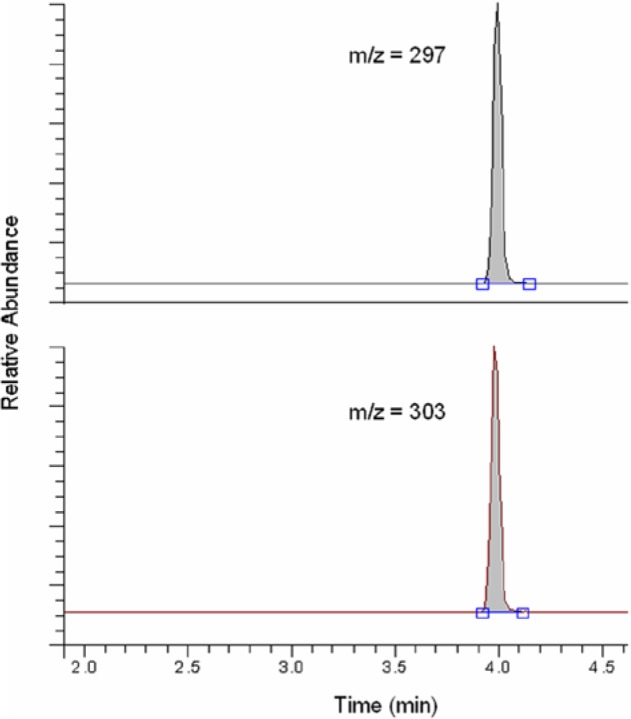
Exemplary chromatogram of a calibrator sample. The peak at m/z = 297 is generated by the 12C-glucose in the sample, while the peak at m/z = 303 is generated by the 13C-glucose in the internal standard.
Figure 4.
Deviations to higher order control material with the ID/GC/MS method observed over time when measuring SRM 965 as control solution at 4 different levels: level 1 = 33.29 mg/dL, level 2 = 75.66 mg/dL, level 3 = 118.5 mg/dL, level 4 = 294.5 mg/dL.
Glucose measurements by means of the ID/GC/MS method using IFCC proficiency testing specimens result in high equivalency in interlaboratory tests as compared to the corresponding target values with a correlation coefficient of r = 1.0000 and mean deviation of 0.2% over the years 2003 to 2011 (Figure 5).
Figure 5.
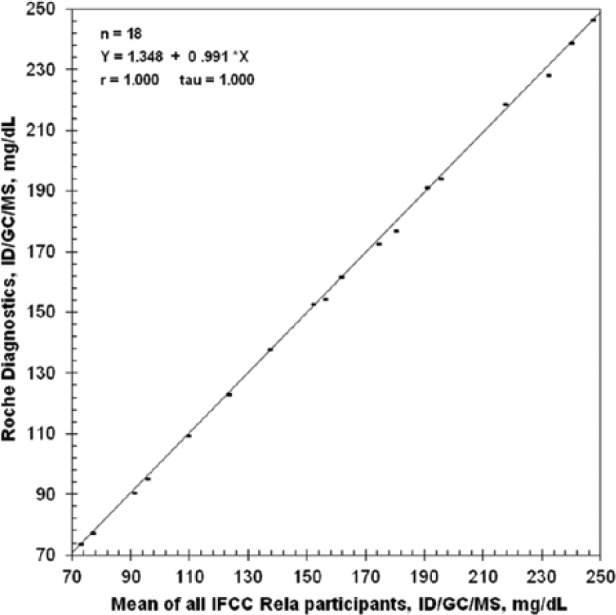
Results of the ID/GC/MS method for glucose utilizing the IFCC proficiency samples over the years 2003 to 2011. r, Spearman’s rank coefficient; tau, Kendall’s tau coefficient.
Glucose Measurements of Blood Specimens
Over a period of 5 years between 2008 and 2012, >500 different whole blood specimens were analyzed with both the hexokinase and ID/GC/MS method (Figure 6). The quantification of glucose measurement results shows a high correlation (r = .997). The mean deviation of the hexokinase method to the ID/GC/MS method is −0.1%.
Figure 6.
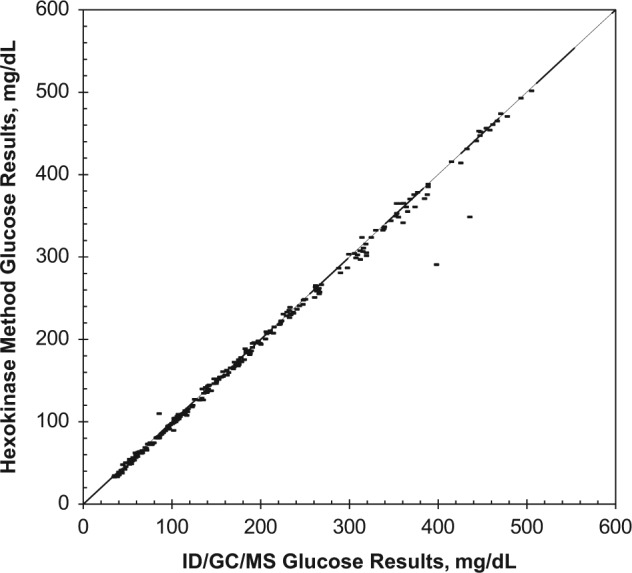
Method comparison between ID/GC/MS and hexokinase method (Hitachi analyzer). Analysis of more than 500 whole blood specimens (r = .997; tau = 0.974; Y = 0.071 + 1.000 *X). The mean deviation between the hexokinase method compared to the ID/GC/MS method was 0.1%. r, Spearman’s rank coefficient, tau, Kendall’s tau coefficient.
To prove the accuracy of BG systems, a direct comparison of the measurement results obtained with such systems with ID/GC/MS measurements is necessary. The mean deviation of >500 individual comparisons between BGM values and ID/GC/MS results was 1.1% (Figure 7).
Figure 7.
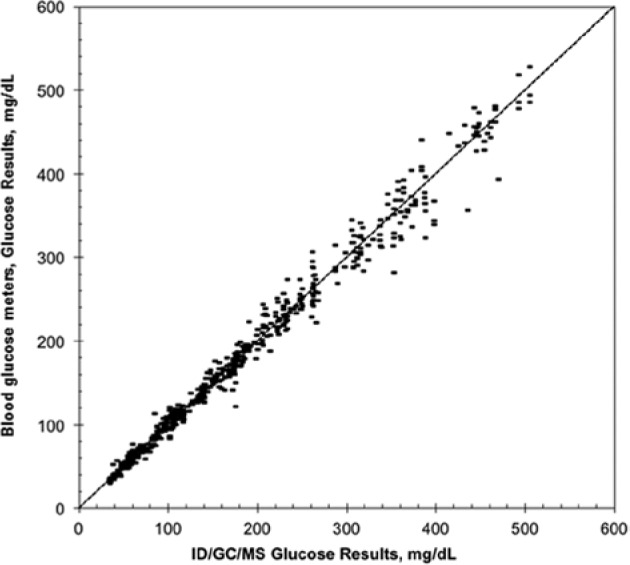
Measurement results obtained >500 whole blood specimens tested on different BG systems manufactured by Roche Diagnostics in comparison to the results obtained with the ID/GC/MS method (r = .993; tau = 0.935; Y = 1.018 + 1.001 *X). The mean deviation between the BG systems compared to the ID/GC/MS method was 1.1%. r, Spearman’s rank coefficient, tau, Kendall’s tau coefficient.
Discussion
Performing BG measurements with the ID/GC/MS method used in the higher-order reference measurement procedure results in more accurate results than other methods, given the same or lower degree of analytical difficulty.20 Isotope dilution is the method of highest metrological order for internal standardization that takes advantage of the almost identical chemical and physical behavior of different isotopes. Due to its insensitivity to substance losses during sample pretreatment, the ID/GC/MS method shows good performance in handling analytical tasks with complex matrices like whole blood, which is the matrix provided to test strips of BG meters for SMBG.5,6
The total uncertainty value of the traceability chain comprises the individual uncertainties of each step of the analytical procedure.21 In case of the ID/GC/MS method, the major uncertainties are generated during standard preparation and pipetting. For this reason special care is taken during these steps. Balances are checked before and after every preparation of a new set of standards to minimize the uncertainty of the weighing step. With the temperature dependence of the volume in mind, only exactly tempered sodium azide solutions are used for the dissolution of the higher-order reference material. All further dilutions are done gravimetrically. Blood samples are deproteinized (using perchloric acid) and glucose is measured from the supernatant with ID/GC/MS. The ID/GC/MS method is calibrated with a higher-order reference material in aqueous solution (NIST SRM 917). The deproteinization step converts the matrix of the blood sample into a matrix that is similar to the matrix of the NIST standard.7
The presented method comparison results of the ID/GC/MS method and the PCA-hexokinase method demonstrates the excellent measurement quality and traceability of these methods. Method validation showed no interferences with matrix elements and demonstrated excellent results in the direct method comparison. Thus, the general requirement of specificity for reference methods to a well-defined analyte is fulfilled. The quality of the hexokinase reference method results is of extraordinary importance with respect to the above-described calibration of the BG systems.18,19
The traceability chain from BG systems at the bottom to PCA-hexokinase and ID/GC/MS in middle to NIST SRM 917 at the top is continuous and guaranties traceability to the “true” glucose value of whole blood samples. In consideration of the mentioned performance parameters of the ID/GC/MS method, it can be concluded that it has all properties of a higher-order reference measurement procedure. The excellent results of direct comparisons between SMBG and ID/GC/MS measurements indicate that interferences with matrix elements are negligible.
Laboratory analyzers that directly use higher-order reference material in aqueous solution for calibrating the instrument measure correct results in aqueous glucose solutions. When whole blood is measured, there is a negative bias compared to a hexokinase method.9 This indicates that the contribution of the blood matrix is not appropriately considered by the method (so-called matrix effect). On the contrary, when the presented reference measurement procedure is being used, the blood samples are deproteinized before the supernatant is measured. This supernatant has the same matrix as the NIST standard SRM 917. So no matrix effect affects the measurement result and the general requirement of specificity for reference measurement procedures to a well-defined analyte is fulfilled.
The essential requirement of the applicable ISO standards 17025 and 1519513,14 is to ensure traceability of values assigned to calibrators and control materials through available reference measurements and available reference materials of a higher order. This is fulfilled with the implementation of this ID/GC/MS method in the reference measurement procedure.10
The establishment of the ID/GC/MS method in the environment of an accredited reference lab according to the ISO standards 17025 and 15195 could be shown as a successful solution to provide traceability. The analytical performance is controlled continuously by proficiency tests as organized by the IFCC. Considering the high relevance of reliable glucose values for the patient’s health, establishment and operation of an in-house measurement procedure that is closely linked to higher-order reference measurement procedure demonstrates a well-controlled and monitored reference program that supports the overall consistency in the calibration of BG systems
In summary, usage of the ID-GC/MS method provides a valuable means of certifying the trueness of both reference methods and BG Systems. A closed traceability chain taking into account the matrix differences to higher-order reference material is a prerequisite for optimal calibration of BG systems according to ISO 15197. All BG systems should be calibrated including adequate reference measurement procedures to improve trueness of BG systems and reduce the risk of potential dangerous biases between BG systems of different origin.
Acknowledgments
We thank Roche Diagnostics GmbH for financial support in implementing the described reference system. We thank Guido Freckmann and Lutz Heinemann for their constructive comments.
Footnotes
Abbreviations: BG, blood glucose; BG systems, blood glucose test systems; CRM, certified reference material; FDA, Food and Drug Administration; GC, gas chromatograph; GML, glucose measuring solution; GUM, guide to the expression of uncertainty in measurement; ID/GC/MS, isotope dilution gas chromatography mass spectrometry; IVDD, in vitro diagnostics directive; JCTLM, Joint Committee for Traceability in Laboratory Medicine; MS, mass spectrometer, NERL, New England Reagent Laboratory; NIST, National Institute of Standards and Technology; SMBG, self-monitoring of blood glucose; SRM, standard reference material.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of Roche Diagnostics, Mannheim, Germany.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Roche Diagnostics GmbH provided for financial support in implementing the described reference system.
References
- 1. Chen E. Performance evaluation of blood glucose monitoring devices. Diabetes Technol Ther. 2003;5:749-768. [DOI] [PubMed] [Google Scholar]
- 2. Hagvik J. Glucose measurement: time for a gold standard. J Diabetes Sci Technol. 2007;1:169-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Organization for Standardization. In vitro diagnostic of medical devices—measurement of quantities in samples of biological origin—description of reference materials. ISO 15194:2002.
- 4. White E, Welch VM, Sun T, et al. The accurate determination of serum glucose by isotope dilution mass spectrometry—two methods. Biomed Mass Spectrom. 1982;9:395-405. [DOI] [PubMed] [Google Scholar]
- 5. Müller MM. Implementation of reference systems in laboratory medicine. Clin Chem. 2000;46:1907-1909. [PubMed] [Google Scholar]
- 6. Hannestad U, Lundblad A. Accurate and precise isotope dilution mass spectrometry method for determining glucose in whole blood. Clin Chem. 1997;43:794-800. [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute. Evaluation of matrix effects; approved guideline EP14-A2. 2005;25(4). [Google Scholar]
- 8. Tietz NW. Accuracy in clinical chemistry—does anybody care? Clin Chem. 1994;40:859-861. [PubMed] [Google Scholar]
- 9. Twomey PJ. Plasma glucose measurement with the Yellow Springs Glucose 2300 STAT and the Olympus AU640. J Clin Pathol. 2004;57(7):752-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greenberg N. Calibrator traceability: the industry impact of the IVD directive’s new requirements. IVD Technol. 2001;2:18-27. [Google Scholar]
- 11. Armbruster D. Measurement traceability and US IVD manufacturers: the impact of metrology. Accred Qual Assur. 2009;14:393-398. [Google Scholar]
- 12. Powers DM. Coming together on traceability. IVD Technol. 2002;8:22-28. [Google Scholar]
- 13. International Organization for Standardization. General requirements for the competence of testing and calibration laboratories. EN ISO/IEC 17025:2005.
- 14. International Organization for Standardization. Laboratory medicine—requirements for reference measurement laboratories. ISO 15195:2003.
- 15. White GH. Metrological traceability in clinical biochemistry. Ann Clin Biochem. 2011;48:393-409. [DOI] [PubMed] [Google Scholar]
- 16. Siekmann L. Metrological traceability—a concept for standardization in laboratory medicine. Clin Chem Lab Med. 2013;51:953-957. [DOI] [PubMed] [Google Scholar]
- 17. Welch MJ. U.S. Government Diabetes Report. NIST’s role in measurement accuracy for diagnosis and monitoring of diabetes. Diabetes Technol Ther. 2002;4:77-79. [DOI] [PubMed] [Google Scholar]
- 18. International Organization for Standardization. In vitro diagnostic Test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. DIN EN ISO 15197:2013.
- 19. International Organization for Standardization. In vitro diagnostic medical devices—measurement of quantities in biological samples—metrological traceability of values assigned to calibrators and control materials ISO 17511:2003.
- 20. Richter W. Primary methods of measurement in chemical analysis. Accred Qual Assur. 1997;2:354-359. [Google Scholar]
- 21. International Organization for Standardization. Uncertainty of measurement—part 3: guide to the expression of uncertainty in measurement (GUM:1995). ISO/IEC Guide 98-3:2008.