Abstract
Optimal coverage of insulin needs is the paramount aim of insulin replacement therapy in patients with diabetes mellitus. To apply insulin without breaking the skin barrier by a needle and/or to allow a more physiological provision of insulin are the main reasons triggering the continuous search for alternative routes of insulin administration. Despite numerous attempts over the past 9 decades to develop an insulin pill, no insulin for oral dosing is commercially available. By way of a structured approach, we aim to provide a systematic update on the most recent developments toward an orally available insulin formulation with a clear focus on data from clinical-experimental and clinical studies. Thirteen companies that claim to be working on oral insulin formulations were identified. However, only 6 of these companies published new clinical trial results within the past 5 years. Interestingly, these clinical data reports make up a mere 4% of the considerably high total number of publications on the development of oral insulin formulations within this time period. While this picture clearly reflects the rising research interest in orally bioavailable insulin formulations, it also highlights the fact that the lion’s share of research efforts is still allocated to the preclinical stages.
Keywords: diabetes, oral insulin, glycemic control, bioavailability, oral delivery, enteral absorption
After more than 90 years of oral insulin research, commercial availability of an insulin product for oral use still seems to be a distant prospect. The quest for an oral insulin therapy started in the early twenties of the past century, almost immediately after the discovery of insulin and initiation of insulin injection therapy. Already the first pilot experiments performed with oral insulin in man in 1922 and 1923 revealed 2 of the major challenges of oral insulin treatment:
The high within-subject variability of the pharmacodynamic effect
The low bioavailability requiring enormous doses as compared to subcutaneously injected insulin
Today, the development of oral peptide-based antihyperglycemic agents has still not overcome these obstacles. Therefore an old statement about oral insulin, which was said “to be of little or no therapeutic value in diabetes mellitus in man,” could hitherto not be proven wrong.1
Despite this lack of success, the interest of the scientific community as well as diabetes patients in an oral insulin therapy is greater than ever. The anticipated advantages of oral insulin therapy appear simply too good and plentiful to be dismissed. Opposed to subcutaneous insulin injections, oral treatment is not associated with any (fear of) pain and would allow more discretion of the practical performance of insulin therapy. Availability of oral insulin would therefore not only ease insulin therapy, it would very likely increase compliance of patients with diabetes.2,3 In addition, oral insulin delivery also holds the promise of a better risk/benefit profile: the oral route of insulin administration, in which insulin passes through the gastrointestinal tract before entering the circulation, gives insulin unique pharmacodynamic advantages. Oral insulin will reach the circulation via the hepatic portal vein similar to endogenous insulin, whereas subcutaneous injections primarily induce high systemic levels of insulin, bearing a greater risk of side effects such as hypoglycemia and weight gain. At the same time, enteral application of the insulin peptide inherently creates unique challenges with regard to the characteristics and design of both oral insulin compounds/formulations and the respective clinical trials to evaluate their pharmacokinetic/pharmacodynamic performance.
In a review published in 2009, Heinemann and Jacques critically described the oral insulin approaches under development at the time.4 They found that the number of publications on clinical studies with oral insulin was surprisingly low, but remained hopeful that oral insulin would stand a good chance of coming to the market as alternative to insulin injection therapy. Now, 5 years and numerous publications in scientific journals later, we observe a still growing interest in oral insulin and an undiminished ambition of companies (this is at least as it appears) to develop oral insulin formulations. This review aims to provide an update on the oral insulin approaches under development with a clear focus on the clinical progress, achieved by way of a structured approach.
Search for Oral Insulin Publications
This review focuses only on oral insulin designed for enteral absorption and does not discuss any other alternative routes of insulin administration. Oral insulin is not only distinctive from conventional insulin injection therapy via the subcutaneous or intravenous route, but also from other novel routes of insulin application (Figure 1).
Figure 1.
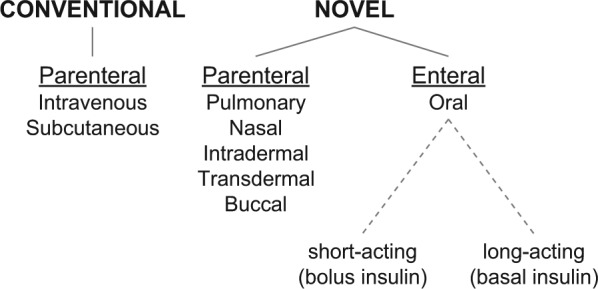
Routes of insulin application. Besides the conventional routes (left), several alternative/novel approaches (right) for insulin administration—characterized in most cases by their noninvasive nature—are under investigation.
Publications of clinical trial data obtained with oral insulin were identified by a literature search in PubMed with the following search terms
“oral insulin”
“insulin” AND “oral delivery”
“insulin delivery” AND “oral” AND “clinical” AND “trial”
“oral spray insulin”
“orally applied insulin”
Duplicate publications and publications that did not specifically focus on development of oral insulin formulations were removed based on abstract screening. Only publications reporting clinical-experimental human trial data focusing on insulin substitution therapy via the enteral (oral) route for the treatment of patients with diabetes were selected (Supplementary Figure 1 available online at http://dst.sagepub.com).
This search revealed that roughly 400 publications on oral insulin have been published to date, covering more than 90 years of oral insulin development. However, most of the publications (360 manuscripts) became available over the past 3 decades, with the majority (285 manuscripts) having been published over the past 10 years (Figure 2). The bulk of these publications (91%) present data from early pharmacological developments published predominantly in pharmacological journals, that is, they present data from in vitro or animal experiments. Clinical trial reports with human data only make up ~5% of all publications. It is remarkable to note that the clinical trial publications have not followed the general trend and remain to be low throughout: In the period from 2009 to 2013, almost half (170 manuscripts) of all the manuscripts on oral insulin were published, but merely 4% (7 manuscripts) presented results from clinical trials (Figures 2 and 3).
Figure 2.
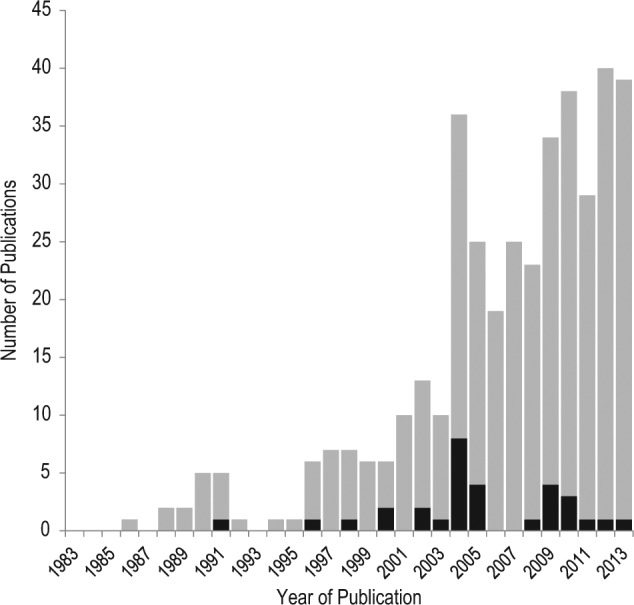
Number of publications on oral insulin that were published per year, as identified by PubMed search (duplicates and inapplicable hits removed). Gray bars, publications of preclinical data; black bars, publications of clinical trial results.
Figure 3.
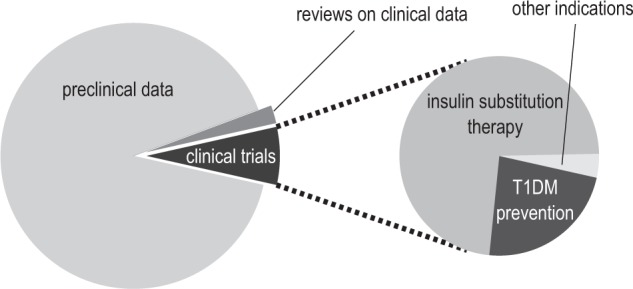
Results of systematic literature survey. The graph displays the general proportion of preclinical and clinical data published on oral insulin as well as the distribution of indications among clinical trials. Besides insulin substitution therapy, oral insulin has been investigated as pharmaceutical prophylaxis for type 1 diabetes as well as for another nondiabetic condition (pediatric short bowel disease).
Efforts to Retrieve Supplementary Data
Due to the scarcity of publicly available clinical trial results, alternative sources of information were consulted. Additional information was gathered from press releases, company websites, and clinical trial registries. The 13 companies that state to clinically develop oral insulin formulations (Table 1) were contacted directly via mail and/or email and asked to provide any amount of published or unpublished information on clinical development of their oral insulin candidate(s). Only 1 company (Oramed, Inc) responded and provided results from 2 recent, unpublished clinical trials.5,6
Table 1.
List of Clinically Tested Oral Insulin Formulations.
| Company | Name | Product | Action | Development phase | Clinical trials | References# |
|---|---|---|---|---|---|---|
| Access Pharmaceuticals, Inc | CobOral™ Insulin | Coated insulin-loaded nanoparticles | Short | Preclinical | accesspharma.com | |
| Aphios Corporation | APH-0907 | Nanoencapsulated insulin/biodegradable polymer nanospheres | Short | Preclinical | aphios.com | |
| Biocon/Bristol-Myers Squibb | IN-105 | Conjugated insulin | Short | II | NCT01035801 CTRI/2008/091/000276 CTRI/2009/091/000479 CTRI/2009/091/001008 | biocon.com, clinicaltrials.gov, ctri.nic.in, Khedkar et al7 |
| Diabetology Ltd | Capsulin™ OAD | Insulin with delivery system Axcess™ | Short | II | diabetology.co.uk, Luzio et al11 | |
| Diasome Pharmaceuticals, Inc | HDV-Insulin | Hepatic-directed vesicle-insulin (nanocarrier) | Short | III | NCT00521378 NCT00814294 | diasome.com, clinicaltrials.gov |
| Emisphere Technologies, Inc | Eligen® Insulin | Insulin with chemical delivery agents (Eligen®) | Short | I | NCT00982254 | emisphere.com, clinicaltrials.gov, Kapitza et al13 |
| Jordanian Pharmaceutical Manufacturing Co PLC | JPM Oral Insulin | Liquid delivery system with insulin chitosan nanoparticles | I | jpm.com.jo, Badwan et al16 | ||
| Novo Nordisk A/S | NN1952 | Insulin analog with oral delivery system GIPET® | Short | NCT01028404 | novonordisk.com, novonordisk-trials.com, clinicaltrials.gov | |
| OI338GT (NN1953) | Insulin analog with oral delivery system GIPET® | Long | I |
NCT01334034 NCT01931137 NCT01796366 |
||
| OI362GT (NN1954) | Insulin analog with oral delivery system GIPET® | Long | I | NCT01597713 | ||
| OI287GT (NN1956) | Insulin analog with oral delivery system GIPET® | Long | I | NCT01809184 | ||
| Oramed, Inc | ORMD-0801 | Insulin with protein oral delivery system POD™ | Short | II | CTRI/2009/091/000371 NCT00867594 NCT01889667 |
oramed.com, clinicaltrials.gov, ctri.nic.in, Eldor et al,17 Eldor et al19 |
| Oshadi Drug Administration Ltd | Oshadi Icp | Insulin, proinsulin, and C-peptide in Oshadi carrier | Short | II | NCT01120912 NCT01973920 NCT01772251 | clinicaltrials.gov |
| NOD Pharmaceuticals, Inc/Shanghai Biolaxy, Inc | Nodlin | Insulin with bioadhesive nanoencapsulation (NOD Tech) | Intermediate | II | ChiCTR-TRC-12001872 | chictr.org, Li et al20 |
| Tamarisk Technologies/Deliv-RX | Insulin with serum-specific nanoencapsulation (SSNe) | deliv-rx.com | ||||
| Transgene Biotek Ltd | TBL1002OI | Proprietary nanotechnology Trabi-Oral™ | Short | Preclinical | transgenebiotek.com |
Companies performing clinical development of oral insulin formulations were identified either through publications as compiled by systematic literature search in PubMed (gray rows) or by way of press releases and/or information on company websites or clinical trial registries in case no published data were available (white rows). #, websites accessed in December 2013.
Status of Oral Insulin Developments
Appraisement of the (true) status of each of these oral insulin developments in 2014 is a rather challenging task in light of the fact that only 6 out of the 13 companies that claim clinical development of an oral insulin formulation published new clinical trial results within the past 5 years. Subsequently the focus will be on the progress made by the companies (in alphabetical order) that did publish new clinical trial reports (Table 1).
In comparison to the situation 5 years ago,4 new companies (Aphios, Diasome, Jordanian Pharmaceutical Manufacturing, Novo Nordisk, Oshadi, NOD Pharmaceuticals/Shanghai Biolaxy, Tamarisk Technologies/Deliv-RX, and Transgene Biotek) declare to have started a clinical development program of oral insulin. It appears as if only Apollo Life Sciences officially ceased development. However, published evidence from the clinical efforts is not available in several cases.
Biocon/Bristol-Myers Squibb
Biocon’s oral insulin compound IN-105 in tablet form is an insulin analog with rapid insulin absorption and pharmacodynamic activity.4,7,8 A polyethylene glycol side chain at position B29 improves the stability and increases the solubility of IN-105, giving the insulin its rapid absorption profile which could make it suitable for control of postprandial glycemic excursions. Clinical trials have shown that timing of IN-105 intake before a meal is crucial to achieve a substantial glucose lowering effect, indicating that intake of IN-105 already 20 minutes before a meal may be sufficient to circumvent the negative effects of food intake on bioavailability/bioefficacy.7,9
In early 2011, Biocon presented first data from a 6-month phase III trial in 264 patients with type 2 diabetes that were poorly controlled (HbA1c between 7.5% and 10%) on a stable dose of metformin (1-2 g per day). Patients were randomized to receive either 4 daily doses of 10 mg IN-105 or placebo in a double-blind manner. This proof-of-concept longer-term trial did not meet its primary efficacy endpoint of achieving a placebo-adjusted HbA1c reduction of 0.7%. Biocon management attributed the negative outcome to a relatively high placebo effect probably due to behavioral modification. Several secondary efficacy endpoints, including 1-hour postprandial glucose, did however significantly improve with IN-105 treatment. No serious adverse events or hypoglycemia concerns were reported, and the oral insulin was found to be weight-neutral, a welcome and potentially important advantage of oral insulin. The full results of this trial are yet to be published.
After the presentation of the somewhat disappointing results of the phase III proof-of-concept trial, Biocon has been relatively sparse with more clinical news of the IN-105 development. In part this may be due to an option agreement that Biocon signed with Bristol-Myers Squibb in November 2012: Biocon will continue the development of IN-105 through phase 2 and then Bristol-Myers Squibb will have an exclusive option to further develop and commercialize IN-105 worldwide, pending outcome of these clinical trials. However, so far no new trials for IN-105 have been entered into clinical trial registries.
Diabetology
For a number of years Diabetology has been developing Capsulin, an oral insulin formulation based on their patented Axcess delivery system. Capsulin is an enteric-coated capsule filled with human insulin and absorption as well as solubility enhancers. The coated capsule is designed to protect the unmodified insulin from gastric degradation and release its contents in the jejunum. A mixture of well-characterized and “generally regarded as safe” (GRAS) substances is supposed to facilitate the transport of the insulin through the intestinal wall and into the circulation.10 Capsulin is being developed for the treatment of patients with type 1 and type 2 diabetes.
In 3 clinical trials performed so far, a clear, dose-dependent plasma insulin response after Capsulin administration could not unequivocally be observed. Having said that, a metabolic effect (ie, improved glucose utilization) was always observed, which has led to the interpretation that this oral insulin primarily lowers blood glucose levels via an effect on liver glucose metabolism.10-12 However, a proof-of-mechanism experiment to support this notion has not been reported to date.
Information on Capsulin intake in combination with food is limited to an investigation in 16 subjects with type 2 diabetes who ingested the oral insulin twice daily—60 minutes before a breakfast and an evening meal—for 10 days. Postprandial blood glucose levels did not return to baseline within 2 hours after neither meal (breakfast, dinner), but typically remained 2-3 mmol/L higher. It is still unclear whether food intake does blunt the metabolic effect of Capsulin.
The latest clinical trial with Capsulin11 was already presented at the Scientific Sessions of the ADA in 2008 and extensively discussed before.4 In recent years, no other clinical trial activity was reported for Capsulin. However, Diabetology announced in July 2012 an exclusive license agreement with USV Limited, an Indian pharmaceutical company, to develop and commercialize oral insulin in India.
Emisphere
The principle for Emisphere’s oral insulin formulation is interaction of the drug-carrier molecule monosodium N-(4-chlorosalicyloyl)-4-aminobutyrate (4-CNAB) with human insulin, facilitating gastrointestinal insulin absorption. Early clinical tests with Emisphere’s oral insulin were performed from 2001 to 2004, and some of the results have been published only recently.13 This first proof-of-concept clamp trial compared oral and subcutaneous administration of human insulin in 10 patients with type 2 diabetes. Oral insulin was absorbed much faster compared to subcutaneous injection (Tmax, 27 vs 161 min) and was also cleared from the circulation much quicker (within 2 hours). The glucose infusion rates during the clamp showed a similar profile suggesting a rapid onset and a short duration of action. Bioefficacy of the oral insulin was however only 3% of the subcutaneous insulin during the entire 6-hour study period. However, the observed preferential action-time profile of the oral insulin could not improve metabolic control in patients with type 2 diabetes during a long-term dosing (90 days) phase II trial.14
After a pause of approximately 5 years, Emisphere announced in 2010 an exclusive license and development agreement with Novo Nordisk to develop oral formulations of Novo’s insulins using Emisphere’s delivery technology (Eligen). It seems that this development is aimed more toward treatment of patients with type 2 diabetes and basal insulin cover, but no clinical results of this collaboration have been presented so far.
Jordanian Pharmaceutical Manufacturing Co
This pharmaceutical company markets products mainly in the Middle East, but also in parts of Africa, Asia, and Europe. This company has developed a patented oral liquid delivery system in which insulin-loaded chitosan nanoparticles are dispersed in an oily vehicle (nanoemulsion).15 The hydrophilic chitosan nanoparticle acts as a drug carrier for regular human insulin and is meant to enhance the absorption of the insulin into the circulation.
Although information on the website of this company refers to 5 separate clinical trials with 50 trial subjects “with excellent results,” these trials do not appear in 1 of the major clinical trial registries, and results on only 1 such trial have been published.16 The respective clinical trial studied the pharmacokinetics and pharmacodynamics of 5 oral insulin formulations during a 6-hour euglycemic clamp in 25 healthy male subjects. The particle size of the nanoemulsions (57, 100, or 220 nm) and dose level (1, 2, or 3 U/kg) varied between the formulations. Subcutaneously injected regular human insulin at a dose level of 0.1 U/kg was used as active control. Although the plasma insulin responses after oral insulin administration were comparable to or even a bit higher than after human insulin injection, the metabolic effect of the oral insulin was less pronounced, especially during the first 3 hours after drug administration. The authors performed an interesting simulation using intestinal physiological parameters from literature and the obtained pharmacokinetic data to calculate the fraction of oral dose absorbed from each part of the gastrointestinal tract. The simulation suggests that insulin is absorbed in almost equal amounts in each part of the intestinal tract with slightly higher absorption in parts of the jejunum. They concluded that the results of the formulations tested are promising, but more pilot studies need to be performed to derive an acceptable insulin formulation.
Oramed
Oramed uses a patented oral delivery system, called POD™ (Protein Oral Delivery) technology. An enteric-coated capsule protects unmodified regular human insulin during transit through the stomach and releases the insulin in the small intestine. Further adjuvants (registered pharmacopoeial or GRAS substances) protect the insulin from degradation and also enhance its absorption across the intestinal wall.
Oramed’s oral insulin formulation ORMD-0801 has undergone extensive clinical testing: up until October 2010 a total of 7 phase I and phase II trials were performed with at least 1444 administrations in 121 subjects (66 healthy subjects, 38 subjects with type 2 diabetes, and 18 subjects with type 1 diabetes). These trials have been presented at a number of diabetes conferences between 2008 and 2013 and results of 2 trials have also been published. In early clinical testing, 5 formulations of ORMD-0801 with varying adjuvant proportions were tested, and lowering of blood glucose concentrations by 11-35% was observed with all formulations in most experiments.17 However, the formulation that showed the most profound glucose lowering effect did not lower the blood glucose concentration at all in 3 out of 8 subjects, which highlights substantial between-subjects variability with regard to the antihyperglycemic effect.17 Another study showed the same level of variability in insulin response: significant increases in insulin concentration were observed in only 61% of the treatment sessions.18 Further clinical findings have shown that ORMD-0801
is absorbed when given 90 to 10 minutes before a meal18
is safe and tolerable over a 6-week treatment period with a once-daily dosing regimen, potentially offering temporary beta-cell relief in patients with type 2 diabetes6
can reduce overall hyperglycemia in poorly controlled (HbA1c >7.5%) patients with type 1 diabetes when given thrice daily premeals in addition to the patient’s usual therapy (eg, oral antidiabetics)19
In the most recently presented phase I trial,5 2 new formulations of ORMD-0801 were tested, demonstrating that Oramed is in the process of optimizing their oral insulin delivery technology because the previous formulation did not show reproducibility of a sufficient pharmacodynamic effect. In 2013, Oramed completed 2 more phase II trials with ORMD-0801 in patients with type 1 and type 2 diabetes, apparently to support safety and efficacy of the formulation prior to initiating a larger multicenter trial in the United States. A full overview of the clinical trial history can be found on Oramed’s website (Table 1).
NOD Pharmaceuticals, Inc/Shanghai Biolaxy, Inc
This drug delivery company and its Chinese subsidiary are developing Nodlin, an oral insulin formulation for basal insulin supplementation. Nodlin consists of insulin nanoparticles embedded in a bioadhesive calcium phosphate enteric-coated capsule. One phase I clinical trial in 12 healthy volunteers tested 3 different doses of oral insulin (50, 100, 200 IU) against a 6 IU dose of subcutaneously injected NPH insulin (Humulin N, Eli Lilly and Company) as the active comparator.20 Time-action profiles were derived from the glucose infusion rates needed to keep a subject’s blood glucose level constant at 90 mg/dL during a manual glucose clamp for 10 hours after drug administration. Overall, the patterns of the pharmacodynamic response to oral and subcutaneous insulin were rather similar. However, a significant difference in the total metabolic effect was found, with NPH insulin exerting an approximately 1.5-fold larger response than that observed after oral insulin administration. Some of the results do, however, put efficacy of this drug as well as conduct of the clamp trial into question. First, the time-action profiles of the 3 oral insulin doses were nearly identical, suggesting that maximum absorption and effect was already achieved at the lowest dose and raising questions about dose proportionality with regard to the pharmacodynamic effect. Second, no responses in serum insulin levels were observed after insulin administration, neither with oral intake nor with subcutaneous insulin administration. While the absence of the pharmacokinetic response with oral insulin may be explained by a primary effect on the liver and substantial hepatic insulin extraction, the absence of the pharmacokinetic response after subcutaneous insulin injection is more difficult to explain. The manufacturer suggested that the NPH dose of 6 IU was simply too low for clinical practice and to detect a response given the continuous background intravenous insulin infusion. In conclusion, further trials are warranted to demonstrate a clinically relevant metabolic effect of this oral insulin formulation.
Other Companies
In addition to the companies presented so far with documented clinical research activity (ie, published trial results) on oral insulin formulations in the past 5 years, a number of other pharmaceutical companies are pursuing development of oral insulin (Table 1). In the following, a very brief overview of these companies is given.
Access Pharmaceuticals is focusing its development effort on its proprietary nanopolymer oral drug delivery (Cobalamin™) technology. This technology utilizes the enteral uptake mechanism for vitamin B12 to transport drugs that otherwise would have little or no oral bioavailability. In April 2011, the company announced to have sold their oral insulin technology to a major undisclosed pharmaceutical company.
APH-0907 is manufactured by Aphios Corporation in a proprietary and patented PNS process and is based on the nanoencapsulation of insulin in biodegradable polymer nanospheres. Animal data demonstrate that the nanoencapsulated insulin was protected in the stomach and then rapidly absorbed. In an in vivo oral gavage study of nanoencapsulated insulin conducted in diabetic mice a significant decrease in glucose levels after oral administration was demonstrated. The company was granted a US patent for their oral insulin delivery technology in October 2013.
The oral insulin formulation developed by Diasome Pharmaceuticals has been extensively discussed in a previous review.4 No additional information has become available since 2009.
Novo Nordisk discontinued development of their prandial oral insulin (NN1952) because they decided that the interactions with food intake were inacceptable. The company recently reports development of long-acting basal insulin analogues designed for oral treatment, utilizing Merrion’s GIPET® Technology. Several early-phase clinical trials in healthy volunteers and in patients with type 2 diabetes have been conducted, but no results have been published to date. In December 2013, completion of a trial investigating different tablet coatings for 1 of their long-acting GIPET® insulins was announced. The company has further partnered with Emisphere (see above) to develop and commercialize the oral delivery of their insulin compounds with Emisphere’s Eligen® technology. The project is currently in the preclinical stage.
Oshadi Drug Administration has developed Icp-insulin, proinsulin and C-peptide in Oshadi carrier, designed for oral administration. The company completed 2 phase I trials in patients with type 1 diabetes. A nonrandomized, open-label trial to investigate safety and pharmacodynamic effects of Oshadi oral insulin in a 4-week multiple-dose regimen has been announced with an estimated primary completion date in November 2014.
Tamarisk Technologies/Deliv-RX claims development of a nanoencapsulated insulin with a patented SSNe vehicle, reaching bioavailability of at least 90%. However, no (clinical) data exist to support this statement. Based on information on the company website, it appears unclear at the moment whether and how Tamarisk will continue their development.
Transgene/Biotek reports discovery of a novel transporter in the mammalian intestine, characterized by relatively high uptake capacity. Their current technology comprises a targeted nanolattice designed to enable both delivery of larger amounts of peptides and protection of the peptides and proteins from proteolysis in the intestine.
Discussions and Future Expectations
Oral insulin replacement therapy remains a very appealing alternative to subcutaneous injections for patients with diabetes. However, it seems that the search for an acceptable insulin formulation is much more difficult than initially thought. After decades of failed attempts to produce an insulin pill, technological innovation and renewed ambition in the past 10 years are driving the pharmaceutical industry to once again try to come up with an oral solution.
The documented progress of oral insulin developments made in the past 5 years is disappointing: although a large amount of data from in vitro and animal experiments was published during this period, clinical trial reports with human data only made up 4% of all the oral insulin publications. In our view, this small number of clinical reports in comparison to published pharmacological studies indicates that oral insulin development is still struggling to move on from the workbench into clinical testing. Moreover, some of the recently published clinical data represent results from trials that were conducted more than 10 years ago and/or from developments that have been discontinued.
In addition to the low number of clinical trial publications, the quality of the clinical trial designs in many cases is also not satisfactory. Furthermore, with the exception of Emisphere’s 3-month phase II trial and Biocon’s 6-month phase III trial, all presented trials are early-phase, feasibility, and proof-of-concept trials with a small number (typically 8-12) of subjects tested. Although the conclusion of these proof-of-concept trials is without exception that oral insulin delivery is feasible and that the results are promising for further development, a closer look at the design and data reveal there is room for improvement:
Many of the trials did not use an active comparator when testing the pharmacodynamic effect of the oral insulin and none of the trials used an insulin analog (either rapid- or long-acting) as state of the art active comparator.
For oral insulin formulations designed to provide prandial glycemic control, the effect of food on insulin absorption should be thoroughly investigated as timing of premeal administration can be key to the observed glucose lowering effect.9 Intake of oral insulin just prior to starting a meal seems to significantly hamper insulin absorption for most formulations. As is true for injectable short-acting insulins, (longer) application-meal intervals are bothersome from a patient perspective. Taking a prandial insulin 60 minutes before food ingestion may work in a trial setting with fixed times for meals,11 but will be less attractive for the majority of patients in real-life, especially in light of available fast-acting injectable insulin analogs.
The variable response to oral insulin intake is quite frequently ignored and was not systematically studied to our knowledge. A reported mean reduction in glucose concentration of up to 35% after oral insulin intake could indeed be regarded as promising, but perhaps more important for treating physicians and patients would be to know that the insulin elicits a reproducible effect in a group of patients (low between-subjects variability) and in the same patient with multiple dosings (low within-subject variability). Published results indicate that between-subjects variability may be high, with approximately 1 out of 4 oral insulin dosings not resulting in a measurable glucose lowering response at all. No recently published study has yet looked into within-subject variability, although data also suggest that the response to oral insulin in the same patient may be highly variable.
With the relatively high doses of insulin that need to be ingested to achieve a significant metabolic effect, there is a theoretical possibility of an insulin overdose if insulin absorption is acutely increased by biological or environmental factors (eg, unique foods, stress or activity). Furthermore, the obvious impact of food intake on bioavailability and pharmacodynamic properties of oral insulin formulations (as well as the variability thereof) would most certainly become less important in the context of a basal oral insulin formulation. In light of the current treatment practices, this may be a true alternative for use of long-acting insulin analogs in addition to other oral antidiabetic drugs in the treatment of patients with type 2 diabetes mellitus.
With at least a dozen companies that to our understanding are actively working on oral insulin formulations in the clinical stage, what may be expected during the next 5 years? Based on the current picture it is hard to imagine that an oral insulin product will be approved for marketing by 2019. Given the limited number of published clinical trial reports so far, the hope clearly is to see more clinical data. Companies like Tamarisk Technologies have released rather bold press statements claiming to have “solved the oral insulin puzzle” and to have “succeeded where others will continue to fail,” but the scientific community has been waiting for years now for conclusive proof in support of these statements. Moreover, in a number of cases, indication of the conduct or completion of a clinical trial in the form of an entry in a national/international database is also missing. Negligence with regard to registering a trial in a clinical database not only is in violation of standard ethical principles of clinical trials as set out in the Declaration of Helsinki, but also makes it difficult to objectively assess the status of a development. A transparent process for clinical trial conduct and publication is key to ensure integrity of data and achieve scientific progress. It is our hope that our structured approach offers some fuel for thought in this regard.
Acknowledgments
The authors wish to thank Dr E. Arbid for providing poster-format publications of clinical trials results on behalf of Oramed, Inc.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LH is partner and scientific consultant, and EZ and LPM are employees of Profil. This institute performs clinical trials in cooperation with many pharmaceutical companies. LH is a member of several advisory boards and speakers bureaus and has received honoraria from such companies. He is not a stockholder in any of the companies with which the institute performs clinical trials.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Harrison GA. Insulin in alcoholic solution by the mouth. Br Med J. 1923;2:1204-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Tabakha MM, Arida AI. Recent challenges in insulin delivery systems: a review. Indian J Pharm Sci. 2008;70:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aronson R. The role of comfort and discomfort in insulin therapy. Diabetes Technol Ther. 2012;14:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinemann L, Jacques Y. Oral and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3:568-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eldor R, Arbit E, Schurr D, Kidron M, Hersko A. Dose response to oral insulin capsules in fasting, healthy subjects. Diabetes. 2013;62(suppl 1):1054-P. [Google Scholar]
- 6. Eldor R, Kidron M, Miteva Y, Arbit E. Decreased CRP levels in response to a six-week, once-daily oral insulin regimen. 81st EAS Congress, Lyon, France. 2013;47. [Google Scholar]
- 7. Khedkar A, Iyer H, Anand A, et al. A dose range finding study of novel oral insulin (IN-105) under fed conditions in type 2 diabetes mellitus subjects. Diabetes Obes Metab. 2010;12:659-664. [DOI] [PubMed] [Google Scholar]
- 8. Iyer H, Khedkar A, Verma M. Oral insulin—a review of current status. Diabetes Obes Metab. 2010;12:179-185. [DOI] [PubMed] [Google Scholar]
- 9. Suryanarayan S, Khedkar A, Vedala A, et al. Pharmacokinetics and pharmacodynamics of a single oral dose of the insulin analog IN-105, in tablet form, in normal healthy volunteers, in the presence of food. Diabetologia. 2007;50(suppl 1):S95-S96. [Google Scholar]
- 10. Phillips J, Russel-Jones DL, Wright J, Brackenridge A, New R, Bansal G. Early evaluation of a novel oral insulin delivery system in healthy volunteers. Diabetes. 2004;53(suppl 2):A113. [Google Scholar]
- 11. Luzio SD, Dunseath G, Lockett A, Broke-Smith TP, New RR, Owens DR. The glucose lowering effect of an oral insulin (Capsulin) during an isoglycaemic clamp study in persons with type 2 diabetes. Diabetes Obes Metab. 2010;12:82-87. [DOI] [PubMed] [Google Scholar]
- 12. Whitelaw DC, Kelly CA, Ironmonger W, Cunliffe CM, New R, Phillips JN. Absorption of orally ingested insulin in human type 1 diabetic subjects: proof of concept study. Diabetes. 2005;54(suppl 1):5-LB. [Google Scholar]
- 13. Kapitza C, Zijlstra E, Heinemann L, Castelli MC, Riley G, Heise T. Oral insulin: a comparison with subcutaneous regular human insulin in patients with type 2 diabetes. Diabetes Care. 2010;33:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldberg M, Dinh S, Castelli C, Majuru S, Arbit E. Improved glycemic control with oral recombinant human insulin in patients with type 2 diabetes (T2DM) inadequately controlled on metformin. Diabetes. 2007;56(suppl 2):A121. [Google Scholar]
- 15. Elsayed A, Al-Remawi M, Farouk A, Badwan A. Insulin-chitosan polyelectrolyte nanocomplexes: Preparation, characterization and stabilization of insulin. Sudan JMS. 2010;5:99-110. [Google Scholar]
- 16. Badwan A, Remawi M, Qinna N, et al. Enhancement of oral bioavailability of insulin in humans. Neuro Endocrinol Lett. 2009;30:74-78. [PubMed] [Google Scholar]
- 17. Eldor R, Kidron M, Arbit E. Open-label study to assess the safety and pharmacodynamics of five oral insulin formulations in healthy subjects. Diabetes Obes Metab. 2010;12:219-223. [DOI] [PubMed] [Google Scholar]
- 18. Eldor R, Arbit E, Miteva Y, Freier R, Kidron M. Oral insulin: type 1 diabetes (T1DM) patient response upon preprandial administration. Diabetes. 2010;59(suppl 1):521-P. [Google Scholar]
- 19. Eldor R, Arbit E, Corcos A, Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLOS ONE. 2013;8:e59524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Wang Y, Han L, Sun X, Yu H, Yu Y. Time-action profile of an oral enteric insulin formulation in healthy Chinese volunteers. Clin Ther. 2012;34:2333-2338. [DOI] [PubMed] [Google Scholar]


