Abstract
In the United States, Spanish-speaking patients with diabetes often receive inadequate dietary counseling. Providing language and culture-concordant dietary counseling on an ongoing basis is critical to diabetes self-care. To determine if automated telephone nutrition support (ATNS) counseling could help patients improve glycemic control by duplicating a successful pilot in Mexico in a Spanish-speaking population in Oakland, California. A prospective randomized open-label trial with blinded endpoint assessment (PROBE) was performed. The participants were seventy-five adult patients with diabetes receiving care at a federally qualified health center in Oakland, California. ATNS, a computerized system that dialed patients on their phones, prompted them in Spanish to enter (via keypad) portions consumed in the prior 24 hours of various cultural-specific dietary items, and then provided dietary feedback based on proportion of high versus low glycemic index foods consumed. The control group received the same ATNS phone calls 14 weeks after enrollment. The primary outcome was hemoglobin A1c % (A1c) 12 weeks following enrollment. Participants had no significant improvement in A1c (–0.3% in the control arm, –0.1% in the intervention arm, P = .41 for any difference) or any secondary parameters. In our study, an ATNS system did not improve diabetes control in a Spanish-speaking population in Oakland.
Keywords: self-care, vulnerable populations, diabetes care, telephone nutrition support, Spanish
Type 2 diabetes (T2DM) is a common chronic condition in the United States,1,2 especially in the Latino community,3,4 with more than 30% of adults affected in some areas.5 Latinos may also be at higher risk for some diabetes complications, while being protected from others.6 Control of blood sugar through diet, exercise, and lifestyle changes, in conjunction with medication use, can reduce complications of diabetes.7-10 However, in the United States, in-person counseling regarding diet and exercise is often suboptimal among limited English proficiency Latino patients.11 Telephone-based counseling interventions can be beneficial for obesity and/or glycemic control12,13 but can also be expensive to provide with high per-user costs.14
In this context, automated telephone counseling, with or without interactive voice response (IVR), with programmable prompts and messages is a cost-effective alternative for improving dietary habits.15-19 It is unclear whether and to what extent an automated telephone nutrition support (ATNS) system might work in the context of traditional Latino diets, how it might best be designed, and whether it could ultimately improve metabolic control.
In 2008 to 2009 our team conducted a small, 10 subject pilot of an ATNS system with language- and culture-concordant counseling. Over a 12-week period, patients received phone calls at least twice a week from a computerized system asking them how many portions of a given food they had consumed, totaling across several categories, tabulating servings of high glycemic index foods, and providing tailored messages to patients based on the number of servings either encouraging them to continue eating lower glycemic index foods or reminding them to limit eating higher glycemic index foods. At study initiation, 6, and 12 weeks, biometric measurements were made and blood samples were obtained. The pilot study showed very promising results in this population, with subjects (all of whom received the intervention) experiencing a drop in hemoglobin A1c % of 1.9 points, from 10.3 to 8.4.
On the basis of this very promising pilot, we then conducted a small follow-up randomized controlled trial in Oakland, California, among patients being treated at a federally qualified health center, of the ATNS intervention.
Methods
Design and Setting
We conducted a prospective, randomized, open-label trial (ClinicalTrials.gov #NCT01040676) with blinded endpoint assessment at, and in partnership with, La Clinica de la Raza (LCR), a large federally qualified health center in Oakland, California, which serves a predominantly Spanish-speaking population. The Institutional Review Board at UCSF and the Quality Assurance Subcommittee of LCR jointly approved this study.
Recruitment and Inclusion/Exclusion Criteria
We requested permission from the adult primary care providers at LCR to directly contact patients for participation in the study, or to opt out on their patients’ behalf (which none did). The LCR investigators then obtained an extract from the electronic record of patients with inclusion criteria of at least 2 visits to LCR in the last 1 year and a Hemoglobin A1c % (A1c) on most recent visit > 8.5, with the patient not being on insulin; the inclusion criteria were later relaxed to A1c > 8.0 and any insulin status as the first query yielded too few patients (<80). An aid at the clinic (MV) then called patients on the list with an upcoming appointment (n = 119) the night prior to an upcoming appointment, asking if they were interested in the study and, if they were, screening them for exclusion if they had end stage heart, liver, or renal disease, were pregnant, or did not speak Spanish. Patients not meeting exclusion criteria then received a brief description of the intervention, followed by an assessment of interest, a request for verbal consent, and an invitation to participate in the study by arriving at the clinic an hour before the appointment to undergo study procedures.
Baseline Visit
All patients who arrived at LCR the day after the screening phone call and provided informed consent to participate in the study (n = 75) watched a 10 minute informational video describing the dangers of uncontrolled diabetes, the benefits of a low-glycemic index diet, and a description of the study protocol. They then underwent baseline anthropomorphic measurements of height, weight, blood pressure, and waist circumference; and had their blood drawn for assessment of A1c and lipid parameters (total cholesterol, triglycerides, high density lipoprotein, and low density lipoprotein). Patients received a $10 gift card for attending this initial visit.
Glycemic Index
Encouraging adults with diabetes to substitute low-glycemic index foods for high-glycemic index foods has a solid pathophysiological basis20,21 and has been associated in practice with small but clinically meaningful improvements in glycemic control as measured by hemoglobin A1c % (A1c).22,23 Accordingly, as they had in the Mexican pilot, all patients in the current study received information, in this case via the educational video described above, regarding the dangers of a Western diet high in refined sugars with the alternative framed as a return to an ancestral diet with low glycemic index foods.24 The main categories of high-glycemic foods to avoid were specified as (1) white breads (pan dulce) and cakes, (2) white rice, (3) potatoes, (4) flour tortillas, (5) refined breakfast cereals, (6) sweets and candies, and (7) sugar-sweetened soft drinks. A special “substitution card” (laminated 8 × 10 inches) with pictures of healthy (and unhealthy) food choices was provided to each participant. The foods represented on the card were chosen specifically to be typical of foods consumed by Latinos in Mexico and the Mexican diaspora (Figure 1).
Figure 1.
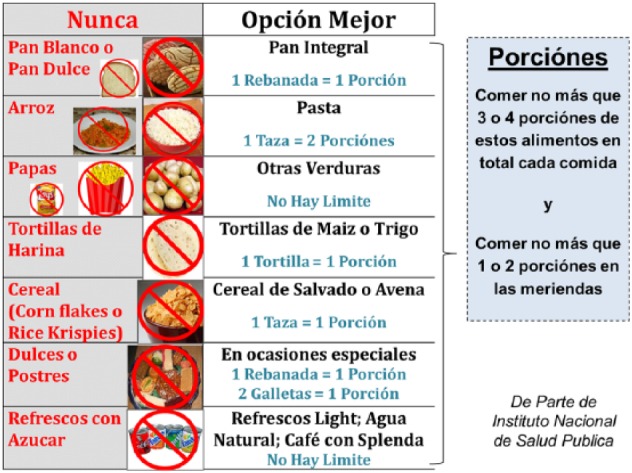
The glycemic index card. Given in Spanish, this card provided both a list of foods high in glycemic index and potential substitutes immediately adjacent to it. During the phone calls patients received, they were asked about portions consumed of each of these foods (including a description of a portion if they had forgotten). The sum was tallied by the machine, and if the result was 0-1 portions of high glycemic index foods, they received a message of congratulations and encouragement to continue the same; for 2-3 portions, they received a message advising them to try to eat fewer portions and a reminder of what effective substitutes might be; and for 4 or more portions, they received a slightly more strongly worded message that also gave information about end-organ damage when diabetes remains uncontrolled.
ATNS Intervention: Framework, Design, Modification, and Implementation
The ATNS intervention was initially designed to meet the needs of low-income, community dwelling adults with a working telephone in Mexico as per the pilot study described above. In accordance with Green and Kreuter’s PRECEDE model25 we targeted specific predisposing, enabling, and reinforcing factors contributing to food choices among the target population with our intervention. To address predisposing factors such as knowledge, attitudes and awareness, we provided education about (1) the importance of minimizing high-glycemic index foods among patients with diabetes, (2) common foods in their diet which have a high glycemic index, and (3) low/moderate glycemic index food substitutes. To address enabling factors (eg, facilitate the intended behavior change) we created the ATNS to provide easy self-assessment and feedback about high-glycemic food intake. To address reinforcing factors we included motivational messages based on each patient’s intake reported through the ATNS.
The ATNS system was designed as follows. After a brief introductory message, participants were asked questions in the format (“How many servings of ___ did you eat yesterday?”) to which they could respond using their telephone key pad. Each serving of each food type was added together to create a “summary” estimate of high-glycemic index food consumption on survey conclusion that was then provided to patients at the conclusion of the call. If the sum of all high glycemic index foods in the previous 24-hour period was 2 or fewer servings, the message was one of congratulations and positive feedback; if 3-4 servings, the message was more cautious and provided some education about appropriate low-glycemic index foods; and if 5 or more servings, then it provided a more educational message regarding high and low-glycemic index foods.25 The ATNS system was designed and implemented using a Dialogic telephone card installed in a desktop computer and connected to a land line, programmed using Telesage software (Boston, MA).
The ATNS system described above was modified for the current study by including a few additional foods our LCR partners (PB, MV, JT) knew to be more commonly consumed among the population in Oakland (as compared to Ahuatepec, Mexico) and voice prompts were re-recorded using MV’s voice to improve local acceptability for the intervention. The scale for providing feedback was also modified so that eating 0-1 serving of high glycemic index foods resulted in positive feedback; 2-3 servings resulted in cautious feedback and suggestions regarding low glycemic index foods; and 4 servings resulted in the more educational/warning message regarding high glycemic index foods—also based on the knowledge of this community of our LCR partners.
Patients were selected into one arm or the other of the study using a random number generator. The ATNS call was delivered to patients in the intervention arm at the phone number they had provided at the baseline visit, attempting to contact them at least twice a week (more frequently if they did not answer or complete a call), enabling them to quantify their 24-hour eating patterns as described above. The control group received usual care and the ATNS phone call 14 weeks after enrollment, after the second and final study visit.
Follow-up
Twelve weeks after the baseline visit, patients were called back in for a second and final study visit, during which anthropometric measurements and lab work were obtained again. If patients could not be reached by an initial phone call, the aid called each patient at least 3 times over the subsequent 2 weeks, mailing cards to their home, and contacting any backup phone numbers in the clinic database. Patients received a $20 incentive card at the follow-up appointment.
Statistics and Power Calculation
In the current study, assuming an 80% power to detect a difference in A1c of approximately 1.2% ± 1.5% (slightly over half the overall reduction seen in phase 1) between groups, and assuming 15% loss to follow-up, we aimed to enroll 80 total participants into our study. For our primary outcome of A1c, we assessed the difference between the ATNS group and the control group in overall change from baseline using the Wilcoxon rank sum test. We also used the Wilcoxon rank sum test for all other outcomes including blood pressure, weight, waist circumference, and lipid levels.
All analyses were carried out using Stata 11 (College Station, TX).
Results
We approached 78 (out of a target 80) patients between August 2010 and October 2011, ultimately enrolling 75 into the study. Patients in the intervention arm were broadly similar to those in the control arm but trended toward being more likely to be male (P = .12), having a larger waist circumference (P = .053), and being on a different number of diabetes medications (P = .07; Table 1). Over the course of the trial, patients in the immediate ATNS group received a mean of 26 calls, or 2.2 per week; they completed, on average, just 10 of these calls (40%), 0.8 per week.
Table 1.
Baseline Parameters, Demographics, and Treatment.
| Parameter | Control (n = 37) | Intervention (n = 38) | P a |
|---|---|---|---|
| Age, mean (SD) | 53 (12) | 51 (12) | .44 |
| Sex, number female (%) | 12 (32%) | 19 (50%) | .12 |
| Diabetes medications, number (%)b | .07 | ||
| 0 | 6 (16%) | 2 (5%) | |
| 1 | 7 (19%) | 16 (42%) | |
| 2 | 20 (54%) | 14 (37%) | |
| 3 | 4 (11%) | 6 (16%) | |
| Taking Insulin, number (%) | 10 (27%) | 15 (39%) | .25 |
| SBP (mm Hg) | 125 (12) | 123 (15) | .31 |
| DBP (mm Hg) | 73 (7) | 72 (10) | .49 |
| BMI (kg/m2) | 33 (7) | 35 (10) | .22 |
| WC (cm) | 41 (7) | 43 (6) | .053 |
| A1c (%) | 8.9 (1.3) | 9.2 (1.9) | .69 |
| TChol (mg/dL) | 176 (43) | 166 (30) | .42 |
| TG (mg/dL) | 182 (154) | 181 (111) | .44 |
| HDL (mg/dL) | 46 (13) | 44 (11) | .95 |
| LDL (mg/dL) | 125 (12) | 123 (15) | .31 |
| Lost to follow-up | 11 (30%) | 15 (39%) | .38 |
BMI, body mass index; DBP, diastolic blood pressure; HbA1c, hemoglobin a1c percentage; HDL, high density lipoprotein; LDL, low density lipoprotein; NR, not reported; SBP, systolic blood pressure.
For sex, number of diabetes medications, number on insulin, and lost to follow-up, by chi-square test; for all others, by rank sum test.
Oral hypoglycemics not including insulin.
Despite numerous attempts to contact patients for follow-up, only 49, or 65%, completed the second and final visit. Primary, non–mutually exclusive reasons for loss to follow-up included “phone disconnected (and no alternate number)” (4 patients), “refused to follow-up (reason not specified)” (4), “did not ever respond to messages left” (5), and “never showed up despite scheduling an appointment” (5). Patients who completed follow-up were similar to those who did not in baseline characteristics except for having higher blood pressure (Supplemental Table 1), with statistically similar numbers lost to follow-up in both the intervention and control groups.
The outcomes of the randomized controlled trial (RCT) are shown in Table 2. Of the 49 patients who followed up at week 12, 26 were in the control group and 23 were in the intervention group. There were no statistically or clinically significant differences between these 2 groups in changes in A1c (–0.3% in the control group, –0.1% in the intervention group), or in any other anthropometric or laboratory measure.
Table 2.
Results.
| Control, change at 12 weeks | Intervention, change at 12 weeks | P valuea | |
|---|---|---|---|
| SBP (mm Hg) | −2 | +4 | .43 |
| DBP (mm Hg) | −1 | +1 | .93 |
| BMI (kg/m2) | −0.1 | +0.4 | .21 |
| WC (cm) | +0.3 | +0.0 | .31 |
| A1c (%) | −0.3 | −0.1 | .41 |
| TChol (mg/dL) | −1 | +4 | .7 |
| TG (mg/dL) | −4 | −17 | .55 |
| HDL (mg/dL) | −1 | −2 | .75 |
| LDL (mg/dL) | −2 | +9 | .08 |
A1c, hemoglobin A1c; BMI, body mass index; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; RCT, randomized controlled trial; SBP, systolic blood pressure; TChol, total cholesterol; TG, triglycerides; WC, waist circumference.
By rank sum test of difference in change between the 2 arms.
Discussion
Despite a successful pilot study in Mexico, we found that a multidimensional intervention deploying video-based and in-person nutrition counseling and subsequently ATNS to promote reductions in high-glycemic food intake had no clinically significant effect on glycemic control and waist circumference for Spanish-speaking community-dwelling adults in care at a large federally qualified health center in Oakland, California. Moreover, patients in the intervention arm did not engage significantly with the intervention, rarely completing the calls they received, and in general following up at a disappointingly low rate despite several attempts to reach them and an incentive for doing so.
There have been multiple studies attempting to improve diabetes control through telephone calls with mixed results.15,16,26,27 This approach often requires both a high degree of motivation among patients and the expense of dedicating nursing or staff time to perform this activity. As information technology and particularly patient-facing information technology continues to improve, interventions seeking to improve patient lifestyle choices without the same expense have proliferated;28,29 yet, most of these interventions continue to rely on patients feeling motivated enough to use the application.
Failure to translate a successful intervention from Mexico to the United States likely represents major differences between the study populations in Mexico and the United States, leading to a mismatch between the intervention design and the health care needs of the target population (Supplemental Table 2). Patients in the Mexico pilot had a higher A1c at baseline (9.8 vs 9.0 averaged across the 2 arms in the current study) and none were using insulin therapy (compared with 27% in the current study). Perhaps even more important than the differences in disease severity was our likely selection of groups differing in Prochaska’s stages of change with regard to nutrition and behavioral modification.30 In Mexico, we recruited community-dwelling, motivated volunteers with limited access to health care, who were eager for more health care advice and support. In contrast, in the United States RCT we recruited volunteers from a diabetes registry of patients who—for a variety of reasons—had uncontrolled diabetes despite attempts at improving glycemic control with medical therapy and physician and dietitian counseling. Thus, in Mexico, a simple culturally concordant, information-based intervention with reminders through the ATNS seemed to meet a critical need among this population; whereas in the United States, usual medical care had not been sufficient to help them achieve control of their diabetes, and neither was the ATNS intervention.
It is possible that the immigrant experience of the US patients could make diabetes management a low priority concern for patients—when faced with issues around deportation, crime and social stress that are likely to be less common or severe in the small, indigenous pueblo of Ahuatepec. The lack of familia and other community networks that are important buffers to stress and anxiety31 are often hard to establish for immigrants in poor urban communities. However, this is total conjecture since we did not measure these factors in our study.
Failure of the ATNS intervention to improve glycemic control in the US study may also be due to modifications that were made to accommodate the randomized controlled trial study design. In the Mexico pilot, there was a period (6 weeks) during which a live person was handling follow-up, whereas in the RCT there was only ATNS. There may have been some trust and engagement created by the live person calls that were never established in the US study. Because A1c continued to fall after the calls were transitioned from the live person to the ATNS, and because we wanted to assess ATNS alone (a much less costly and scalable program than one requiring nursing or dietitian time), we did not include any component of live person phone calls in our follow-up. The other key intervention difference that could explain some of the difference in intervention impact was the live, group educational session led by a dietitian in the Mexico pilot versus the DVD educational module used in the US RCT.
Our study has important limitations. The ATNS intervention was delivered entirely automatically, and there was limited engagement with individual phone calls. A shorter call, or one for which patients had been better prepared prior to receiving it for the first time (perhaps with an in-person walk-through) might have been more effective, though these modifications may have reduced the generalizability of an otherwise low-cost, “easy” intervention. Moreover, there was significant loss to follow-up despite several attempts to reach patients. The clinic population is known to travel frequently, which may have played a role both in driving down engagement and also reducing our ability to assess our patients at a return visit.
In summary, in the current RCT community-dwelling Spanish-speaking adults living in the Oakland area did not experience any benefit from a seemingly promising, low-cost ATNS intervention. The failure to translate evidence generated in Mexico to the United States, while disappointing, underscores the importance of tailoring health care interventions to one’s target audience and specific population. Further work is needed to better understand the ideal settings in which an ATNS system is beneficial, as if it can be shown to be effective in these other settings, its cost and scalability will be particularly attractive as an adjunct to disease management programs for patients with diabetes in limited resource settings.
Footnotes
Abbreviations: A1c, hemoglobin A1c %; ATNS, automated telephone nutrition support; FQHC, federally qualified health center; IVR, interactive voice response; RCT, randomized controlled trial; T2DM, type 2 diabetes; UCSF, University of California, San Francisco.
Authors’ Note: Preliminary results of this study were presented at the 32nd annual meeting of the Society for General Internal Medicine, May 13, 2009, Miami Beach, FL, and at the 35th annual meeting of the Society for General Internal Medicine, May 10, 2012, Orlando, FL.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a UCMEXUS Visiting Scholar Award (RG) and a UCMEXUS-CONACYT Collaborative Research Grant (RG, SB). Work done in the United States was supported by Department of Medicine discretionary account (RG). DS was supported by the National Institute of Diabetes and Digestive and Kidney Diseases for Diabetes Translational Research (CDTR) at Kaiser Permanente and University of California, San Francisco (P30DK092924). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
References
- 1. Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006;113:2914-2918. [DOI] [PubMed] [Google Scholar]
- 2. National Institutes of Health. National diabetes statistics, 2011. Available at: http://diabetes.niddk.nih.gov/dm/pubs/statistics/dm_statistics.pdf. Accessed September 9, 2014.
- 3. Beard HA, Al-Ghatrif M, Samper-Ternent R, Gerst K, Markides K. Trends in diabetes prevalence and diabetes-related complications in older Mexican Americans from 1993/1994 to 2004/2005. Diabetes Care. 2009;32:2212-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Markides KSEK, Ray LA, Peek MK. Census disability rates among older people by race/ethnicity and type of Hispanic origin. In: Angel JL, Whitefield KE, eds. Health of Aging Hispanics. New York, NY: Springer; 2007:26-39. [Google Scholar]
- 5. Graham JE, Stoebner-May DG, Ostir GV, et al. Health related quality of life in older Mexican Americans with diabetes: a cross-sectional study. Health Qual Life Outcomes. 2007;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519-2527. [DOI] [PubMed] [Google Scholar]
- 7. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 8. United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83-88. [PMC free article] [PubMed] [Google Scholar]
- 9. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] [Google Scholar]
- 10. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103-117. [DOI] [PubMed] [Google Scholar]
- 11. Lopez-Quintero C, Berry EM, Neumark Y. Limited English proficiency is a barrier to receipt of advice about physical activity and diet among Hispanics with chronic diseases in the United States. J Am Diet Assoc. 2009;109:1769-1774. [DOI] [PubMed] [Google Scholar]
- 12. Eakin EG, Reeves MM, Winkler E, et al. Six-month outcomes from living well with diabetes: a randomized trial of a telephone-delivered weight loss and physical activity intervention to improve glycemic control. Ann Behav Med. 2013;46:193-203. [DOI] [PubMed] [Google Scholar]
- 13. Oh JA, Kim HS, Yoon KH, Choi ES. A telephone-delivered intervention to improve glycemic control in type 2 diabetic patients. Yonsei Med J. 2003;44:1-8. [DOI] [PubMed] [Google Scholar]
- 14. Schechter CB, Cohen HW, Shmukler C, Walker EA. Intervention costs and cost-effectiveness of a successful telephonic intervention to promote diabetes control. Diabetes Care. 2012;35:2156-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piette JD. Interactive voice response systems in the diagnosis and management of chronic disease. Am J Managed Care. 2000;6:817-827. [PubMed] [Google Scholar]
- 16. Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000;108:20-27. [DOI] [PubMed] [Google Scholar]
- 17. Piette JD, Weinberger M, McPhee SJ. The effect of automated calls with telephone nurse follow-up on patient-centered outcomes of diabetes care: a randomized, controlled trial. Med Care. 2000;38:218-230. [DOI] [PubMed] [Google Scholar]
- 18. Schillinger D, Handley M, Wang F, Hammer H. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care. 2009;32:559-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Handley MA, Shumway M, Schillinger D. Cost-effectiveness of automated telephone self-management support with nurse care management among patients with diabetes. Ann Fam Med. 2008;6:512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fabricatore AN, Wadden TA, Ebbeling CB, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract. 2011;92:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludwig DS, Ebbeling CB. Weighing the data in studies of the glycaemic index. Int J Obes. 2008;32:1190. [DOI] [PubMed] [Google Scholar]
- 22. Ventura E, Davis J, Byrd-Williams C, et al. Reduction in risk factors for type 2 diabetes mellitus in response to a low-sugar, high-fiber dietary intervention in overweight Latino adolescents. Arch Pediatr Adolesc Med. 2009;163:320-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261-2267. [DOI] [PubMed] [Google Scholar]
- 24. Neuhouser ML, Thompson B, Coronado GD, Solomon CC. Higher fat intake and lower fruit and vegetables intakes are associated with greater acculturation among Mexicans living in Washington State. J Am Diet Assoc. 2004;104:51-57. [DOI] [PubMed] [Google Scholar]
- 25. Green LW, Kreuter M. Health Program Planning: An Educational and Ecological Approach. 4th ed. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 26. Navicharern R, Aungsuroch Y, Thanasilp S. Effects of multifaceted nurse-coaching intervention on diabetic complications and satisfaction of persons with type 2 diabetes. J Med Assoc Thailand/Chotmaihet thangphaet. 2009;92:1102-1112. [PubMed] [Google Scholar]
- 27. Nunn E, King B, Smart C, Anderson D. A randomized controlled trial of telephone calls to young patients with poorly controlled type 1 diabetes. Pediatr Diabetes. 2006;7:254-259. [DOI] [PubMed] [Google Scholar]
- 28. Rusin M, Arsand E, Hartvigsen G. Functionalities and input methods for recording food intake: a systematic review. Int J Med Inform. 2013;82:653-664. [DOI] [PubMed] [Google Scholar]
- 29. Hieftje K, Edelman EJ, Camenga DR, Fiellin LE. Electronic media-based health interventions promoting behavior change in youth: a systematic review. JAMA Pediatr. 2013;167:574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prochaska JO, DiClemente CC. Self change processes, self efficacy and decisional balance across five stages of smoking cessation. Prog Clin Biol Res. 1984;156:131-140. [PubMed] [Google Scholar]
- 31. Jung M, Choi M. Impact of community capacity on the health status of residents: understanding with the contextual multilevel model. Health Care Manag (Frederick). 2013;32:77-86. [DOI] [PubMed] [Google Scholar]


