Abstract
Although test strips for self-monitoring of blood glucose (SMBG) represent around 50% of diabetes treatment cost in Argentina, little is known about their current use and relationship with different types of treatment. We therefore aimed to estimate the current use of test strips and identify the major use drivers and the percentage they represent of total prescription costs in 2 entities of the social security system (SSS) of Argentina. Observational retrospective study measuring test strip prescriptions delivered by pharmacies from the province of Buenos Aires (8115 records collected during 3 months provided by the Colegio de Farmacéuticos de la Provincia de Buenos Aires) of affiliates with type 2 diabetes (T2DM) from 2 large entities of the SSS system. The average monthly test strips/patient used for SMBG was 97.5 ± 70.1. This number varied according to treatment: monotherapy with oral antidiabetic drugs (OAD) < combined OAD therapy < insulin treatment. Test strips represented a higher percentage of the total prescription cost in people under OAD monotherapy (84.6%) and lower in those with insulin analogs (46.9%). In our population, the type of hyperglycemia treatment was the main driver of test strip use for SMBG and its impact on the total prescription cost depends on the kind of such treatment. Since it has been shown that patients’ education and prescription audit can optimize test strip use and treatment outcomes, implementation of such strategies could appropriately support, optimize, and reduce ineffective test strip use in people with T2DM.
Keywords: prescription audit, self-monitoring blood glucose, self-monitoring blood glucose relative cost, type 2 diabetes treatment
Self-monitoring of blood glucose (SMBG) is a major component of diabetes management; it provides information about current glycemic status, which is necessary to trigger immediate treatment adjustments to improve glucose control. Its incorporation into daily practice has represented an important step forward in diabetes care, because it helps to optimize treatment outcomes1-3 and promotes the active participation of patients in the control and treatment of their disease, the development of self-confidence, and motivation.4,5 Consequently, test strip use for SMBG has risen sharply over the past decade,6 leading to an increase in the cost of the metabolic control of people with diabetes.7 In fact, the cost of test strips can represent up to 41.6% of the total pharmacy cost.8 On the other hand, reported evidence has shown that SMBG would facilitate the achievement of treatment goals and decrease the costs of care.9,10
While the benefits of SMBG in people with type 1 (T1DM) or type 2 diabetes (T2DM) treated with insulin are widely accepted, its performance in patients with T2DM treated with oral antidiabetic drugs (OAD) or lifestyle changes has been seriously challenged.11 Given the high cost of the current T2DM care and the fact that its prevalence continues to grow worldwide, many efforts have been made to determine whether economic support to SMBG in non-insulin-treated T2DM patients is justified and effectively applied. Despite these concerns, test strip use in real world conditions and its financial implications have not been extensively explored. The issue is important particularly in developing countries, where health budgets are not always sufficient to cope with the constant demand of care for costly chronic diseases such as diabetes. However, neither the affordability of SMBG cost in these countries nor the implementation of any policy to cover its cost can be established when there are no current use and cost data.
In an attempt to provide such evidence, we currently studied test strip use for SMBG and its relative impact on total prescription cost (OAD and insulin) to treat people with diabetes in the social security system (SSS) of Argentina.
Methods
We performed an observational retrospective study collecting information from prescription data of 2 entities belonging to the SSS, provided by the Colegio de Farmacéuticos de la Provincia de Buenos Aires (COLFARMA).
COLFARMA systematically records online all the test strip prescriptions delivered by the pharmacies of the province of Buenos Aires to the SSS affiliates at a variable out-of-pocket cost. This organization provided 8297 anonymous registries of affiliates from 2 large entities of the SSS; the data included drug and test strip prescriptions collected in the province during 3 months (February to April 2012). Before starting the statistical analysis of these data, we reviewed the whole database searching for atypical values (number of test strips beyond a reasonable daily use and based on an objective cut-off value). To identify those values, we applied a common statistical model using the interquartile ranges (IQRs), defining an outlier as any observation outside the range [Q1 – (k × IQR); Q3 + (k × IQR)], with Q1, Q3 = first and third quartiles, IQR = Q3 − Q1. We set k = 3 to only remove “extreme” outliers in the data. Accordingly, we considered as atypical utilization more than 300 test strips per month for people treated with insulin, and more than 200 strips per month for those treated only with OADs. These values were considered as the consequence of data loading mistakes. Using this criterion, we excluded 182 records, thus including 8115 records in the final statistical analysis.
Statistical analyses were done using the Statistical Package for Social Sciences version 15 (SPSS Inc, Chicago, IL, USA). Descriptive statistics are presented as percentages with 95% confidence intervals (CIs) and mean ± standard deviation (SD). Group comparisons for continuous variables were performed by ANOVA, Student’s t test, Mann–Whitney U test and Kruskal–Wallis test according to the data distribution profile. Chi-square test was performed for proportions. The level of significance was established at P ≤ .05.
We also estimated the impact of test strip use on total prescription cost for hyperglycemia treatment. For that purpose, we designed a model that represents an average of prescriptions provided by COLFARMA. The monthly cost of a given medical prescription was estimated by micro-costing. Drug and test strip costs were obtained from Alfabeta.net, a private Internet database which is the main source of pharmaceutical product pricing in the Argentine market. Values are expressed in Argentine pesos as of December 2012.
Results
The average monthly use of test strips/person was 97.5 ± 70.1 (Table 1); 75.4% of patients were treated with either insulin alone or associated with OADs. The average monthly test strip use was larger in people treated with insulin than in those treated only with OADs (P < .001). In the latter group, 52.9% of patients were treated with a single OAD and the number of test strips was lower than that of patients treated with a combination of OAD (P < .001).
Table 1.
COLFARMA Data: Test Strip Use for SMBG According to Type of Treatment.
| Mean | SD | Median | IQR | n | P | |
|---|---|---|---|---|---|---|
| DM total | 97.5 | 70.1 | 67 | 33-133 | 8115 | — |
| Treatment | ||||||
| Only OADs | 70.2 | 46.3 | 67 | 33-100 | 1973 | <.001* |
| Insulin (with or w/o OADs) | 106.3 | 74.1 | 67 | 50-133 | 6142 | |
| Only OADs | ||||||
| Monotherapy | 66.2 | 45.0 | 67 | 33-83 | 1043 | <.001* |
| Combined therapy | 74.7 | 47.3 | 67 | 33-100 | 930 | |
| Insulin with OADs | 89.0 | 62.5 | 67 | 33-133 | 1313 | — |
| Only insulin | ||||||
| Long acting (LA) | 112.0 | 76.2 | 100 | 67-167 | 4200 | .037† |
| Crystalline (C) | 105.9 | 79.4 | 67 | 33-133 | 496 | |
| LA + C | 98.4 | 65.4 | 83 | 50-133 | 133 |
DM, diabetes; IQR, interquartile range; OAD, oral antidiabetic drugs. *Student’s t test. †ANOVA.
Within the group of patients treated only with insulin, 87% used only long-acting insulin, 10% used only crystalline insulin, and 3% used both (basal bolus). Test strip use in the last group was significantly lower than that of patients using only long-acting insulin (P = .042) (Table 1).
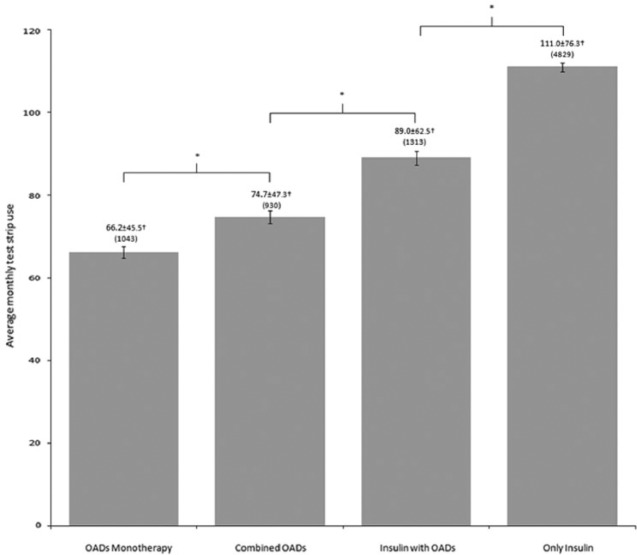
People treated with a combination of insulin plus OAD used significantly less test strips than those treated only with insulin (P < .001; Figure 1), regardless of the type of insulin used.
Figure 1.
COLFARMA test strip use according to type of treatment. Values represent the mean ± SD. Number of cases in brackets. Error bars represent standard error of the mean (SEM). † P < .001 (ANOVA; F statistic = 178.97) between all groups. *Statistically significant (P < .001) between the indicated groups.
Impact of Test Strip on the Total Cost of the Medical Prescription
Test strips for SMBG represented in average 66.3% of the total cost of each physician prescription. This percentage varied according to the type of treatment and the frequency of SMBG. While the highest value was achieved with OAD monotherapy (84.6%), it decreased as a function of the number of drugs used to treat hyperglycemia, reaching the lowest in cases of intensified insulin therapy with analogs (46.9%; Table 2).
Table 2.
Impact of Test Strip Use for SMBG on the Total Cost of a Given Treatment Prescription.
| Prescription | Monthly cost (Argentine pesos) | Test strips (%) |
|---|---|---|
| Metformin (850 mg × 2)—Glucophage 850® Strips—Accu-Chek Performa 25® (COLFARMA: 66.2) |
90.6 497.3 |
84.6 |
| Total | 587.9 | |
| Metformin (850 mg × 2)—Glucophage 850® Gliclazide (80 mg 2/day)—Diamicron® Strips—Accu-Chek Performa 25® (COLFARMA: 74.7) Total |
90.6 106.0 561.1 757.7 |
74.0 |
| DPP4i + Metformin (50/850 mg 2/day)—Janumet® Strips—Accu-Chek Performa 25® (COLFARMA: 74.7) Total |
336.1 561.1 897.2 |
62.5 |
| NPH Insulin (30 IU/day)—Insulatard® Strips—Accu-Chek Performa 50® (COLFARMA: 106.3) Total |
187.6 632.3 819.9 |
77.1 |
| Insulin Analog (30 IU/day)—Levemir® Strips—Accu-Chek Performa 50® (COLFARMA: 106.3) Total |
562.5 632.3 1194.8 |
52.9 |
| Insulin Analog—Basal (20 IU/day)—Levemir® Insulin Analog—Bolus (20 IU/day)—Humalog® Strips—Accu-Chek Performa 50® (COLFARMA: 106.3) Total |
375.0 342.0 632.3 1349.3 |
46.9 |
Discussion
This study analyzed the use of test strips for SMBG in a group of people with diabetes whose care and treatment is covered by 2 different entities of the SSS in Argentina. Results show that the monthly use of SMBG test strips was significantly associated with the type of hyperglycemia treatment considered: while it was higher in people receiving insulin, followed by those treated with a combination of OAD, the lower figures corresponded to people receiving a single OAD. Thus, under our study conditions, type of treatment was apparently an important driver of the SMBG frequency and test strip use pattern.
SMBG is considered a core component of effective diabetes self-management of either T1DM or insulin-treated T2DM patients. Accordingly, the 2013 American Diabetes Association (ADA) guidelines for SMBG frequency state that “patients on intensive insulin regimens might perform SMBG at least before meals and snacks, as well as occasionally after meals, at bedtime, before exercise and before critical tasks, when hypoglycemia is suspected and after treating hypoglycemia until normoglycemia is achieved.”12 This recommendation means that these patients might frequently—though not regularly—perform 8 or more daily tests. These figures are far from those currently recorded in our insulin-treated patients.
On account of the ADA and other recommendations, the use and frequency of SMBG has progressively increased in the last decade, facilitating diabetes self-management and patient empowerment, but also increasing its economic cost.6 While there is general agreement regarding SMBG benefits for people with T1DM or T2DM treated with insulin, there are divergent views on such benefits in people with T2DM on oral treatment. Three recent reviews have shown mixed opinions on the issue; 1 of them does not find any benefit,11 and the other 2 do, but only under certain circumstances, that is, SMBG is useful in non-insulin-treated T2DM patients only when the glucose information is understood correctly and accurately interpreted by the patient, and the process results in therapy adjustments.13,14 A similar position was adopted by the International Diabetes Federation (IDF) and the Canadian Diabetes Association (CDA) in their guidelines for SMBG.15,16 The ADA also recommends that clinicians must not only educate these people on how to interpret their SMBG data, but they should also reevaluate the ongoing need for and frequency of SMBG at each routine visit.12 Therefore, most organizations share the concept that it is worth using SMBG in people with T2DM not treated with insulin only when it results in a proactive therapeutic adjustment, that is, when patients have been educated to make their own appropriate therapeutic decisions. Unfortunately, it has been shown that when blood glucose is high or low, many patients do nothing, and only some of them take an active attitude toward its correction.17 In those cases, SMBG increases the cost of care, but it does not necessarily help to achieve treatment targets.
A lower SMBG frequency has been recorded among people paying higher out-of-pocket expenditures for test strips or having limited access to them.18,19 This would not be the case in our study because we did not find any significant association between these 2 variables, despite the different percentage of coverage within the SSS.
In brief, our study provides important data to estimate the cost and the affordability of SMBG potential full coverage either in entities of our SSS or from other countries with similar health care characteristics. In addition, and on account of previous reports, it could serve to associate SMBG to patient education and prescription audit to optimize their favorable impact on treatment outcomes and to decrease ineffective test strip use.20 Further studies are necessary to obtain a complete view of the impact of SMBG on treatment outcomes and establish a reasonable coverage policy based on real world needs and benefits of its use in developing countries.
Limitations
Although our results provide information that was not previously available, they should be considered with caution because (1) the data analyzed were obtained from an observational retrospective study performed in a population from 2 entities of our SSS rather than from a population-based study, and (2) as previously mentioned, data included only test strip use and type of diabetes, but not clinical or laboratory results. Thus, it was not possible to evaluate the impact of SMBG on treatment goal achievement.
Acknowledgments
The authors greatly appreciate the contribution of the Colegio de Farmacéuticos de la Provincia de Buenos Aires authorities. They also thank Andrea Diaz Vidal for her active participation in data loading and processing and Adriana Di Maggio for careful manuscript editing.
Footnotes
Abbreviations: ADA, American Diabetes Association; CI, confidence interval; COLFARMA, Colegio de Farmacéuticos de la Provincia de Buenos Aires; IQR, interquartile range; OAD, oral antidiabetic drugs; SD, standard deviation; SMBG, self-monitoring of blood glucose; SSS, social security system; T1DM, type 1 diabetes; T2DM, type 2 diabetes.
Declaration of Conflicting Interests: JFE, ER, and JJG are members of the Health Economics Research Unit at CENEXA. LG is a research fellow of the National University of La Plata. JJG is a member of the Research Career of CONICET. The authors declare that they have no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported with an unrestricted grant provided by Novo Nordisk.
References
- 1. Garg S, Hirsch IB. Self-monitoring of blood glucose. Int J Clin Pract Suppl. 2010;166:1-10. [DOI] [PubMed] [Google Scholar]
- 2. Klonoff DC, Blonde L, Cembrowski G, et al. Consensus report: the current role of self-monitoring of blood glucose in non-insulin-treated type 2 diabetes. J Diabetes Sci Technol. 2011;5:1529-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virdi N, Daskiran M, Nigam S, Kozma C, Raja P. The association of self-monitoring of blood glucose use with medication adherence and glycemic control in patients with type 2 diabetes initiating non-insulin treatment. Diabetes Technol Ther. 2012;14:790-798. [DOI] [PubMed] [Google Scholar]
- 4. Fisher L, Polonsky WH, Parkin CG, Jelsovsky Z, Petersen B, Wagner RS. The impact of structured blood glucose testing on attitudes toward self-management among poorly controlled, insulin-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96:149-155. [DOI] [PubMed] [Google Scholar]
- 5. Schnell O, Alawi H, Battelino T, Jelsovsky Z, Petersen B, Wagner RS. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther. 2011;13:959-965. [DOI] [PubMed] [Google Scholar]
- 6. Evans JM, Mackison D, Emslie-Smith A, Lawton J. Self-monitoring of blood glucose in type 2 diabetes: cross-sectional analyses in 1993, 1999 and 2009. Diabet Med. 2012;29:792-795. [DOI] [PubMed] [Google Scholar]
- 7. Gagliardino JJ, de la Hera M, Siri F. Grupo de Investigacion de la Red Qualidiab. Evaluación de la calidad de la asistencia al paciente diabético en America Latina. Rev Panam Salud Publica/Pan Am J Public Health. 2001;10:309-317. [DOI] [PubMed] [Google Scholar]
- 8. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635-2645. [DOI] [PubMed] [Google Scholar]
- 9. Gagliardino JJ, Olivera E, Etchegoyen GS, et al. PROPAT: a study to improve the quality and reduce the cost of diabetes care. Diabetes Res Clin Pract. 2006;72:284-291. [DOI] [PubMed] [Google Scholar]
- 10. Gagliardino JJ, Etchegoyen G. PEDNID-LA Research Group. A model education program for people with type 2 diabetes: a cooperative Latin American implementation study (PEDNID-LA). Diabetes Care. 2001;24:1001-1007. [DOI] [PubMed] [Google Scholar]
- 11. Malanda UL, Bot SD, Nijpels G. Self-monitoring of blood glucose in noninsulin-using type 2 diabetic patients: it is time to face the evidence. Diabetes Care. 2013;36:176-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association Position Statement. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(suppl 1):S11-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parkin CG, Buskirk A, Hinnen DA, Axel-Schweitzer M. Results that matter: structured vs. unstructured self-monitoring of blood glucose in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:6-15. [DOI] [PubMed] [Google Scholar]
- 14. Benhalima K, Mathieu Ch. The role of blood glucose monitoring in non-insulin treated type 2 diabetes: what is the evidence? Primary Care Diabetes. 2012;6:179-185. [DOI] [PubMed] [Google Scholar]
- 15. International Diabetes Federation Clinical Guidelines Taskforce and International SMBG Working Group. Global guideline on self-monitoring of blood glucose in noninsulin treated type 2 diabetes; 2009. www.idf.org. Accessed March 1, 2011.
- 16. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(suppl 1):S1-S201. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Zgibor J, Matthews JT, Charron-Prochownik D, Sereika SM, Siminerio L. Self-monitoring of blood glucose is associated with problem-solving skills in hyperglycemia and hypoglycemia. Diabetes Educ. 2012;38:207-218. [DOI] [PubMed] [Google Scholar]
- 18. Karter AJ, Ferrara A, Darbinian JA, Ackerson LM, Selby JV. Self-monitoring of blood glucose. Language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23:477-483. [DOI] [PubMed] [Google Scholar]
- 19. Kjome RLS, Granas AG, Nerhus K, Roraas TH, Sandberg S. The prevalence of self-monitoring of blood glucose and costs of glucometer strips in a nationwide cohort. Diabetes Technol Ther. 2010;12:701-705. [DOI] [PubMed] [Google Scholar]
- 20. Giaccari A, Grassi G, Ozzello A. Self-monitoring of blood glucose: guideline application rather than utilization restrictions on testing strips has potential to reduce diabetes healthcare costs in Italy. Diabetes Technol Ther. 2012;14:862-867. [DOI] [PubMed] [Google Scholar]