Abstract
We analyzed the pretransplant continuous glucose monitoring (CGM) data of 45 patients that underwent total pancreatectomy followed by autologous islet transplantation (AIT) at the University of Arizona Medical Center. Traditional and novel metrics of CGM time series were correlated to the total islet count (TIC), islet equivalents (IEQs), and weight-normalized IEQs (IEQ/kg). In a subset cohort (n = 26) we analyzed the relationship among the infused number of islets, the CGM indicators, and the first recorded insulin requirement after the procedure. We conclude that receiving a high islet yield is sufficient yet not necessary to achieve low or null insulin requirements within the first 50 days after surgery. Furthermore, CGM inertia and CGM length of curve (2 novel CGM indicators) are shown to be correlated to islet yield, and the CGMs normalized area (Ao) and time ratio above hyperglycemic level (To) are strongly correlated to insulin requirement. A screening test based on To is shown to have 100% sensitivity and 88% specificity discriminating insulin independence upon discharge.
Keywords: continuous glucose monitoring, autologous islet transplantation, chronic pancreatitis, pancreatectomy
Chronic pancreatitis (CP) is a major medical problem with few available medical therapies or reliable, long-term surgical options. Total or near total pancreatectomy is the most effective means of alleviating intractable pain in CP patients.1,2 Autologous islet transplantation (AIT) has the potential to minimize the major drawback of pancreatectomy, brittle diabetes, and thus greatly enhance the expected quality of life. AIT immediately following pancreatectomy is now recognized as one of the most effective symptomatic treatments for patients with CP.3,4 Even though AIT is well established, its success depends delicately on many factors at each step of the process. Characterizing these factors has always been a major concern for both autologous and allogenic transplants.5 It is widely accepted that there is a correlation of islet yield with postsurgical insulin requirements,4,6,7 as well as with the period of insulin independence posttransplantation.8 This has justified considerable effort to find clinical predictors of yield to indirectly assess the probabilities of successful engraftment and survival.9-12 From our experience at the University of Arizona with 61 AIT patients, this trend was observed but was not exclusive to high IEQ groups, as some patients who received lower yields required relatively low doses of insulin at discharge. This stresses the role of other factors besides current islet count assessments in outcome. A similar observation was published by Schrader et al,13 in which the eventual development of diabetes after partial pancreatectomy was not found to correlate to the fractional beta cell mass determined directly from tissue samples.
In this study, we examine the use of continuous glucose monitoring (CGM) as a possible preoperatory indicator of islet function and islet yield. We also reevaluate the relationship between transplanted islet mass and postsurgical glycemic control as per our experience.
Methods
A group of 45 patients (group I) was selected from the total 61 that were transplanted at our center at the University of Arizona Medical Center from 2009 to 2013 based on the availability of satisfactory CGM and laboratory records within 90 days pretransplant. The information gathered from these CGM time series was analyzed and correlated with the patients’ total islet count (TIC), islet equivalents (IEQs), and weight-normalized islet equivalents (IEQs/kg). These same CGM coefficients were also correlated to the first daily average insulin recorded within the first 50 days (first insulin average, FIA) after surgery for the subset (group II, n = 26) of group I that had such records. Patients were instructed to try to maintain at most a 120 mg/dL fasting glucose target. FIA is the first sliding-scale + carb-counting basal daily insulin units average that patients reported at their weekly clinical examination after discharge from hospital. None of these patients were on parental or enteral feeds posttransplant. No adjunct therapies such as Exenetide or dipeptidyl peptidase were used. The 50-day interval was deemed to be indicative of the immediate success of the AIT, even if in the literature there is a subset of patients whose β cell functionality improved over time. It should be added that this quantity excludes the drip insulin that patients are given postsurgically to induce islet metabolic rest.14,15
Continuous Glucose Monitoring
Blood glucose (BG) data from CGM devices (iPro, Medtronic, Northridge, CA ) were analyzed for patients who wore the device for more than 2 consecutive days (mean and standard deviation of total days device was worn 4.20 ± 0.87). Some patients reported interruptions in the use of the CGM. None of these brief discontinuities were deemed to hinder our study, since we are treating the BG variations as a steady process. Capillary BG was monitored every 6 hours. CGMs registered an average interstitial glucose level every 5 minutes and thus yielded time series {BGt } consisting of thousands of points from which a number of coefficients were derived. The following preliminary definitions are needed: the BG rate of change is defined as Δ BGt= BGt+1 BGt, the time interval between the t-th and the t+1-th measurements is denoted (in this study minutes for all i), and N denotes the total number of BG measurements in each series.
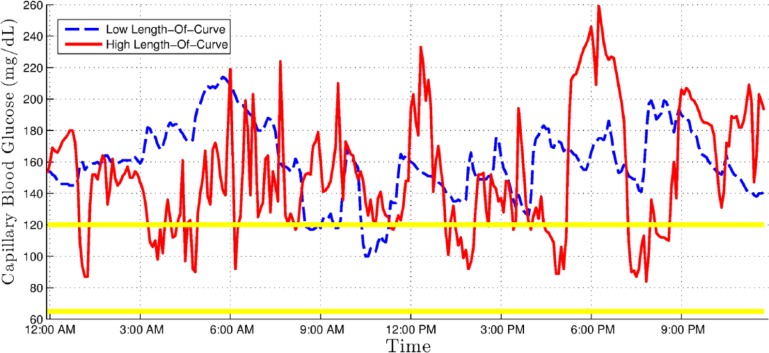
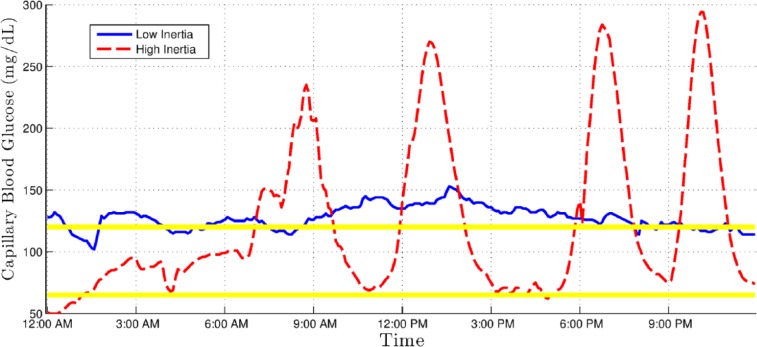
All of these coefficients are commonplace in clinical practice and have been previously described in the literature,16,17 except for the CGM length of curve (LoC) and CGM inertia (CGMI) values, which were developed by our group. Table 1 lists these metrics, gives a short description, and contains the strict mathematical definition or a reference to it. LoC is essentially the length of the polygonal formed by the graph of a CGM series. It is then normalized by dividing it by the sum of all the time intervals, that is, by the total time. It addresses the need to characterize broad and quick glycemic excursions that wouldn’t appreciably change the more commonly used area over/under curve or time over/under limit markers. Figure 1 shows 2 CGM series with similar area under curve but high and low CGM LoC. CGMI is a complementary measurement to LoC. It is defined as the ratio of all the consecutive pairs of BG rate of change values with identical signs, to the total number of such pairs. In other terms, CGMI increases for every pair of consecutive BG measurements in which both increased or both decreased relative to the previous measurement. Thus, a hypothetical BG graph that steadily increases (or decreases) throughout the recorded time interval would have a CGMI value 1, whereas a one-to-one alternation of increases and decreases of BG would have a CGMI value of 0. This indicator therefore intuitively corresponds to the steadiness of glycemic control. Figure 2 shows 2 examples of patients showing similar area under curve but high and low CGMI values.
Table 1.
Different Metrics Derived From CGM Data.
| Metric | Definition/reference | Equation |
|---|---|---|
| CGM LoC | Normalized sum of the lengths of the polygonal segments formed by the CGM data over time | |
| CGMI | Ratio of BG changes (ΔBG) that followed the trend (positive or negative) of the previous value in time | |
| Area over limit | Normalized area between the upper BG threshold (120 mg/dL) and dL the BG values above this limit | |
| Area under limit | Normalized area between the lower BG threshold (65 mg/dL) and dL the BG values below this limit | |
| Time ratio over limit | Ratio of time spent over the hyperglycemic level (120 mg/dL) to total dL time | |
| Time ratio under limit | Ratio of time spent under the hypoglycemic level (65 mg/dL) to total dL time | |
| MAGE | Average of the size of glycemic excursions greater than 1 standard deviation of the entire BG range | See Molnar et al25 |
| BG average | Average BG value over the whole data range | |
| BG standard deviation | Standard deviation of the BG data | |
| BG rate of change standard deviation | Standard deviation of the consecutive changes of BG |
BG, blood glucose; CGM, continuous glucose monitoring.
Figure 1.
Continuous glucose monitoring (CGM) graph showing an example with low CGM length of curve (dashed line) and one with high CGM length of curve (solid line)
Figure 2.
Continuous glucose monitoring (CGM) graph showing an example with low CGM inertia (solid line) and one with high CGM inertia (dashed line).
It is noteworthy to point out that even though in principle the standard deviation of the rate of change and LoC represent different features of any given time series, they will be strongly correlated among sets of series whose rate of change have similar means. So, for our data LoC and CGM rate of change standard deviation are highly correlated with ρ = .91, P < .0001. Other noteworthy correlations are LoC and the standard deviation of BG (ρ = .86, P < .0001), and between CGMI and the standard deviation of BG (ρ = .60, P < .0001).
Autologous Islet Transplantation
The surgical procedure employed is described in detail by Rilo et al.3 The degree of islet liberation was determined by routine sampling by sequential in vitro staining with Dithizone (Diphenylthiocarbazone, Sigma-Aldrich, St Louis, MO, USA). Upon completion of digestion (ie, after islets are adequately liberated from the remaining exocrine tissue), the flow from the continuous enzymatic isolation device was rerouted to a separate, temperature controlled collecting flask. The majority of enzymatic reactions were quenched by diluting the islet-containing solution with 5% human serum albumin (HSA) and by lowering its temperature to approximately 7°C. None of the islet preparations in our series were purified to avoid further islet loss, since the vast majority of our cases display a high degree of exocrine atrophy and fibrosis. The islets were collected and washed in Roswell Park Memorial Institute 1640 media and then resuspended in HSA containing 70 IU/kg of heparin. This preparation was then loaded into one 600-ml blood infusion bag for intraportal infusion into the liver. Portal venous pressure was measured before, during, and after islet infusion. Arterial and central venous pressures were monitored in all patients.
Statistical Analysis
All correlations were Spearman rank coefficients. All values reported as mean ± standard deviation unless noted otherwise. Significance level was set at P < .05.
Results
The clinical characteristics of the groups are summarized in Table 2. All the coefficients listed in Table 1 were calculated for every patient in group I and then correlated to TIC, IEQs, and IEQ/kg. All the CGM indicators whose correlation coefficients showed significance (P < .05) are reported in Table 3.
Table 2.
Clinical Characteristics of Cohorts Pretransplant.
| Group I | Group II | |
|---|---|---|
| Total number (n) | 45 | 26 |
| Sex (n; M/F) | 18/27 | 11/15 |
| Age (years) | 37 ± 4.2 | 33 ± 8.5 |
| BMI | 24.5 ± 5.0 | 21.3 ± 0.7 |
| HbA1c (%) | 5.8 ± 0.3 | 5.4 ± 0.4 |
| C-Pep (ng/mL) | 1.9 ± 0.1 | 1.3 ± 0.1 |
| MAGE (mg/dL) | 46.2 ± 29.5 | 45.50 ± 27.3 |
| CGM Average (mg/dL) | 111.8 ± 13.5 | 111.7 ± 15.2 |
| Etiology (%) | ||
| Idiopathic | 60 | 58 |
| Alcohol | 11 | 19 |
| Familial | 11 | 15 |
| Pancreas divisum | 9 | 4 |
| Genetic/autoimmune/other | 9 | 4 |
| Beta score composition (%)26 | ||
| 8 | 60 | 61 |
| 7 | 31 | 31 |
| 6 | 7 | 8 |
| 5 | 2 | — |
Values are mean ± standard deviation, unless otherwise indicated.
Table 3.
Correlations Between CGM Metrics and Their Relationship to Quantitative Islet Indicators.
| Metric | TIC | IEQ | IEQ/kg |
|---|---|---|---|
| CGM length of curve | ρ = –.31 | — | — |
| CGM inertia | ρ = –.36** | ρ = –.35** | ρ = –.34* |
| Area over limit | — | ρ = –.32 | ρ = –.33 |
| Area under limit | — | — | — |
| Time over limit | — | ρ = –.31 | ρ = –.34* |
| Time under limit | — | — | — |
| MAGE | ρ = –.30 | — | — |
| BG average | — | — | ρ = –.31 |
| BG standard deviation | — | — | — |
| BG rate of change standard deviation | — | — | — |
Blank indicates P > .05; all other values are P < .05. *P < .025. **P < .02. IEQ, islet equivalents; TIC, total islet count.
It was clear from these first results that the CGM derived markers that better correlated to islet number or islet mass (IEQs) were CGMI and the 2 coefficients of the group that are directly associated with hyperglycemia, namely and .
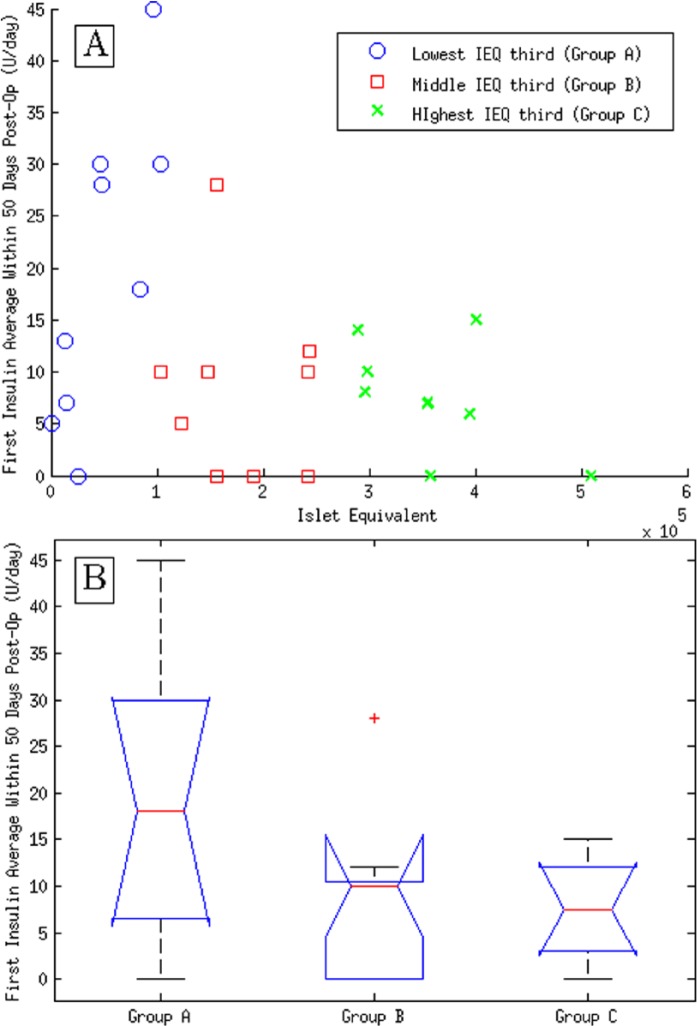
As previously mentioned, the general motivation to try to predict Islet yield stems from the fact that successful restoration of normoglycemia (as measured by off-insulin times or the average amount of insulin required) after AIT is significantly associated to greater values of the quantitative islet indicators (particularly IEQs11). This association was tested with the data from group II. Figure 3A shows a plot of the IEQ values against the FIA.
Figure 3.
(A) Plot of islet equivalents (IEQ) versus first insulin average (FIA) in group II. Different marker types designate the 3 different subsets corresponding to the lowest, middle, and highest IEQs. (B) Whisker plot of groups A, B, and C.
Patients in group II were divided in 3 subgroups corresponding to the lowest, middle, and highest IEQ scores. The null hypothesis that these groups show the same insulin requirement means was discarded by ANOVA at a significance level of P < .05 (lower inset of Figure 3). The relationship between these 2 variables was found to be neither linear nor monotonous (Pearson and Spearman correlations did not reach P < .05). Although patients among the higher scores in IEQ showed a clear tendency to be within the group of relatively low or null initial insulin requirements, the converse was not at all evident. Rather, insulin averages show a sufficiency dependence on IEQ, that is, good outcomes are not exclusive to higher yields but poor glycemic control does only arise after transplanting a reduced number of islets.
The markers calculated from the CGM data of patients in group II were correlated directly to FIA values. Table 4 lists all markers that correlated significantly to FIA.
Table 4.
CGM Metrics Correlated to First Insulin Registered Within First 50 days After AIT (Group II).
| Indicator | P value | Spearman’s ρ |
|---|---|---|
| CGM time ratio over limit | <.001 | .61 |
| CGM area over limit | <.005 | .56 |
| CGM mean | <.005 | .55 |
| CGM standard deviation | <.005 | .54 |
| CGM MAGE | <.01 | .51 |
| CGM LoC | <.05 | .42 |
| CGM BG variability standard deviation | <.05 | .39 |
AIT, autologous islet transplantation; CGM, continuous glucose monitoring.
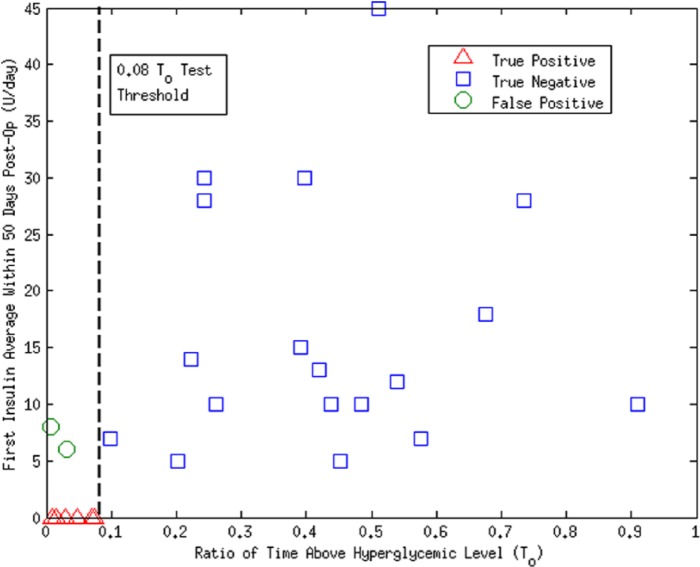
Correlations to FIA (group II) are visibly higher than those to TIC, IEQs, and IEQ/kg (group I). Again, CGM markers of hyperglycemia resulted the more accurate predictors of better initial normoglycemia, not surprising since it has been proven that hypoglycemic episodes are eradicated with minimal β cell function.18 An important result is that, as a screening test, CGM time ratio over limit of less than 0.08 () showed a sensitivity of 100% and a specificity of 88% predicting null insulin requirements within the 50 days (positive and negative groups were tested through 1-way ANOVA; null hypothesis was rejected with P = .0013; see Figure 4).
Figure 4.
Plot of versus first insulin average (FIA) use in group II. A value of separates insulin independent from nonindependent at 50 days with 100% sensitivity and 88% specificity. Positive is defined as being identified as insulin independent by the test.
Discussion
In our series of analysis we established the relationship between islet mass indicators and outcomes as far as insulin independence. Although previously reported,9 this was done to illustrate the complexity of the way in which posttransplantation normoglycemia depends on islet mass, thus stressing how predicting yield to then predict insulin averages is not as desirable as somehow trying to directly make a prognostic on insulin use. We then demonstrated that presurgical CGM markers commonly used for assessing deviations from normoglycemia, together with the CGMI, are not only a fair indicators of yield, but more importantly that they can be used more effectively as such direct insulin use prognostic tools. The importance of finding such direct markers should be stressed for a number of reasons. First, screening accuracy is in principle higher if it doesn’t depend on an intermediary on which another inference has to be made. This is especially important if this intermediary is IEQs, as we have shown that the relationship between IEQs and immediate insulin requirements is not one-to-one, so that even very accurate prediction of yield could translate into an uncertain forecast of glycemic control. See, for example, Takita et al,19 where excellent metabolic results were reported in low- and high-yield cohorts in their CP/AIT patients. Second, direct markers can cast light on the nature of factors, other than yield, relevant to restoration of normoglycemia: general health of the patient, number of years between CP diagnosis and surgery, etiology of the disease, comorbidities, and other aspects of isolation and/or engraftment may play an important role in AIT outcomes. Such was the case with CGMI, as it was significantly (negatively) associated with higher yields, but was not present at all in the list of important FIA estimators. This suggests the possibility that the responsiveness of BG homeostasis is more closely associated with β cell mass variation than with islet cell functionality within the CP cohort. Viability and functional assays have also been used to discern the major factors affecting AIT success.20,21
The test shows promise in the prediction of AIT outcomes. The levels of hyperglycemia it characterizes () are certainly subdiabetic,22 the margins it uses to rate blood sugar levels (60-120 mg/dL) are also more stringent than the ones normally used to evaluate departures from normoglycemia in diabetic patients (in Clarke and Kovatchev16 the suggested range to assess departure from normoglycemia is 61-180 mg/dL). These ranges should therefore be better bound by contrasting them with those from healthy patients. The levels of HbA1c and the average CGM values in groups I and II were found to be just marginally over healthy standards,23 and comply with the Diabetes Control and Complications trials equivalents of HbA1c and BG average24 .
Conclusions
Even if AIT has been investigated for over 30 years as a means of preventing diabetes in persons undergoing pancreatectomy for severe diseases of the pancreas, it has been difficult to achieve full insulin independence in most patients, and evidence suggests that islet function decreases over time in some of the patients.5 It is therefore of importance to identify the underlying factors affecting islet graft functionality. Islet quantitative estimators were the first such factors to be acknowledged, and have been successfully associated with efficient, long-lasting restoration of normoglycemia. Our work addresses the need to diversify the efforts to find islet yield predictors, and points to the fact that CGM technology can serve as powerful means to do so.
Acknowledgments
The authors wish to thank the reviewers for their valuable observations and time.
Footnotes
Abbreviations: AIT, autologous islet transplantation autologous islet transplantation; BG, blood glucose; CGM, continuous glucose monitoring; CGMI, CGM inertia; CP, chronic pancreatitis; FIA, first insulin average; HSA, human serum albumin; IEQ, islet equivalents; LoC, length of curve; TIC, total islet count.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Frey CF, Child CG, Fry W. Pancreatectomy for chronic pancreatitis. Ann Surg. 1976;184(4):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frey C, Suzuki M, Isaji S, Zhu Y. Pancreatic resection for chronic pancreatitis. Surg Clin North Am. 1989;69(3):499-528. [DOI] [PubMed] [Google Scholar]
- 3. Rilo HLR, Ahmad SA, D’Alessio D, et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7(8):978-989. [DOI] [PubMed] [Google Scholar]
- 4. Wahoff DC, Papalois BE, Najarian JS, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222(4):562-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robertson RP. Islet transplantation as a treatment for diabetes, a work in progress. N Engl J Med. 2004;350(7):694-705. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi T, Manivel JC, Bellin MD, et al. Correlation of pancreatic histopathologic findings and islet yield in children with chronic pancreatitis undergoing total pancreatectomy and islet autotransplantation. Pancreas. 2010;39(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chinnakotla S, Radosevich DM, Dunn TB, et al. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg. 2014;218(4):530-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaufman DB, Morel P, Field MJ, Munn SR, Sutherland DE. Purified canine islet autografts: functional outcome as influenced by islet number and implantation site. Transplantation. 1990;50(3):385-390. [PubMed] [Google Scholar]
- 9. Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060-2069. [DOI] [PubMed] [Google Scholar]
- 10. Bellin MD, Blondet JJ, Beilman GJ, et al. Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis. Pediatr Diabetes. 2010;11(4):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lundberg R, Beilman G, Dunn T, et al. Metabolic assessment prior to total pancreatectomy and islet autotransplant: utility, limitations and potential. Am J Transplantation. 2013;13(10):2664-2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Street CN, Lakey JR, Shapiro AJ, et al. Islet graft assessment in the Edmonton Protocol implications for predicting long-term clinical outcome. Diabetes. 2004;53(12):3107-3114. [DOI] [PubMed] [Google Scholar]
- 13. Schrader H, Menge B, Zeidler C, et al. Determinants of glucose control in patients with chronic pancreatitis. Diabetologia. 2010;53(6):1062-1069. [DOI] [PubMed] [Google Scholar]
- 14. Jimbo T, Inagaki A, Imura T, et al. A novel resting strategy for improving islet engraftment in the liver. Transplantation. 2014;97(3):280-286. [DOI] [PubMed] [Google Scholar]
- 15. Koh A, Senior P, Salam A, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89(4):465-471. [DOI] [PubMed] [Google Scholar]
- 16. Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther. 2009;11(S1):S-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molnar G, Ackerman E, Rosevear J, Gatewood L, Moxness K. Continuous blood glucose analysis in ambulatory fed subjects. I. General methodology. In: Mayo Clinic Proceedings. Vol. 43 No.12. Mayo Clinic; 1968:833. [PubMed] [Google Scholar]
- 18. Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab. 2012;97(11):E2078-E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takita M, Naziruddin B, Matsumoto S, et al. Variables associated with islet yield in autologous islet cell transplantation for chronic pancreatitis. Proc (Baylor Univ Med Cent). 2010;23(2):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papas K, Colton C, Nelson R, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplantation. 2007;7(3):707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hubert T, Strecker G, Gmyr V, et al. Acute insulin response to arginine in deceased donors predicts the outcome of human islet isolation. Am J Transplantation. 2008;8(4):872-876. [DOI] [PubMed] [Google Scholar]
- 22. Geiger M, Ferreira J, Hafiz M, et al. Evaluation of metabolic control using a continuous subcutaneous glucose monitoring system in patients with type 1 diabetes mellitus who achieved insulin independence after islet cell transplantation. Cell Transplantation. 2005;14(2-3):2-3. [DOI] [PubMed] [Google Scholar]
- 23. Group JDRFCGMS, et al. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33(6):1297-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275-278. [DOI] [PubMed] [Google Scholar]
- 25. Molnar GD, Rosevear JW, Ackerman E, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644-655. [DOI] [PubMed] [Google Scholar]
- 26. Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AJ. β-score: an assessment of β-cell function after islet transplantation. Diabetes Care. 2005;28(2):343-347. [DOI] [PubMed] [Google Scholar]