On June 27, 2014, the US Food and Drug Administration approved Afrezza (MannKind, Danbury, CT), an ultra-rapid-acting inhaled insulin to improve postprandial glycemic control in adults with diabetes mellitus.1 This is the only ultra-rapid-acting insulin on the market with faster pharmacokinetics and pharmacodynamics than the 3 rapid-acting insulin analogs currently on the market, which are insulin aspart, insulin glulisine, and insulin lispro.
The name “Afrezza” refers to the drug/device combination product consisting of Technosphere insulin powder (known as TI), the inhaler, and the cartridges containing TI which is referred to as the Afrezza Inhalation System or the TI Inhalation System. TI is composed of recombinant human insulin and fumaryl diketopiperazine (FDKP), which is an inert excipient. Insulin powder particles are adsorbed onto uniform-sized (approximately 2 microns) carrier Technosphere particles which contain mostly crystallized FDKP. Upon inhalation, the Technosphere particles carry the insulin into the alveoli where the particles dissolve. Both the insulin and the FDPK are rapidly absorbed across the alveolar walls independently of each other. The insulin uptake into the blood stream is faster than that of any other approved insulin. The absorbed FDPK is not metabolized and is excreted in an inert form mostly by the kidneys.2
This approval was in response to the company’s third submission in 2013. After 2 previous cycles of review MannKind had received complete response letters on March 12, 2010, and January 18, 2011, because of multiple deficiencies identified by FDA in the prior applications.3 At one point in 2010, the company elected to drop their first generation delivery system (Medtone) and seek approval for their smaller second-generation delivery system (Gen2 also known internally as Dreamboat). FDA responded that a head-to-head comparison between the 2 inhalers would be needed, as well as a dose-proportionality study and a human factors study.2
The pharmacokinetic properties of TI are uniquely rapid of all approved insulins. The median time to maximum concentration in most subjects is 12-15 minutes. The PK of TI in subjects with type 1 diabetes, type 2 diabetes, and even smokers or those with chronic obstructive pulmonary disease is no different than that in unaffected controls. In asthmatic subjects insulin absorption may be decreased.4
Regarding the pharmacodynamics of TI, this drug has a more rapid onset of action and a shorter duration of action compared to rapid-acting insulin analogs. In glucose clamp studies, the maximum glucose infusion rate (tie of peak glucose-lowering activity) was 53 minutes for TI, 108 minutes for the analog insulin, and 3-4 hours for regular human insulin. The duration of Afrezza action is 2.5-3 hours.4
In their FDA submission, MannKind evaluated the safety and effectiveness of Afrezza in a total of 3017 subjects—1026 had type 1 diabetes and 1991 had type 2 diabetes. In 4 pivotal trials TI was either noninferior to comparator insulin in 2 of 3 trials and on the margin of noninferiority versus inferiority in 1 study (TI had a higher A1c than comparator with a 95% confidence interval as extreme as 0.40% worse than comparator with the prespecified noninferiority target set at <0.40%). In a placebo-controlled trial TI was superior to placebo.5
The most common adverse reactions associated with Afrezza in clinical trials were hypoglycemia, cough, and throat pain or irritation. In the approval, Afrezza was asked to print a boxed warning advising that acute bronchospasm has been observed in patients with asthma and chronic obstructive pulmonary disease (COPD). Regarding hypoglycemia, in all 3 active comparator-controlled trials of this drug, subjects had overall 20-65% less hypoglycemia, and based on an A1C stratification analysis, it turned out that the decreased hypoglycemia occurred irrespective of subjects’ A1C levels.2
The 2 most interesting trends in my opinion were the decreased postprandial excursions that were demonstrated with 7-point glucose curves and the decreased incidence of hypoglycemia in the trials where comparator insulin was used and in the placebo-controlled trials, the event rate for subjects on metformin plus sulfonylurea was the same as metformin plus TI.2 Although TI had PK/PD advantages compared to insulin aspart, these 2 benefits were not statistically significant. The early onset of action of Afrezza compared to insulin aspart likely helped control the postprandial rise in glucose and the early disappearance of Afrezza compared to insulin aspart likely led to less late postprandial hypoglycemia after a meal has been absorbed (Figure 1). This type of data would support the position that a valid effectiveness endpoint could be a reduction in such type of hypoglycemic events if a novel insulin or insulin delivery system is associated with ultra rapid absorption. At the same time, an ultra rapidly acting insulin like Afrezza might be expected to produce less hyperglycemia immediately after a meal and less hypoglycemia late after a meal and result in little decline in the A1C. Such a drug could still be helping not only to mitigate glycemic lows and highs, but to also decrease glycemic variability (GV), even though GV has not been proven to be a true problem requiring treatment.
Figure 1.
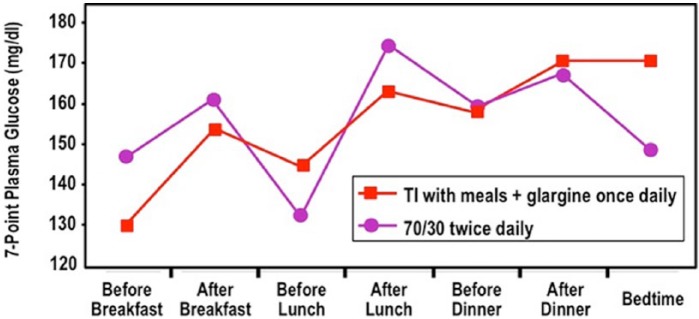
Seven-point glucose profile at week 52 in T2DM subjects in a trial of Technosphere Insulin vs insulin comparator. Red square and red line = TI with each meal plus basal glargine insulin once daily. Purple circle and purple line = 70/30 insulin twice daily. This figure was adapted from data provided by Dr Anders Boss from a study by MannKind.7
The risk of lung cancer has been raised as a safety concern to the use of inhaled insulin. In the registration trials 2 heavy smokers developed lung cancer—1 while in a controlled comparative clinical trial and 1 while in an extension. This incidence of lung cancer (0.8 cases per 1000 patient-years)2 was felt by the company to be within the range of lung cancer observed in the general population (approximately 0.23-1.22 cases per 1000 patient-years, according to the American Lung Association).5 These incidences with Afrezza can be compared to the incidence of primary lung cancer and the mortality of primary lung cancer which were observed in a follow-up study of users of Exubera, another type of inhaled insulin that was available from September 2006 through October 2007. Comparing Exubera to comparator agents according to person-years of use, the relative increased lung cancer incidence and mortality with Exubera were 3.75 and 2.81 respectively and FDA classified pulmonary malignancies as adverse events of special interest for inhaled insulins.3 At the Afrezza panel hearing on April 1, 2014, the FDA reviewers raised the issue of whether inhaled insulin causes preexisting lung cancers to grow more quickly than they would otherwise grow, but neither FDA nor MannKind officials could think of a good way to prove or disprove that hypothesis.
The FDA is requiring the following postmarketing studies for Afrezza:1
a clinical trial to evaluate pharmacokinetics, safety, and efficacy in pediatric patients
a clinical trial to evaluate the potential risk of pulmonary malignancy with Afrezza (this trial will also assess cardiovascular risk and the long-term effect of Afrezza on pulmonary function)
2 pharmacokinetic-pharmacodynamic euglycemic glucose-clamp clinical trials, 1 to characterize dose response and 1 to characterize within-subject variability.
Afrezza will fill a needed role for patients who do not wish to dose themselves for prandial insulin coverage with insulin from a needle injection. The rapid onset and offset of this insulin may well prove to be a great advantage in minimizing glucose fluctuations without much of the risk of late postinjection hypoglycemia. Now that MannKind signed a marketing agreement with the world’s largest insulin company, Sanofi,6 it is likely that Afrezza will soon be made available in many countries by way of a well-established distribution system. I look forward to seeing Afrezza used for mealtime insulin therapy in a closed-loop system and for many other situations where bolus insulin therapy is needed but late postprandial hypoglycemia is intolerable.
Footnotes
Abbreviations: COPD, chronic obstructive pulmonary disease; FDKP, fumaryl diketopiperazine; GV, glycemic variability; PK, pharmacokinetic; TI, Technosphere insulin.
Declaration of Conflicting Interests: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DCK is a consultant to Google, Insuline, Roche, Sanofi, and Voluntis.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. FDA approves Afrezza to treat diabetes. June 30, 2014. Available at: http://www.fda.gov/newsevents/newsroom/press-announcements/ucm403122.htm. Accessed September 1, 2014.
- 2. Briefing document. Endocrinologic and metabolic drug advisory committee April 1, 2014. NDA 022472 Afrezza (insulin human [rDNA origin]) inhalation powder. An ultra-rapid acting insulin treatment to improve glycemic control in adult patients with diabetes mellitus. Available at: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm390865.pdf. Accessed September 1, 2014.
- 3. FDA briefing document. Endocrinologic and metabolic drugs advisory committee meeting. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM390864.pdf. Accessed September 1, 2014.
- 4. Afrezza prescribing information. Available at: http://www.mannkindcorp.com/Collateral/Documents/English-US/Afrez-za_PrescribingInformation.pdf. Accessed September 1, 2014.
- 5. American Lung Association. Lung cancer fact sheet. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html. Accessed September 1, 2014.
- 6. Sanofi to pay MannKind up to $925m for inhaled insulin. August 11, 2014. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html. Accessed September 1, 2014.
- 7. Rosenstock J, Lorber DL, Gnudi L, et al. Prandial inhaled insulin plus basal insulin glargine versus twice-daily biaspart insulin for type 2 diabetes: a multicentre randomized trial. Lancet. 2010;375:2244-2253. [DOI] [PubMed] [Google Scholar]


