Abstract
Gestational diabetes mellitus (GDM) is defined as new onset or recognition of glucose intolerance in pregnancy. Evidence supports tight blood glucose regulation to prevent adverse maternal and fetal outcomes. Finger-prick blood glucose (BG) testing with frequent clinic review remains the most common method of managing diabetes in pregnancy. The prevalence of GDM is rising globally, pressuring resource-limited services. We have developed an intuitive, interactive, reliable, and accurate management system to record BG measurements and deliver management of GDM remotely. Following an initial scoping phase, a prototype software application was developed using an Android smartphone with BG meter linkage via Bluetooth. A custom website was built for clinician review of the data transmitted by the smartphone. After system refinement, further evaluation was undertaken for usability and reliability in a 48-patient service development project. Women used the system for an average of 13.1 weeks. In all, 19 686 BG measures were transmitted, 98.6% of which had a meal tag. A total of 466 text messages were transmitted. A mean of 30 BG readings per woman per week were transmitted, and 85% of women submitted the minimum requirement of 18 readings per week. We have developed a novel, real-time, smartphone-based BG monitoring management system that allows clinician review of real-time patient-annotated BG results. Results indicate high usage and excellent compliance by women. Robust clinical, economic, and satisfaction evaluations are required. To address these requirements, we are currently conducting a randomized controlled pilot trial.
Keywords: gestational diabetes, self monitoring, digital health, complex intervention development
Gestational diabetes (GDM) is defined by the World Health Organization as “carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy.”1 Maternal hyperglycemia results in excess transfer of glucose to the fetus resulting in fetal hyperinsulinemia. The effects of fetal hyperinsulinemia include an overgrowth of insulin-sensitive tissues, causing accelerated growth and large babies, which increases the risk of delivery complications such as shoulder dystocia, birth trauma, and the need for caesarean section. Fetal hypoxemia is also more common, which may increase the risk of intrauterine fetal death, fetal polycythemia, and hyperbilirubinemia.2
Recognizing and treating GDM to achieve tight glycemic control, has been shown in randomized controlled trials to reduce these obstetric and fetal complications.3,4 However, in the rapidly changing physiology of pregnancy, the development of GDM and its progression to requiring treatment are difficult to accurately predict. Once on treatment, further and rapid titration of medication is often required to maintain optimal glycemic control. Moreover, GDM is most often diagnosed in the last trimester of pregnancy therefore providing a short time frame for intervention to improve glycemic control—typically 10-16 weeks only.
Therefore, women with GDM are required to be assessed by a maternity diabetes team, most often in a hospital setting, at frequent and regular intervals—typically every 2 to 4 weeks depending on the indication and their glucose control. This constitutes a significant burden to the patient of having to attend many antenatal clinics and to the health care system in providing the service.
In recent years there has been a widening of screening criteria,5 a lowering of diagnostic thresholds,6 and underlying demographic changes with an increasing proportion of overweight or obese pregnant women. This has led to a rise in the prevalence of GDM, from a baseline of around 4% in 2008,5 predicted to reach over 16% in the United Kingdom if the new diagnostic thresholds are widely adopted.7 Taking these factors together, it is likely that resource-limited services for women with GDM could rapidly become overwhelmed.
The concept of monitoring blood glucose levels measured at home, with results transmitted in real-time to a health care provider is appealing. Technology used to deliver health care at a distance using an electronic means of communication was traditionally referred to as telehealth, but is now more frequently referred to as digital health, as this description covers a wide range of technology. M-health is a subcategory of digital health for which smartphones are used to transfer data and run software applications to integrate with health technology and services.
The evidence-base for m-health as an intervention to improve glycemic control in diabetes is now becoming increasingly well established.8 Our team has previous experience using these approaches with management of type 1 and type 2 diabetes.9-11
Published studies of digital health solutions used to support the management of GDM are few and utilize a range of technologies, including toll-free telephone lines, short messaging service (SMS) data transfer and web-based applications, making direct comparison difficult.
Superior glycemic control in pregnant women with type 1 diabetes using telehealth solutions compared to controls has been demonstrated by some groups.12-14 One of the larger telehealth studies enrolled 276 women with GDM and type 1 diabetes. Women in the intervention group submitted glucose data by phone weekly and were reviewed in clinic monthly, as opposed to the control group, which had a clinic review every 2 weeks. Their results revealed that the telehealth solution was associated with better glucose control in the third trimester in GDM women, as well as lower rates of macrosomia and caesarean section.14 Telehealth delivery of care has also been shown to be more acceptable to women as shown by improved quality of life measures.14-16
Yet several studies have not shown an impact upon pregnancy outcomes or glycemic control.15-18 One such study showed no difference in measures of glycemic control and obstetric and neonatal outcomes in 97 women with GDM randomized to a telehealth solution versus usual clinic care; however, they did show a significant reduction in outpatient clinic attendance in the telehealth group.18 Many studies highlight considerable technical challenges that have often made compliance poor and wider adoption unlikely.
Our objectives were to develop an intuitive, interactive, reliable and accurate digital health solution for women with GDM to record BG measurements and facilitate delivery of GDM management that was convenient and potentially cost-saving for women and health care providers. The system was designed to incorporate several features of digital health technology that have only been evaluated in isolation before. The functional objectives included the ability to
Allow women to accurately and easily record BG measurements, which are then automatically uploaded to a website
Allow health care professionals to access these measurements remotely and respond quickly to them, thus potentially improving glycemic control without the need for more intensive face-to-face contact
Allow 2-way communication between women and health care professionals
Promote user participation (empowerment) of pregnant women in their medical management
Methods
Development of the Digital Health Solution
The GDM management system was designed to allow women to monitor their BG using a glucose meter with a smartphone running a preloaded software application. Readings are automatically transferred from the glucose meter via Bluetooth to the phone and from the phone to a National Health Service (NHS) server using the 3G network (Figure 1). The use of Bluetooth allows automatic real-time upload of BGs without the need for patients to enter their data manually or upload it at regular intervals. This removes the possibility of errors that may occur when patients type in their own readings. The clinical team can access the BG readings from each patient using the application through a purpose-built website.
Figure 1.
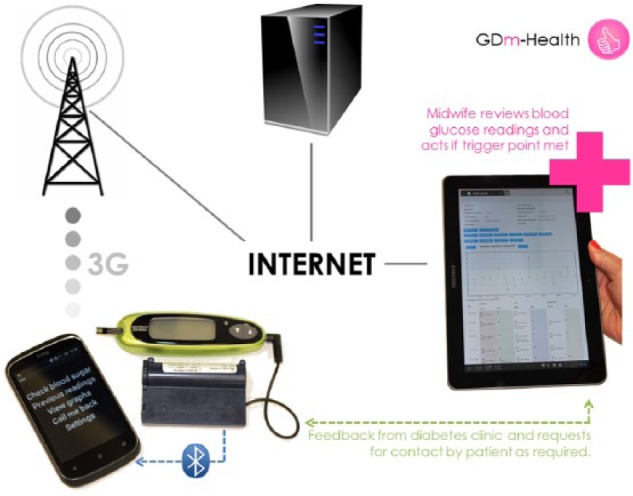
Diagram of m-health system for the management of gestational diabetes mellitus (GDM).
Following initial codesign of the system with patients and health care professionals to ensure it fulfilled the requirements of users, and early prototyping of the technology, a brief phase of testing was carried out in which minor technical issues were resolved. Implementation in a clinical setting with patients and health care professionals was carried out in 2 phases; beta testing and service development. Figure 2 shows an overview of the different phases. Initially, 7 volunteers were approached to complete a beta test of the system, alongside normal paper-based recording of their BG. Further changes to the system were made throughout the duration of beta testing in response to patient feedback collected through participant group discussion and feedback from health care professionals. In a second phase, the self-monitoring smartphone solution was offered as part of a service development initiative to all women in the hospital maternity diabetes clinic with GDM not immediately requiring treatment with insulin or metformin. These women relied on the self-monitoring smartphone solution for recording their BG (ie, did not record BG on paper) and management of their GDM.
Figure 2.
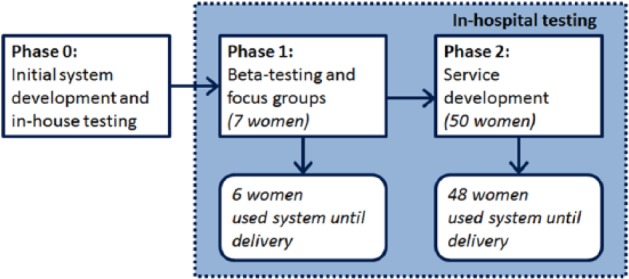
System development phases.
During both phases of the development process, diabetic clinical care was provided to women in accordance with recommendations from the National Institute for Care Excellence5 and local hospital guidelines. Women were asked to check their BG up to 6 times a day, and medication initiation and modification was in accordance with standard hospital practice.
Ethical approval was not required for this service development initiative, and the usual diabetes care of women was not changed whilst they used the self-monitoring smartphone solution.
Details of the System
Women received a smartphone (HTC Desire C, Samsung Electronics Co. Ltd., Seoul, South Korea, Android OS version 4.1) and a blood glucose meter (One Touch UltraEasy, Lifescan Inc., Milpitas, California, USA) coupled to a Bluetooth device (Polytel Glucose Meter Accessory, Polymap Wireless LLC, Tucson, Arizona, USA). BG readings are transmitted to the phone upon accessing the software application (preloaded on the phone), and the user is prompted to enter an annotation (label) with the applicable mealtime tag. The readings and tags are transmitted in real time to a server, which resides behind the NHS firewall.
We stipulated that the minimum number of BG readings per week should be 18. Readings transmitted to the server were viewed by a member of the health care team on a webpage on the secure server. The website was password protected, and each member of the health care team was assigned a unique username and password.
Additional functionalities of the smartphone application and website that were added in response to patient and health care professional feedback are described below.
Changes to the Software Application on the Phone
The initial version of the smartphone application only allowed the patient to upload readings from the glucose meter and label these before the readings were automatically transmitted to the server. To make the BG records easier to review for patients and health care professionals (from the website), a prompt for patients to enter information about their medication was added. Medication doses are now transmitted together with each BG reading. To help prevent mistyping, a “confirm medication” message is displayed when the medication dose entered is different from those entered previously for that specific meal (breakfast, lunch, or supper).
Women are not able to delete any readings. However, in response to patient feedback, the interactivity of the application has been extended with patients being able to add a comment to each reading—often using this to explain a high reading or to record what they have just eaten (see Appendix 1A).
Communication with the health care team was further enhanced by allowing patients to request a callback from a member of the team. The patient indicates the level of urgency by requesting a call either within 3 days or 24 hours (or following working day). The health care team is then alerted of the request through the website and is able to text the patient directly from the website to deliver feedback (see Appendix 1B).
To remind patients to perform a BG reading after their meal, an optional alert was implemented. This can be activated when the woman submits a preprandial reading. This causes an alert to sound and a reminder to be displayed at the time of the recommended postprandial reading (Appendix 1C).
In the final iteration, several changes were made to the user interface on the smartphone. The displays were changed to show BG readings in both graphical and tabular formats. The graphical representation is designed with color-coded thresholds (eg, a green dot indicates a BG reading in range for that particular time; a red dot indicates a BG reading that is too high; a blue dot indicates a BG reading that is too low). Graphical displays are available for all readings (Appendix 1D) and for each meal (pre- and postprandial BG values), displayed over a 1-week period (Appendix 1E). The complete information for each BG reading (including time of reading and medication dose) can be viewed by placing the cursor over a reading on the graph.
To make the application more intuitive to navigate, illustrations have been added to on-screen buttons to illustrate key functions (Appendix 1F). Moreover, user instructions are embedded in the application in the form of videos that automatically run to illustrate specific actions. For example, when a user chooses to take a new BG reading, a video that explains how to operate the glucose meter is shown. The user can also choose whether to skip or replay these videos.
Changes to the Website Contents
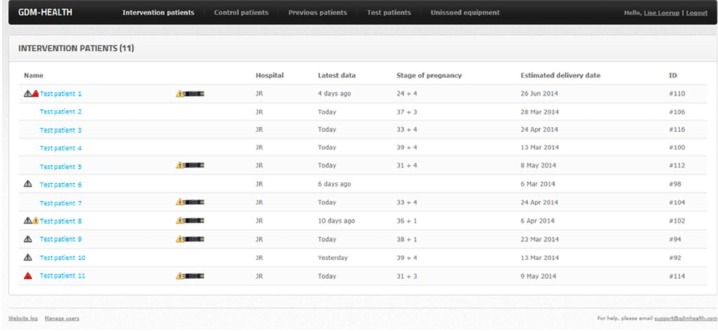
For the health care professional, the website opens on a summary page that displays the names of patients together with their current gestation, date of last BG reading and their estimated date of delivery (Appendix 2A). Selecting a patient links to a webpage displaying their hospital number, contact details, and details of their diabetes medication and expected number of BG recordings per week.
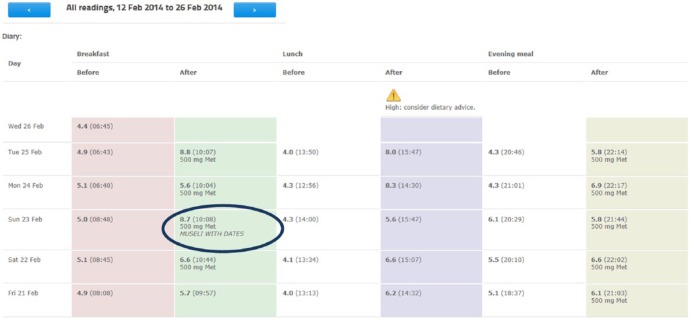
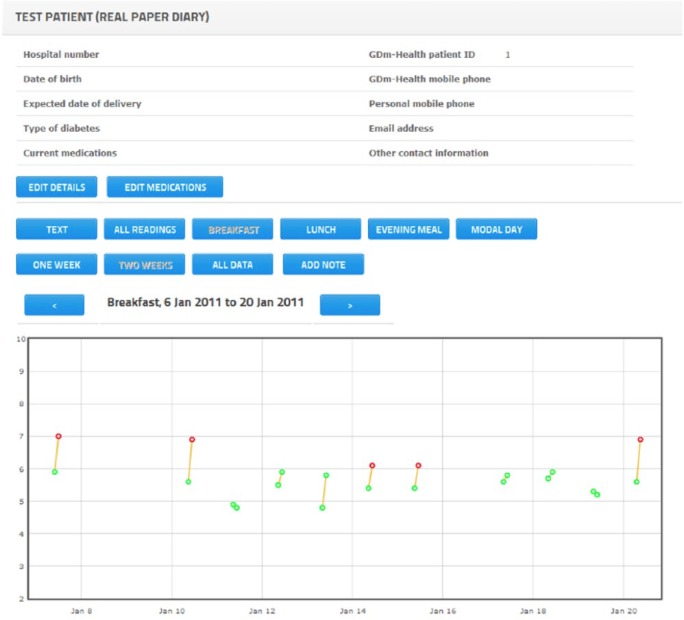
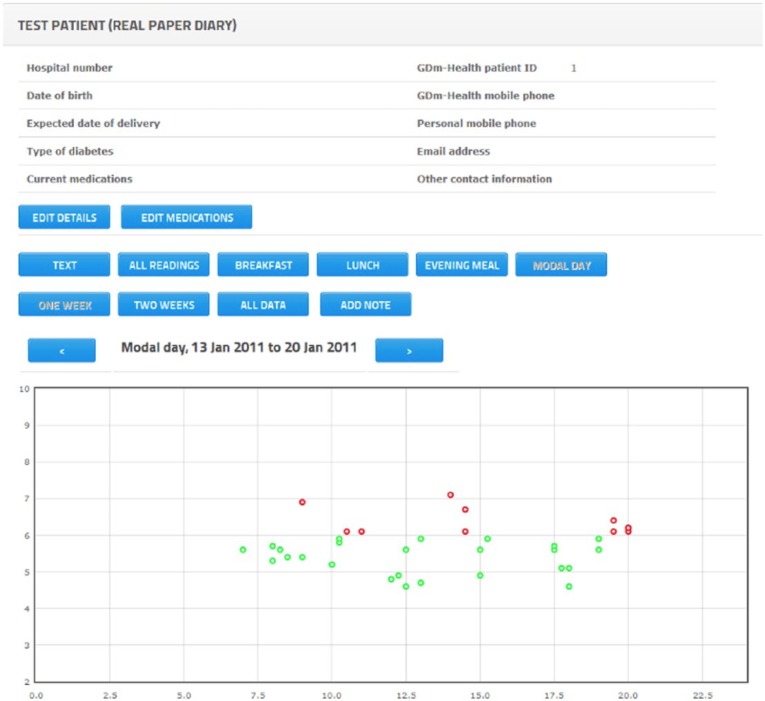
Once a patient is selected, health care professionals can see a tabular display of BG readings that mimics paper diaries (Appendix 1A). Different column colors are used to indicate times of BG reading (eg, pre-breakfast, post-breakfast, pre-lunch, post-lunch, pre-dinner, post-dinner). Graphical representations similar to the graphs displayed on the phone are also implemented on the website, with dots used to represent individual BG readings (Appendices 2B, 2C). Holding the cursor over a dot displays the actual value and time of the BG reading.
In response to health care professional feedback, members of the clinical team were given the facility to add a note on the website, for example, to record a medication change or dietary advice intervention. Such comments are not made available to patients but are designed to aid health care professionals when the management of patients is shared between several members of the clinical team.
Alerts were added to help prioritize and highlight patients who required an intervention. The alerts show as small triangles next to the patient’s name on the summary page (Appendix 2A) and above the column in which the abnormal reading is to be found (Appendix 1A). The alerts are for high or low readings and set to identify specific patterns; for example, 3 consecutive high BG readings after breakfast give a red alert (suggesting the patient’s breakfast medication needs to be increased). Gray alerts were added to show when a patient is not submitting the recommended number of readings in a week—the number of readings expected being adjustable by a member of the clinical team.
Action buttons were developed to record if medication was increased or decreased, dietary advice was given, or no action was taken. Pressing these buttons resets any alert and also allows the health care professional to add a description of the action taken.
A “strip” counter was added to help the midwife know when more BG measurement strips needed to be sent to a patient. If a patient requires more strips, a picture of a strip is shown on the summary page next to the patient’s name (Appendix 2A). Patients are also able to place a request for strips from the midwife using either the free-text comment or phone-request functionalities.
Results
Seven women participated in the beta testing phase and 50 of the 104 women approached volunteered to test the system in the service development phase. The reasons for not participating in the service development phase are detailed in Table 1.
Table 1.
Reasons for Not Participating in the Service Development Phase.
| Reason | Number of women |
|---|---|
| Late gestation | 20 |
| Language | 6 |
| Required immediate treatment | 16 |
| Other obstetric/medical complications | 5 |
| Practical considerations | 4 |
| Nonattendance/no time in clinic | 3 |
During beta testing, 6 of 7 women used the system until the end of their pregnancy. One woman stopped using the system due to issues not related to her diabetes management. During the service development phase, 48 of 50 women used the system until the end of their pregnancy; 1 woman left the country and 1 woman did not engage with the system at all.
System Usage
Details of system usage during the beta testing and service development phase are presented in Table 2. Note that some of the system functionalities, such as the ability to send text messages to patients, were added only during the service development phase.
Table 2.
System Usage.
| Beta test phase (n = 6) | Service development phase (n = 48) | Combined (n = 54) | |
|---|---|---|---|
| Weeks of usage, mean per woman (SD) | 14.5 (6.4) | 12.9 (7.2) | 13.1 (7.1) |
| Blood glucose submitted, total (n) | 3152 | 16 534 | 19 686 |
| BG submitted, mean per woman per week (SD) | 35.8 (8) | 29 (10) | 30 (10) |
| Women submitting min 18 BG mean per week, number (%) | 6 (100%) | 40 (83%) | 46 (85%) |
| Overall percentage of BG with additional information | |||
| - With meal labels (%) | 99.9 | 98.3 | 98.6 |
| - With free text comments (%) | 11.0 | 15.7 | 14.9 |
| Text messages sent from website | |||
| Text messages to patients (n) | N/A | 466 | 466 |
| Text messages to patients, mean per woman (SD) | N/A | 10 (8) | 10 (8) |
| -With medication adjustments (%) | N/A | 26 | 26 |
The average time women used the system was 13.1 weeks, and an average of 30 BG readings per week were recorded, which represents over 4 readings per day. 85% of women submitted the minimum requirement of 18 readings per week, for every week of use. While the BG readings are automatically transmitted, women were required to manually tag the BG reading as preprandial or postprandial. This was successfully done in 98.6% of BG readings.
Discussion
Women with GDM provide a compelling scenario for telehealth solutions as they are young and generally technically literate; they are usually highly motivated to engage with health care professionals and improve their health to nurture their growing fetus. Moreover, this intervention is for a short and finite time, and therefore technology fatigue is not a concern. Finally, for women with mild GDM, the frequent clinic visits are entirely for the review of BG measurements, and therefore there is scope to reduce the number of outpatient clinic appointments and provide a cost saving to both patients and the health care system.
We set out to develop a simple system to improve monitoring of BG in women with GDM, with the aim of ultimately reducing the number of visits to the hospital. Using repeated cycles of clinician and patient feedback, we have developed a novel, real-time, smartphone-based GDM management system that enables real-time transmission of patient-annotated BG results to a secure website. Real-time transmission of data from the phone to the server allows rapid clinician evaluation with potential for early modification of treatment.
Our data demonstrate high usage and excellent compliance with the system, with 85% of women recording the minimum required number of BG readings each week. The levels of use and compliance were much higher than in previously reported studies,18 suggesting that the combination of digital health technologies may well lead to a system that can be used successfully at scale.
This is the first system described in the literature to use several aspects of digital health technology, which previously have only been evaluated in isolation, to ensure BG values are transferred to a central server in real time allowing patients and health care professionals to have access to the same information and to communicate via the website or by text message. This facilitates the provision of immediate and repeated feedback of blood glucose, dietary, and lifestyle information, which fosters desired behavioral change. Sucessful intervention to allow lifestyle modification is of paramount importance for the short- and longer-term health of women with GDM.
This system is also novel in its ability to allow communication between health care professionals. As health care services move more and more toward a team-based approach, accurate yet simple means to ensure good communication between members of a clinical team are of utmost importance. Finally, this system allows capture of data for audit purposes, which is rightly becoming a mandatory requirement for all clinical services.
Conclusion
Responding to user feedback and allowing for a service development phase have been cited as important steps to ensure buy-in and integration of new technologies into clinical practice.19 The development of the GDM management system described in this article took feedback from both health care professionals and patients into account at every stage of the development process. This created a user-friendly system with evidence of high usage and compliance in a subsequent evaluation with 48 patients. The next phase is to evaluate the effects of the system on clinical, economic, and satisfaction outcomes, and this is currently under investigation in a randomized controlled pilot trial (clinicaltrials.gov NCT01916694).
Appendix
Appendix 1A.
Patient’s tabular view with patient’s free text annotation and an alert to indicated 2 consecutive high readings at the same time of day.
Appendix 1B.
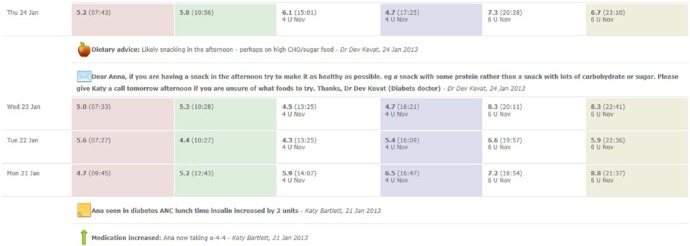
Example of using text messaging from the website to communicate with the patient. The doctor notices a pattern of pre-evening meal high BG readings and advises dietary modification via text message, and in so doing reinforces desired health behaviors.
Appendix 1C.
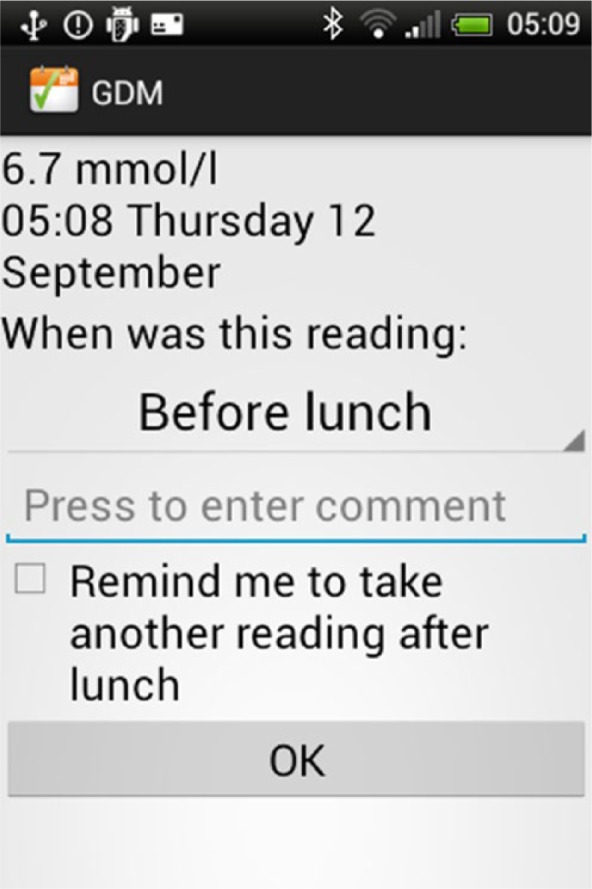
Alarm function.
Appendix 1D.
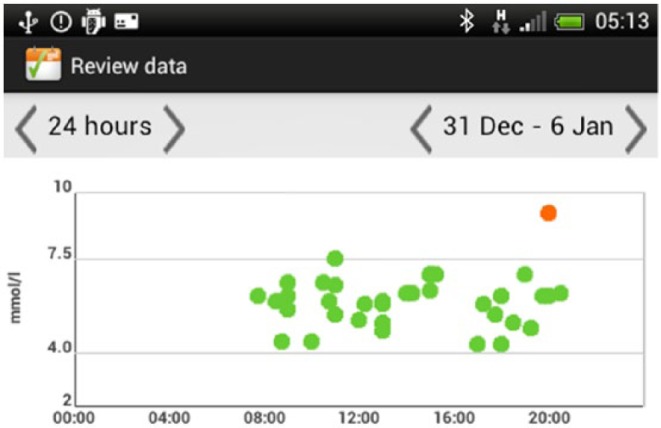
Display of all BG.
Appendix 1E.
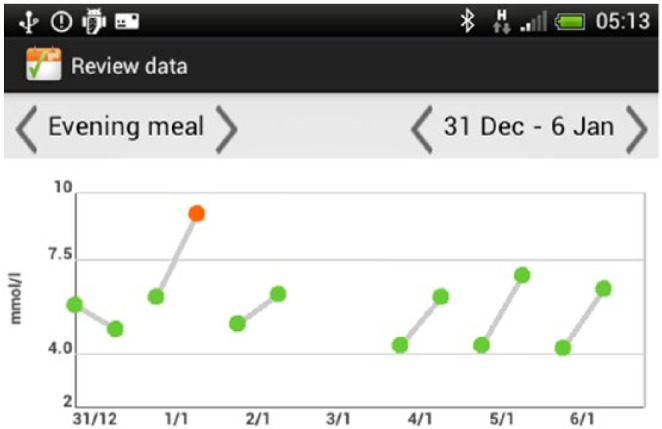
Display of meal time BGs over 1 week.
Appendix 1F.
Application welcome screen.
Appendix 2A.
Summary page on website showing alerts and strip counter.
Appendix 2B.
Example of graphical cumulative readings.
Appendix 2C.
Example of graphical representation of modal day.
Footnotes
Abbreviations: BG, blood glucose; GDM, gestational diabetes mellitus; NHS, National Health Service; SMS, short messaging service.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AF is an NIHR senior investigator.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was funded by the National Institute of Health Research Biomedical Research Centre Programme, Oxford. LL is funded by the RCUK Digital Economy Programme grant number EP/G036861/1 (Oxford Centre for Doctoral Training in Healthcare Innovation) and the Clarendon Fund.
References
- 1. World Health Organization and Department of Noncommunicable Disease Surveillance. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 2. Hod M, Rabinerson D, Peled Y. Gestational diabetes mellitus: is it a clinical entity? Diabetes Rev. 1995;3(4):602-613. [Google Scholar]
- 3. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486. [DOI] [PubMed] [Google Scholar]
- 4. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute of Clinical Excellence. Diabetes in Pregnancy. Management of Diabetes and Its Complications From Preconception to the Postnatal Period. London, UK; 2008. Available at: www.nice.org.uk/CG63. [PubMed]
- 6. International Association of Diabetes and Pregnancy Study Groups. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Royal College of Obstetricians and Gynaecologists Scientific Impact Paper 23. Diabetes and Treatment of Gestational Diabetes. 2011. Available at: http://www.rcog.org.uk/files/rcog-corp/uploaded-files/SIP_No_23.pdf.
- 8. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabetic Med. 2011;28:455-463. [DOI] [PubMed] [Google Scholar]
- 9. Larsen ME, Turner J, Farmer A, Neil A, Tarassenko L. Telemedicine-supported insulin optimisation in primary care. J Telemed Telecare. 2010;16:433-440. [DOI] [PubMed] [Google Scholar]
- 10. Turner J, Larsen M, Tarassenko L, Neil A, Farmer A. Implementation of telehealth support for patients with type 2 diabetes using insulin treatment: an exploratory study. Inform Prim Care. 2009;17:47-53. [DOI] [PubMed] [Google Scholar]
- 11. Farmer A, Gibson O, Dudley C, et al. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes. Diabetes Care. 2005;28(11):2697-2702. [DOI] [PubMed] [Google Scholar]
- 12. Wojcicki JM, Ladyzynski P, Krzymien J, et al. What we can really expect from telemedicine in intensive diabetes treatment: results from 3-year study on type 1 pregnant diabetic women. Diabetes Technol Ther. 2001;3(4):581-589. [DOI] [PubMed] [Google Scholar]
- 13. Frost D, Beischer W. Telemedicine in the management of pregnancy in type 1 diabetic women. Diabetes Care. 2000;23(6):863-864. [DOI] [PubMed] [Google Scholar]
- 14. Dalfra MG, Nicolucci A, Lapolla A. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. J Telemed Telecare. 2009;15(5):238-242. [DOI] [PubMed] [Google Scholar]
- 15. Homko CJ, Santamore WP, Whiteman V, et al. Use of an Internet-based telemedicine system to manage underserved women with gestational diabetes mellitus. Diabetes Technol Ther. 2007;9(3):297-306. [DOI] [PubMed] [Google Scholar]
- 16. Kruger DF, White K, Galpern A, et al. Effect of modem transmission of blood glucose data on telephone consultation time, clinic work flow, and patient satisfaction for patients with gestational diabetes mellitus. J Am Acad Nurse Pract. 2003;15(8):371-375. [DOI] [PubMed] [Google Scholar]
- 17. Homko CJ, Deeb LC, Rohrbacher K, et al. Impact of a telemedicine system with automated reminders on outcomes in women with gestational diabetes. Curr Diabetes Rep. 2013;13:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez-Ferre N, Galindo M, Fernandez MD, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol. 2010;2010:386941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders C, Rogers A, Bowen R, et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Serv Res. 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]