Abstract
There is room for improvement in the algorithms used in closed-loop insulin therapy during the prandial period. This pilot study evaluated the efficacy and safety of the Diabeloop algorithm (model predictive control type) during the postprandial period. This 2-center clinical trial compared interstitial glucose levels over two 5-hour periods (with/without the algorithm) following a calibrated lunch. On the control day, the amount of insulin delivered by the pump was determined according to the patient’s usual parameters. On the test day, 50% or 75% of the theoretical bolus required was delivered, while the algorithm, informed of carbohydrate intake, proposed changes to insulin delivery every 15 minutes using modeling to forecast glucose levels. The primary endpoint was percentage of time spent at near normoglycemia (70-180 mg/dl). Twelve patients with type 1 diabetes (9 men, age 35.6 ± 12.7 years, HbA1c 7.3 ± 0.8%) were included. The percentage of time spent in the target range was 84.5 ± 20.8 (test day) versus 69.2 ± 33.9% (control day, P = .11). The percentage of time spent in hypoglycemia < 70 mg/dl was 0.2 ± 0.8 (test) versus 4.4 ± 8.2% (control, P = .18). Interstitial glucose at the end of the test (5 hours) was 127.5 ± 40.1 (test) versus 146 ± 53.5 mg/dl (control, P = .25). The insulin doses did not differ, and no differences were observed between the 50% and 75% boluses. In a semi-closed-loop configuration with manual priming boluses (25% or 50% reduction), the Diabeloop v1 algorithm was as successful as the manual method in determining the prandial bolus, without any exposure to excessive hypoglycemic risk.
Keywords: type 1 diabetes mellitus, artificial pancreas, closed-loop insulin therapy, prandial bolus, algorithm
There has been considerable progress recently in closed-loop insulin therapy during the interprandial period, and particularly overnight.1-3 However, closed-loop delivery of insulin therapy during the prandial and postprandial periods remains a real challenge since calculation by the algorithm of inappropriate and excessive delivery commands related to early postprandial elevation of glucose levels exposes patients to a risk of hypoglycemic episodes.4,5
In addition to the problems of forecasting the inherent glycemic variations associated with meals (inter- and intraindividual variation in carbohydrate digestion), there is the question of the inertia of a closed-loop system, combining the absorption time for subcutaneously administered insulin and the delayed dynamics of interstitial glucose compared to plasma glucose levels.4,5
Several technological and strategic choices must be made to address control during the prandial period: type of algorithm (model predictive control [MPC], proportional integral derivative [PID], fuzzy logic),6,7 meal announcement or not, entering carbohydrate intake into the algorithm, manual bolus priming or not.8 Thus, 1 difference between MPC algorithms and PID algorithms is that the structure of the model was designed to integrate the inherent delay in carbohydrate metabolism.6,7 Information on carbohydrate intake and a small insulin priming bolus in a semi-closed-loop configuration was suggested as a way to address the postprandial glucose peaks observed in other closed loop studies.6-10
In the present study (Diabeloop WP6-0), our aim was to test the efficacy and safety of the Diabeloop v1 algorithm during the postprandial period. This algorithm is of the MPC type, and is informed of carbohydrate intake (semiclosed configuration). We also aimed at comparing different levels of manual bolus priming. This study was an exploratory pilot trial.
Materials and Methods
This clinical study (clinical trial registry NCT01754181) was conducted between March 11 and May 24, 2013, at 2 university hospital centers in Grenoble and Toulouse, France. Interstitial glucose profiles 5 hours after ingestion of a calibrated lunch were compared under 2 different circumstances: insulin therapy was delivered using an open loop (on the control day) followed by a semi-closed-loop (on the test day).
Study Population
Twelve patients with type 1 diabetes using an external insulin pump, and aged at least 18 years, were included in the study after providing informed consent. The exclusion criteria were type 2 diabetes, HbA1c >8.5%, insulin resistance (defined as body mass index > 30 kg/m2; and/or insulin requirements > 2 IU/kg/d), pregnancy, or any serious illnesses potentially interfering with the study.
The study protocol was approved by the local ethics committees and the study was authorized by the French national medicine agency (ANSM, Agence nationale de sécurité du médicament et des produits de santé).
Study Protocol
Following inclusion in the study, patients were assigned by the investigator to either the “flexible insulin therapy” (FIT, carbohydrate counting) group or the “fixed dietary plan” (FDP, non–carbohydrate counting) group, according to their ability to accurately determine their carbohydrate intake and adapt their prandial insulin dose accordingly. The specifications of the Diabeloop program state that the controller will be informed of carbohydrate intake on a semiquantitative scale (usual, increased, lower intake). To compensate for an incorrect estimation given by the patient, the current study tested the impact of a mealtime primer bolus, that is, a reduced theoretical bolus dose. Two levels of priming were assessed: 75% for FIT patients with a low presumed risk of error regarding assessment of carbohydrate intake and 50% for FDP patients with a higher risk of error.
A standard meal without excess protein or lipids, adapted to the patient’s tastes and identical for the 2 study days, was ordered by the dietician.
The carbohydrate content of the meal varied according to the subgroup to which each patient belonged, and was selected by the investigator from the following profiles:
I: normal carbohydrate content.
II: low carbohydrate content (50% reduction in normal carbohydrate level).
III: high carbohydrate content (50% increase in normal carbohydrate level).
IV: bolus omitted and low carbohydrate content (target postprandial excursion of between 60 and 120 mg/dl, based on the formula “20 g of carbohydrates = elevation in blood glucose of 80 × 60/body weight mg/dl”).
Details of the quantity of carbohydrate ingested (including in subgroup IV) and of the insulin boluses administered were entered in the algorithm.
At least 24 hours before the first assessment day, 2 subcutaneous CGM DexCom G4 sensors were placed on the patient’s abdomen, and were calibrated according to the manufacturer’s instructions. The most reliable sensor (in the absence of any difference noted at the furthest point from the pump catheter) was designated as the reference sensor, the only one used by the Diabeloop algorithm, and also used in the statistical analysis of the results. Patients were asked to change their insulin pump catheter, filling it with their usual ultra-rapid analogue, at the latest on the day before the control day.
The 2 assessment days (the control visit was conducted 24 hours to 48 hours before the test visit) followed the same pattern:
Patients arrived at the clinical investigation center at 9 am after having breakfast no later than 8 am.
Capillary glucose monitoring was performed hourly (more frequently if hypoglycemia was experienced by the patient or detected by CGM) using the patient’s measuring device, which was less than 2 years old.
If capillary glucose was > 180 mg/dl at 10 am, a corrective bolus was administered based on the patient’s usual insulin sensitivity and lunch was postponed until 1 pm (to reduce a cumulative effect of corrective bolus and lunch bolus as much as possible).
If capillary glucose was < 180 mg/dl at 10 am, lunch was served at 12 pm.
In the event of hypoglycemia in the morning, carbohydrates were given and the basal pump rate was temporarily reduced in accordance with the patient’s normal habits. After correction of hypoglycemia, the meal was started only after a period for stabilization of interstitial glucose levels of at least 30 minutes.
Correction of symptomatic hypoglycemia or capillary blood glucose value <70 mg/dl was standardized (the algorithm for the expected increase in blood glucose per 20 g carbohydrate portion was: delta blood glucose [mg/dl] = +80 × 60/kg body weight).
On the control day, a mealtime bolus was injected based on the patient’s usual insulin-carbohydrate ratio and adjusted for the preprandial capillary glucose measurement (except for group IV, in which the bolus was deliberately omitted). The bolus assistant of the pump was not used.
On the algorithm test day, a mealtime primer bolus was administered (except in group IV). Patients in the FIT group, with a low presumed risk of error regarding assessment of carbohydrate intake, were given a bolus comprising 75% of their normal bolus. Patients in the FDP group were given a bolus comprising 50% of their normal bolus.
The bolus (control day) or primer bolus (test day) was given 10 minutes before the meal if preprandial capillary glucose was > 72 mg/dl for the first 2 patients and > 120 mg/dl for the next 10 patients.11 Conversely, the bolus was injected at the start or the end of the meal in the event of moderate asymptomatic hypoglycemia with no corrective carbohydrate intake.
Interstitial glucose data were collected for the 5-hour period following the beginning of the meal, as it has been shown that 5 hours are necessary for complete glucose absorption after a mixed meal.12
Functioning of the Algorithm
The algorithm is based on the MPC approach and was developed in the CEA-LETI laboratory (Grenoble, France). The general idea underlying the algorithm is not to take total control of the insulin delivery, but rather to adapt the insulin delivery on top of the patient’s usual treatment whenever possible, to increase the time spent in normoglycemia. The 3 steps in the functioning of the Diabeloop v1 algorithm were as follows.
The model is the one of Hovorka et al,13 which takes into account the patient’s body weight, the basal rate levels, the administered boluses, and the quantified carbohydrate intake.
A parameter estimation method is implemented and fed with the historical data from 7 pm on the previous evening to time t, to obtain the patient-dependent values of the model’s parameters. The Prediction of glucose variations over a 3-hour window was performed with meal announcement and the subject’s usual basal pump rate. In the case where the parameter estimation failed, no glucose prediction was made and the patient usual treatment was delivered.
The control consisted of the potential modification of the insulin delivery in the absence of predicted normal glycemia (90-145 mg/dl). The control horizon was 1 hour and the control format consisted of a bolus, and/or a change in the basal rate for the first half hour and/or a change in the basal rate for the second half hour. In fact, the basal rate for the second half hour was not implemented, since a new prescription occurred before then.
In practice, the 3 new interstitial glucose values (obtained every 5 minutes) were entered manually into the algorithm software program (installed on a portable computer) every 15 minutes by the engineers. The algorithm was functional as of administration of the primer bolus and throughout the 5-hour postprandial period. Every 15 minutes, the algorithm reassessed the quantity of insulin to be delivered. All prescriptions were subject to validation by a diabetologist, who manually modified the programming of the patient’s insulin pump.
The algorithm used during this pilot experiment was slightly modified during the course of the study (versions 1.1 to 1.3).
Statistical Analysis
The primary evaluation criterion was the percentage of time with interstitial glucose values between 70 and 180 mg/dl. The study of Hovorka et al focused on the issue of assessing performance of closed-loop studies by CGM values.14 They showed that glucose mean and variability could be estimated by unmodified CGM levels with acceptable clinical accuracy. They also suggested that stochastic CGM transformation provided better and unbiased estimate of time when glucose is in target and below target in closed loop studies. However, Phillip et al did not observe a change in statistical significance when using stochastic transformation.2 The results in the present article are thus calculated on the unmodified CGM levels. Analysis of the 12 patients yielded a statistical power of at least 80% for the demonstration of a difference of at least 21% based on the hypothesis of a standard deviation of 30% or less and a type 1 alpha risk of 0.05. Analysis of the primary criterion was performed using a crossover model (General Linear Model [GLM]). A per protocol analysis, which excluded patients in failure at the algorithm “modeling” step, was conducted in addition to the intention-to-treat analysis.
The secondary evaluation criteria were as follows: percentage of time with interstitial glucose levels between 80 and 140 mg/dl, <70 mg/dl, <80 mg/dl, >140 mg/dl, >180 mg/dl; maximum interstitial glucose; interstitial glucose at H5 (end of the study period); area under the curve (AUC) from H0 to H5; insulin doses.
Results are expressed as mean ± standard deviation.
Results
Participants
Twelve patients with type 1 diabetes (9 men and 3 women) were included. Their characteristics were as follows: age 35.6 ± 12.7 years; BMI 23.9 ± 2.4 kg/m2; HbA1c 7.3 ± 0.8 %; duration of diabetes 19.8 ± 12.7 years; total daily insulin dose 42.5 ± 11.6 units. Each subgroup (bolus omitted, normal/increased/decreased daily carbohydrate intake) included 3 patients, with a total of 6 patients in FIT and 6 patients in FDP groups.
Comparison of the Preprandial Periods (Excluding the Study Period)
Nocturnal hypoglycemic events occurred once in 3 subjects before the control day and once in 2 subjects before the test day. Eight episodes of hypoglycemia requiring treatment were documented between 9 am and 1 pm, on the control day (in 5 subjects) and 4 episodes on the test day (in 4 subjects). The capillary glucose reading at 10 am was 140 ± 70 mg/dl on the control day versus 130 ± 40 mg/dl on the test day. A corrective bolus was given at 10 am to 2 patients (1.7 and 2 units) on the control day and 1 corrective dose (1.8 units) on the test day. At the start of the test (H0), the capillary glucose readings were identical (100 ± 20 mg/dl on the control day and 100 ± 30 mg/dl on the test day). The quantity of carbohydrates ingested was the same for the 2 meals (62.9 ± 43.4 g on the control day and 62.8 ± 43.5 g on the test day).
Primary and Secondary Outcomes
The results are given for the total population (n = 12) and for the per protocol population (n = 9), excluding the 3 patients for whom the parameter estimation step systematically failed. In the latter case, the algorithm reverts to the patient’s usual treatment. The results (intent-to-treat analysis) are given in summary form in Table 1.
Table 1.
Comparison of Interstitial Glucose Profiles for Open-Loop Versus Semi-Closed-Loop Systems.
| Control day (open-loop system) (n = 12) | Algorithm day (semi-closed-loop system) (n = 12) | P | |
|---|---|---|---|
| Primary evaluation criterion | |||
| Time in target range (70-180 mg/dl) (%) | 69.2 ± 33.9 | 84.5 ± 20.8 | .11 |
| Secondary evaluation criteria | |||
| Time in target range (80-140 mg/dl) (%) | 49.3 ± 32.9 | 54.7 ± 28.9 | .51 |
| Time in hypoglycemia | |||
| <70 mg/dl (%) | 4.4 ± 8.2 | 0.2 ± 0.8 | .18 |
| <80 mg/dl (%) | 10.1 ± 12.2 | 4.3 ± 6.8 | .17 |
| Number of carbohydrate intakes | 5 | 1 | |
| Time in hyperglycemia | |||
| >180 mg/dl (%) | 26.4 ± 36.5 | 15.2 ± 20.9 | .56 |
| >140 mg/dl (%) | 40.6 ± 42.4 | 40.9 ± 30.7 | .97 |
| Maximum glucose level from H0 to H5 (mg/dl) | 168.1 ± 49.7 | 180.6 ± 41.9 | .34 |
| Change in glucose level (mg/dl) | 65.7 ± 41.0 | 79.8 ± 47.0 | .39 |
| Interstitial glucose at H5 (mg/dl) | 146 ± 53.5 | 127.5 ± 40.1 | .25 |
| Area under the curve (H0-H5) (mg/dl/min) | 40 062 ± 14 072 | 40 401 ± 7920 | .92 |
| Total insulin dose (units) | 9.0 ± 5.1 | 8.9 ± 4.3 | .91 |
Change in glucose level = maximum glucose (H0-H5) – preprandial glucose.
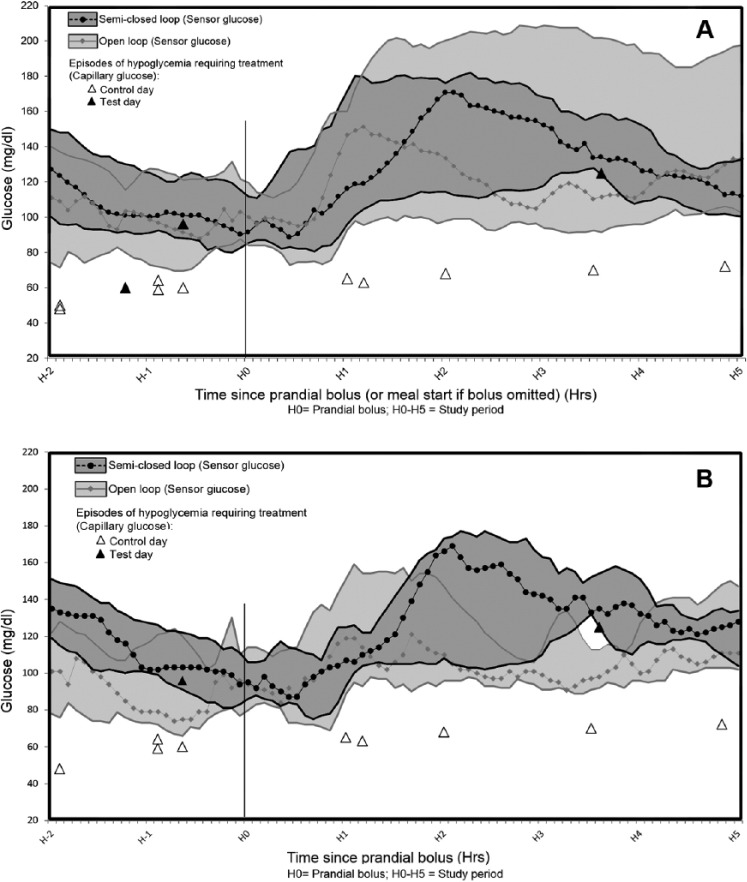
The results (intention-to-treat analysis) of the primary outcome are shown in Figure 1A (inclusion of bolus omitted group) and Figure 1B (exclusion of bolus omitted group).
Figure 1.
Comparison of medians and interquartile variations for interstitial glucose in open-loop versus semi-closed-loop systems. (A) Group IV “bolus omitted” included; (B) group IV “bolus omitted” excluded. Median (curves) and 25-75% quartiles (shaded areas) of CGM glucose comparing open loop CSII and semi-closed-loop data.
In the per protocol analysis, the primary outcome (percentage of time in the target range of 70-180 mg/dl) was 90.4 ± 17.7% (test day) versus 69.6 ± 34.1% (control day) (P = .08).
Regarding hypoglycemic events occurring during the 5-hour period following the beginning of the meal: on the test day only 1 patient was given carbohydrates once (interstitial glucose 125 mg/dl, decreasing kinetics) at the request of the diabetologist, while on the control day 5 carbohydrate intakes for hypoglycemia were necessary (4 patients in all) (Figure 1).
Influence of the Type of Bolus Given on the Test Day (50% for FDP or 75% for FIT)
The percentage of time in the target range (70-180 mg/dl) was 79.3 ± 21.8%, with a 75% bolus on the test day versus 65.9 ± 35.4% on the control day (P = .31). This was 89.8 ± 20.4% for a 50% bolus on the test day versus 72.5 ± 35.3% on the control day (P = .19).
The type of bolus given (50 or 75%) had no significant effect on the secondary evaluation criteria (time in hypoglycemia, time in hyperglycemia, maximum interstitial glucose and interstitial glucose at H5).
Effect of the Quantity of Carbohydrates Ingested
This effect did not differ significantly between the 2 study days. The results are summarized in Table 2. The percentage time in the target range (70-180 mg/dl) was greater for patients with lower overall 2 day carbohydrate intakes.
Table 2.
Influence of the Quantity of Carbohydrate Ingested on Interstitial Glucose Profiles for Open-Loop Versus Semi-Closed-Loop Systems.
| Subgroup | Low carbohydrate intake (n = 3) | Normal carbohydrate intake (n = 3) | Increased carbohydrate intake (n = 3) | Bolus omitted (n = 3) | ||||
|---|---|---|---|---|---|---|---|---|
| Carbohydrate intake (g) | 39.4 | 61.2 | 123.3 | 27.7 | ||||
| Visit | Control | Algorithm | Control | Algorithm | Control | Algorithm | Control | Algorithm |
| % time in target range (70-180 mg/dl) | 90.1 ± 13.2 | 96.5 ± 6.0 | 86.3 ± 6.8 | 88.5 ± 19.9 | 74.1 ± 44.9 | 78.4 ± 29.4 | 26.3 ± 13.9 | 74.7 ± 25.5 |
| % time in hypoglycemia <70 mg/dl | 9.9 ± 13.2 | 0.0 ± 0.0 | 7.7 ± 8.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.9 ± 1.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| % time in hyperglycemia >180 mg/dl | 0.0 ± 0.0 | 3.5 ± 6.0 | 6.0 ± 10.4 | 11.5 ± 19.9 | 25.9 ± 44.9 | 20.7 ± 30.0 | 73.7 ± 13.9 | 25.3 ± 25.5 |
| Total insulin dose (units) | 7.5 ± 2.6 | 6.7 ± 3.3 | 9.5 ± 4.3 | 7.3 ± 3.5 | 15.2 ± 4.4 | 14.4 ± 3.2 | 3.8 ± 0.5 | 7.2 ± 2.2 |
In the particular case of the omitted bolus subgroup, the percentage of time spent in the target range (70-180 mg/dl) was 74.7 ± 25.5% (test day) versus 26.3 ± 13.9% (control day).
Refusal of Proposals Made by the Algorithm
For 3 different patients, 4 proposals made by the algorithm were refused by the diabetologist, who considered them to present a risk of hypoglycemia.
Discussion
The Diabeloop v1 algorithm, in a semi-closed-loop control system that required a meal announcement and a partial manual premeal bolus, was shown to be as effective in the postprandial period as the conventional prandial bolus procedure. Our results support the feasibility and safety of the system since the incidence of hypoglycemia seemed not to be increased (5 episodes of hypoglycemia experienced on the control day versus 1 impending hypoglycemic event and 4 overrides for potentially excessive insulin delivery on the test day).
The limitations of this pilot study were as follows:
Low statistical power (due to the small study population), preventing demonstration of statistically significant differences, among patients with a good glycemic control (HbA1c 7.3%).
Absence of randomization at inclusion: The control day had to precede the test day in all cases to ensure the collection of adequate historical data for entering in the algorithm to facilitate the modeling step.
Duration limited to 5 hours, precluding the possibility to studying a potential meal stacking effect.
Changes made to the algorithm during the study restricted the relevance of comparison of glycemic profiles between patients.
Absence of regulation by the algorithm of the preprandial period, which could have had an impact on its performance. Postprandial interstitial glucose elevation is partly the result of the choice of the basal pump rate at the end of the morning (on which hepatic glucose production depends), which in this study was set by the diabetologist and not by the algorithm.
Intervention of the supervising diabetologist, overriding the algorithm in 3/12 experiments. This was allowed in the setting of our pilot trial, but demonstrated a failure of this version of the algorithm.
The failure of the parameter estimation step in 3/12 experiments, precluding any prediction, and leading the algorithm to revert to the patient’s usual treatment. This had no impact on safety, but could bring efficacy into question. This limitation will have to be addressed before further studies.
The closed-loop delivery of insulin in the prandial period is aimed at reducing constraints on patients with type 1 diabetes by relieving them of the need to perform a precise calculation of their carbohydrate intake in particular. It is expected that such a system, capable of adapting to unforeseeable changes in blood glucose levels, may improve glycemic equilibrium and reduce hypoglycemic risk.5
However, in the first clinical studies that used a PID algorithm, delays in subcutaneous insulin absorption and glucose sensing contributed to early postprandial hyperglycemia and late postmeal hypoglycemia.15 Further modifications of this PID algorithm incorporating the use of insulin feedback to compensate for delays in the insulin action led to improved postprandial glucose values, that were found to be similar to open-loop therapy values in a study in young children.16,17 Meanwhile, improvements of MPC algorithms incorporating safety components (insulin on board constraint) led, in a short term pilot evaluation, to overcoming small unannounced meals without postprandial hypoglycemia.18 In a pilot experiment, another team showed that the regulation of blood glucose was feasible in a closed-loop system without any meal announcement.19 Another team reported that their MPC controller was at least as effective as conventional therapy in managing rises in blood glucose following breakfast.20 The fuzzy-logic-based approach has been shown to also have the potentiality to identify meals that require special treatment, in a feasibility study where the mean detection time after meal consumption was 23 minutes and the mean peak postprandial glucose level was 224 mg/dl.21 The bihormonal artificial pancreas manipulating both insulin and glucagon is another approach to achieve completely automated closed-loop control. A recent study reported a glucose control that was comparable after lunch, but tended to give higher values after breakfast, in closed-loop compared to open-loop mode.22
In an alternative approach, delays in subcutaneous insulin absorption have led many investigators to explore the use of a hybrid approach with mealtime bolus or announcements. Better control can be achieved with this strategy.9 A small manual priming bolus, given 15 minutes before the meal decreased the mean peak postprandial glucose level.10 A recent report, using rule-based algorithms that require a meal-priming bolus of 50% of the usual value and 2 different strategies to control basal or postmeal periods, showed that postprandial results, although not inferior to those obtained with a conventional therapy, did not avoid excessive glycemic excursions.23 Nevertheless, manual administration of the entire meal bolus was associated with a risk of hypoglycemia despite the reduction (up to -100%) in the preprogrammed basal rate performed by an MPC algorithm in a single-hormone closed-loop study.24 Yet meal-priming insulin boluses dramatically restrict the reduction in burden of care, if they require accurate carbohydrate estimations. Partial weight-based meal-priming insulin boluses are an alternative approach that is less burdensome to patients. The glycemic control achieved with 2 different meal-priming insulin doses (0.035 vs 0.05 units/kg/meal) did not differ in a bihormonal closed-loop study.25 A weight-based meal-priming bolus (0.047 units/kg/meal) was safe and feasible, in another bihormonal closed-loop study, but not as effective as a carbohydrate-matching bolus.26 The third-generation bihormonal artificial pancreas uses simple meal notification since it includes an adaptive meal-priming insulin bolus capability that automatically adjusts the size of meal doses by administering 75% of the average prandial insulin provided from previous meals. It improved mean plasma glucose, without increasing the hypoglycemic risk, relative to an entirely automated closed-loop system.27
Modeling of the prandial period based on a longer observation period lasting several days would enable us to mimic the prescription of a bolus as is done by a diabetologist in real life. Such an approach has already been shown to be feasible though its superiority has not yet been demonstrated.28,29
There is as yet no consensus in the literature on the strategic question of whether any 1 artificial pancreas design has more advantages than another. The type of priming bolus used (50% vs 75%) had no significant effect in our study, which may have a beneficial impact on the safety of the treatment in future closed-loop designs. There are plans for testing of an algorithm configuration that is less burdensome to patients, operating in “semiquantitative” mode, based on an evaluation scale of the type “standard / high /low carbohydrate content.” Finally, in the case of snack-type carbohydrate intakes in which a bolus is frequently deliberately omitted, the Diabeloop algorithm enabled a favorable glucose profile to be restored by doubling the quantity of insulin delivered in 5 hours (75% of time in euglycemia vs 26% on the control day in the “omitted bolus” subgroup). The next step will consist of testing the efficacy and safety of the algorithm in cases of unannounced carbohydrate snacks.
Conclusion
This initial clinical study testing the Diabeloop v1 algorithm during the prandial period has shown it to be a safe and effective semiautomatic insulin delivery system. Thanks to the promising results of this pilot study, randomized multicenter controlled studies may be envisaged in larger populations over longer observation periods.
Acknowledgments
CERITD (Centre d’Études et de Recherches pour l’Intensification du Traitement du Diabète) is a nonprofit clinical translational research center located in Corbeil hospital. CERITD was fully involved in the design and coordination of the study. Part of the investigations was conducted within the Grenoble Clinical Research Centre (CIC-INSERM 1406, Grenoble University Hospital) under the supervision of Pr Jean Luc Cracowski. Another part of the investigations was conducted within the Toulouse Clinical Research Centre (CIC-INSERM, Toulouse University Hospital). The authors would like to thank Lydie Canipel, Caroline Peschard, Ilham Xhaard from CERITD, Laure Nasse, Adeline Paris, Claire Cracowski, Enkelejda Hodaj, Dominique Abry, Anne Tournier, Marlene Dreux, Stephanie Roudet, from Grenoble University Hospital, Eric Guillaume, Marie-Blanche Arhainx, Laurent Cazals, Nelly Puech, Sandrine Bonnet, Lisa Lefebvre, Angelika Vaccaro from Toulouse University Hospital, for involvement in the management of the study. Statistical analysis was performed by Didier Not (www.rcts.fr).
Footnotes
Abbreviations: AUC, area under the curve; CGM, continuous glucose monitoring; FDP, fixed dietary plan; FIT, flexible insulin therapy; MPC, model predictive control; PID, proportional integral derivative.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support of the study was provided by a contract grant from AVIESAN (Contract 201101, National Alliance for Life and Health Sciences, www.aviesan.fr) and by CERITD.
Contributor Information
on behalf of the Diabeloop Consortium:
Guillaume Charpentier, Sylvia Franc, Alfred Penfornis, Yves Reznik, Pierre Yves Benhamou, Denis Raccah, Eric Renard, Bruno Guerci, Nathalie Jeandidier, Helene Hanaire, Chantal Simon, Maeva Doron, Michel Antonakios, and Regis Guillemaud
References
- 1. Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824-833. [DOI] [PubMed] [Google Scholar]
- 3. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61:2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60:2672-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7:385-395. [DOI] [PubMed] [Google Scholar]
- 6. Steil GM. Algorithms for a closed-loop artificial pancreas: the case for proportional-integral-derivative control. J Diabetes Sci Technol. 2013;7:1621-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bequette BW. Algorithms for a closed-loop artificial pancreas: the case for model predictive control. J Diabetes Sci Technol. 2013;7:1632-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control. 2012;36:255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery vs. semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934-939. [DOI] [PubMed] [Google Scholar]
- 11. Luijf YM, van Bon AC, Hoekstra JB, DeVries JH. Premeal injection of rapid-acting insulin reduces postprandial glycemic excursions in type 1 diabetes. Diabetes Care. 2010;33:2152-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pehling G, Tessari P, Gerich JE, Haymond MW, Service FJ, Rizza RA. Abnormal meal carbohydrate disposition in insulin-dependent diabetes. Relative contributions of endogenous glucose production and initial splanchnic uptake and effect of intensive insulin therapy. J Clin Invest. 1984;74:985-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25:905-920. [DOI] [PubMed] [Google Scholar]
- 14. Hovorka R, Nodale M, Haidar A, Wilinska ME. Assessing performance of closed-loop insulin delivery systems by continuous glucose monitoring: drawbacks and way forward. Diabetes Technol Ther. 2013;15:4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344-3350. [DOI] [PubMed] [Google Scholar]
- 16. Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96:1402-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dauber A, Corcia L, Safer J, Agus MS, Einis S, Steil GM. Closed-loop insulin therapy improves glycemic control in children aged <7 years: a randomized controlled trial. Diabetes Care. 2013;36:222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15:386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol. 2009;3:1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol. 2012;6:1114-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Capel I, Rigla M, García-Sáez G, et al. Artificial pancreas using a personalized rule-based controller achieves overnight normoglycemia in patients with type 1 diabetes. Diabetes Technol Ther. 2014;16:172-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elleri D, Allen JM, Kumareswaran K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care. 2013;36:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haidar A, Farid D, St-Yves A, et al. Post-breakfast closed-loop glucose control is improved when accompanied with carbohydrate-matching bolus compared to weight-dependent bolus. Diabetes Metab. 2014;40:211-214. [DOI] [PubMed] [Google Scholar]
- 27. El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ., III Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007;30:1131-1136. [DOI] [PubMed] [Google Scholar]
- 29. Rossetti P, Ampudia-Blasco FJ, Laguna A, et al. Evaluation of a novel continuous glucose monitoring-based method for mealtime insulin dosing—the iBolus—in subjects with type 1 diabetes using continuous subcutaneous insulin infusion therapy: a randomized controlled trial. Diabetes Technol Ther. 2012;14:1043-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]