Abstract
The InsuPad is a medical device to accelerate insulin resorption by applying local heat at the insulin injection site. This crossover study examined the impact of the InsuPad use on postprandial glucose excursions under daily life conditions. In 1 study phase, diabetic patients used the InsuPad when injecting bolus insulin before breakfast and dinner and measured their blood glucose 5 times daily (before breakfast, lunch, and dinner and after breakfast and dinner). In the other study phase, blood glucose measurements were maintained without using the InsuPad. The order of the study phases was randomized. Twenty patients with a high insulin demand took part (30% type 1 diabetes, age 53.7 ± 8.9 years, diabetes duration 14.9 ± 7.4 years; HbA1c 8.3 ± 0.8%; total daily insulin demand 0.97 ± 0.32 IU per kg). Postprandial glucose excursion was reduced by 15.4 mg/dl (95% CI 9.7-21.2 mg/dl; P = .011) after breakfast and dinner if InsuPad was used. The mean blood glucose was lower by 8.8 mg/dl (95% CI 0:3-18:0 mg/dl; P = .099) when using the InsuPad. Safety parameters and the percentage of hypoglycemic (< 60 mg/dl) or hyperglycemic (> 300 mg/dl) blood glucose measurements were not negatively affected by InsuPad use (hypoglycemic values 1.4% vs 1.5%, P = .961; hyperglycemic values 2.6% vs 4.0%, P = .098). Local heating of the insulin injection site by use of the InsuPad device is an effective and safe method to reduce postprandial blood glucose excursions under daily life conditions without negative side effects on the occurrence of low or high blood glucose values.
Keywords: blood glucose values, glycemic variability, InsuPad, postprandial glucose excursion
The insulin action profiles of subcutaneously injected fast-acting insulin analogues are still slow compared to the physiological release of human insulin. Thus, postprandial glucose excursions cannot be avoided.1 The InsuPad is a medical device designed to apply local heat around the site of the insulin injection. When an insulin injection is given, the skin surface temperature is heated locally up to 40.0 C° for 30 minutes. Pharmacokinetic studies demonstrated that local heating around the insulin infusion site for 30 minutes after a prandial insulin bolus resulted in accelerated uptake of insulin from the subcutaneous tissue.2-4 Clamp studies investigating the pharmacodynamic effect of local heating demonstrated that the glucose-lowering effect of insulin was earlier and more pronounced when heating was applied around the local infusion site.2-4 Inpatient studies, using standardized meals in type 1 diabetic subjects on continuous subcutaneous insulin infusion (CSII) therapy, showed that postprandial glucose values could be significantly reduced by using a local heating device with CSII therapy.2,5 However, until now, the impact of local heating at the injection site has not been demonstrated under real-life conditions.
In this crossover study, the efficiency of InsuPad on glycemic control was studied in an outpatient setting under real-life conditions. The primary objective of this study was to test the hypothesis that the use of the InsuPad has a significant effect on the reduction of postprandial blood glucose excursions after breakfast and dinner under daily life conditions of insulin-resistant diabetic patient on multiple daily injection (MDI) therapy. Secondary outcomes were the effects of InsuPad use on glycemic control and on patient-reported outcomes (diabetes-related distress and diabetes treatment satisfaction). Safety parameters were incidences of hypo- and hyperglycemia.
Methods
Design and Setting
This single-center, open-label, crossover study consists of 2 study phases, of 4 weeks each. In the intervention phase patients were instructed to use the InsuPad at least while injecting the prandial insulin dose at breakfast and dinner; in the control phase participants did not use the InsuPad. The order of these 2 study phases was randomized. In both phases participants were instructed to measure their blood glucose at least 5 times a day, before breakfast, lunch, and dinner as well as 90 minutes after breakfast and after dinner. Participants were also asked to document insulin doses, time of meal start, and possible adverse events (eg, hypoglycemia, device failures, and reactions at the injection site) in their log books.
During the first visit, participants were instructed on the study procedures. Participants were also familiarized with the Diasend® system. Diasend is a standalone system for easy uploading of information from most point-of-care blood glucose meters (BGMs) and transferring them to a central computer. The subjects were instructed to connect their BGMs to the Diasend transmitter at least once a week. All point-of-care BGM of the patients were subjected to a quality control procedure by using appropriate control material to check if the BGM results were within the manufacturers accuracy limits.
Participants, randomized to use the InsuPad during the first study phase, were also instructed in how to use this device. After 2 weeks, a phone call was scheduled to ensure that participants were complying with the study protocol and to address any problems. After 4 weeks, the first phase ended and a second visit took place. Participants were instructed to measure their blood glucose in the same frequency as during the first phase and to connect their BGM to the Diasend transmitter at least once a week. Patients without InsuPad during the first period were now instructed in how to use the InsuPad; patients with InsuPad during the first phase handed back their devices. After week 6, another phone call was made. After 8 weeks the close-out took place for all participants during visit 3.
The study took place in an outpatient setting of the Research Institute of the Diabetes Academy Mergentheim, Bad Mergentheim, Germany.
Subjects
Eligible for this study were participants who met the following inclusion criteria:
Age ≥ 18 and ≤ 70 years
Type 1 diabetes mellitus or insulin-treated type 2 diabetes with a daily insulin demand ≥ 0.7 insulin units per kg body weight
Use of short-acting prandial insulin analogues and therapy with multiple daily insulin injections
HbA1c ≥ 6.0 % and ≤ 9.5 %
Exclusion criteria were these:
Life-threatening diseases, known gastro- or enteroparesis, or other unstable chronic diseases other than diabetes mellitus for the last 6 months before start of the study
Excessive fibrosis, lipohypertrophy, or eczema at injection sites
Under guardianship or with other interfering compliance issues
All participants signed informed consent. The study was approved by the local ethics committee (Lande-saerztekammer Baden-Württemberg, Stuttgart, Germany).
Outcomes
Primary Outcome
The primary outcome was the combined outcome of blood glucose excursions after breakfast and dinner as measured by the patients’ BGM. Blood glucose excursions were calculated with respect to premeal baseline levels. Only postprandial glucose excursions, which were not earlier than 75 minutes and not later than 135 minutes after the meals were analyzed.
Secondary Outcomes
Overall glycemic control was assessed by the daily average of all BGM measurements during the 2 study phases.
The impact of the InsuPad on postprandial glucose control was analyzed separately for breakfast and dinner.
As a subsequent analysis, the amount of self-reported carbohydrate consumption and prandial insulin doses at breakfast and dinner were evaluated with and without InsuPad.
Patient-reported outcomes were assessed by self-report scales. Diabetes-related distress was evaluated via the Problem Areas in Diabetes (PAID) Questionnaire;6 treatment satisfaction was tested via the Diabetes Treatment Satisfaction Questionnaire (DTSQ).7
The DTSQ measures overall satisfaction with diabetes treatment. However, the DTSQ contains also some items that can be considered to be at least sensitive to assess the impact of InsuPad use more specifically. Satisfaction regarding the occurrence of hypo- and hyperglycemic blood glucose values as well as the questions regarding if the patient would recommend his or her current diabetes treatment for other patients and if the patient would continue his or her current diabetes treatment could be related to the InsuPad use.
Safety parameters were the percentage of hypo- or hyperglycemic BGM measurements and the number of severe hypo- or hyperglycemic events requiring third-party assistance or medical assistance for recovery.
Sample Size
The sample size estimation was based on a laboratory study using standardized liquid meals (2). In this study, the use of a heating device similar to the InsuPad had an effect size of Cohen’s d = 0.85 on postprandial glucose excursions. We assumed a somewhat lower effect size of Cohen’s d = 0.65 as this study was conducted under daily life conditions. To detect an effect of this size in a within-group comparison with a 2-sided 5% significance level and a power of 80%, a sample size of 20 pairs was necessary.
Statistical Methods
Only blood glucose excursions with a time difference between 75 and 135 minutes between preprandial and postprandial measurement, documented by the Diasend system, were regarded as valid postprandial glucose excursions and were included in the statistical analysis; no data clearing methods were applied. For each subject an individual mean of postprandial glucose excursion and total glucose values were calculated.
Also the mean percentage of biochemically defined hypo- and hyperglycemic events was calculated for each subject prior to the use of inference statistical analysis. Based on the blood glucose logbook, mean amount of self-reported carbohydrates and insulin doses also were calculated.
The primary and secondary outcomes were analyzed using repeated measures ANOVA. The use of the InsuPad device (yes/no) was the within factor, and the order of the periods was the between factor. Thus, this analysis controlled for the order effect.
Results
Study Sample
Twenty patients completed the study. The sample characteristics are described in Table 1.
Table 1.
Patient Characteristics.
| Parameter | All (N = 20) | Type 2 diabetes (n = 14) | Type 1 diabetes (n = 6) | P |
|---|---|---|---|---|
| Mean age (years) ± SD | 53.7 ± 8.9 | 56.3 ± 8.4 | 47.8 ± 7.7 | .048 |
| # female (%) | 9/20 (45) | 6/14 (42.9) | 3/6 (50) | .783 |
| Mean diabetes duration (years) ± SD | 14.9 ± 7.4 | 14.9 ± 7.5 | 14.8 ± 8.0 | .980 |
| Mean HbA1c (%) ± SD | 8.3 ± 0.8 | 8.3 ± 0.8 | 8.3 ± 0.9 | .942 |
| Mean basal insulin dose (IU) ± SD | 36.2 ± 15.4 | 33.5 ± 10.0 | 42.5 ± 23.9 | .242 |
| Mean bolus insulin dose (IU) ± SD | 55.3 ± 27.1 | 59.3 ± 27.1 | 46.0 ± 27.2 | .328 |
| Mean body weight (kg) ± SD | 94.5 ± 17.3 | 94.6 ± 16.7 | 94.1 ± 20.2 | .950 |
| Mean BMI (kg/m2) ± SD | 33.2 ± 4.9 | 33.7 ± 4.9 | 32.1 ± 5.3 | .542 |
| Mean insulin demand (IU/Kg) ± SD | 0.97 ± 0.32 | 0.98 ± 0.34 | 0.92 ± 0.26 | .710 |
| # of patients with combination treatment with antidiabetic oral drugs (%) | 8/20 (40) | 7/14 (50) | 1/6 (16.7) | .181 |
| Mean diabetes related distress score ± SD | 26.7 ± 18.0 | 27.1 ± 19.3 | 26.0 ± 16.3 | .907 |
| Mean diabetes treatment satisfaction score ± SD | 28.7 ± 5.3 | 29.3 ± 4.4 | 27.3 7.3 | .465 |
This is a rather young sample with a majority of type 2 diabetic patients and suboptimal glycemic control. Individual insulin dose of 0.97 IU per kg body weight suggests rather high insulin resistance. Also, most study participants are overweight, as indicated by their body mass index (BMI 33.2 kg/m2). Nearly half of the sample received a combination treatment of insulin and antidiabetic oral drugs. In all cases the oral antidiabetic medication was Metformin, no participant received DPP-4 inhibitor or incretin treatment in addition to insulin treatment. The participants reported a rather low amount of diabetes-related distress and a high diabetes treatment satisfaction at baseline. Table 1 also provides sample characteristics for type 1 and type 2 diabetic patients separately. Except for the expected difference in age, there were no other important differences between these subgroups.
In Table 2, the numbers of valid pre- to postmeal comparisons, the mean carbohydrate intake, and the mean bolus insulin dose before breakfast and dinner are reported. The numbers of valid pre- to postmeal comparisons are highly comparable. This also applies to the amount of carbohydrates consumed and the number of prandial insulin units. In addition to these combined data for breakfast and dinner, the number of pre-to postmeal comparisons, the mean carbohydrate intake, and the mean bolus insulin dose are also separately reported at breakfast and dinner. The amounts of carbohydrates consumed and the number of prandial insulin units are rather similar at each of the 2 meals.
Table 2.
Number of Valid Pre- to Postmeal Comparisons and Treatment Factors at Breakfast and Dinner Combined and Separately.
| Without InsuPad | With InsuPad | P a | |
|---|---|---|---|
| Breakfast and dinner | |||
| # of valid pre- to postmeal comparisons | 30.3 ± 17.7 | 31.7 ± 13.8 | .784 |
| Carbohydrates (g) | 46.0 ± 14.6 | 44.1 ± 15.0 | .684 |
| Bolus insulin per meal (IU) | 20.1 ± 11.0 | 20.7 ± 11.2 | .406 |
| Pre- to postprandial time difference (min) | 105.8 ± 6.4 | 104.6 ± 9.9 | .570 |
| Breakfast | |||
| # of valid pre- to postmeal comparisons | 15.6 ± 8.8 | 16.3 ± 6.8 | .985 |
| Carbohydrates (g) | 44.3 ± 16.2 | 38.2 ± 11.8 | .289 |
| Bolus insulin (IU) | 23.8 ± 15.7 | 24.3 ± 14.2 | .805 |
| Pre- to postprandial time difference (min) | 105.4 ± 7.5 | 104.1 ± 10.0 | .657 |
| Dinner | |||
| # of valid pre- to postmeal comparisons | 15.5 ± 8.9 | 15.4 ± 8.0 | .696 |
| Carbohydrates (g) | 46.1 ± 16.8 | 43.0 ± 15.7 | .812 |
| Bolus insulin (IU) | 17.8 ± 10.3 | 18.4 ± 9.4 | .421 |
| Pre- to postprandial time difference (min) | 106.6 ± 7.3 | 104.8 ± 11.35 | .486 |
Controlled for order effects.
Effect on Postprandial Glucose Control
In Figure 1, the impact of InsuPad use on combined postprandial glucose excursion after breakfast and dinner is shown. Local heating of the skin at the injection site could reduce postprandial glucose excursions by approximately 15 mg/dl. Variance analysis controlling the order effect showed a significant effect of InsuPad use (P = .011), whereas there was no significant interaction effect between order and InsuPad use (P = .377).
Figure 1.
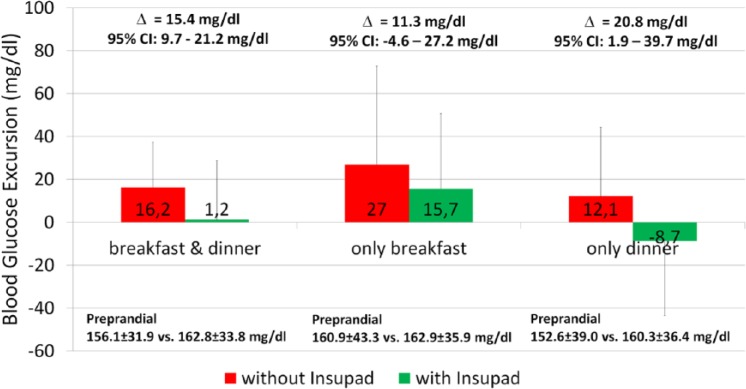
Effect of InsuPad use on postprandial glucose control after breakfast and dinner.
In a secondary analysis the impact of InsuPad use on postprandial glucose excursions was analyzed separately for breakfast and dinner. A reduction in postprandial glucose excursion was seen after breakfast when the InsuPad device was used, but it was not statistically significant (P = .199). InsuPad use after dinner was associated with a significant better postprandial glucose control. Order effects as indicated by significant interaction between the order of InsuPad use and postprandial glucose excursions were not significant for breakfast (P = .799) or for dinner (P = .320).
Overall Glycemic Control
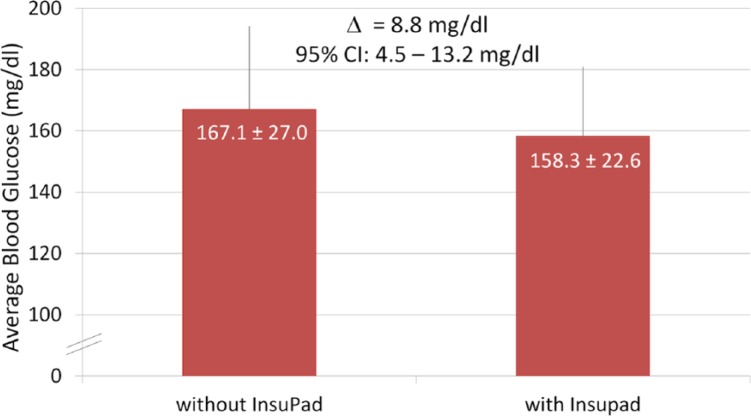
The impact of the InsuPad use on the overall glycemic control is based on results of blood glucose self-monitoring during the 2 study phases with and without the InsuPad use. Phases length were highly comparable (without InsuPad 30.0 ± 5.1 days vs with InsuPad 28.0 ± 1.0 days, P = .142). Figure 2 displays mean blood glucose levels as captured by BGM measurements with and without InsuPad use. Although the overall mean blood glucose level was approximately 9 mg/dl lower during the intervention phase using the InsuPad, the difference was not statistically significant (P = .099); there was also no significant interaction effect between order and InsuPad use (P = .101).
Figure 2.
Effect of InsuPad on the mean blood glucose levels (patient data from BGM).
Safety Parameters
No severe adverse events were recorded during the study. Similarly, no events were recorded in both study periods regarding severe hypoglycemic episodes (requiring third-party or medical assistance) as well as diabetic ketoacidosis (requiring medical assistance).
Table 3 shows the number of biochemically defined hypo- and hyperglycemic events per week during study phases with and without InsuPad use. Different biochemical thresholds to define these hypo- or hyperglycemic episodes were used. All these results are based on the data of patients’ BGM.
Table 3.
Percentage of Biochemically Defined Hypo- and Hyperglycemic Events.
| Without InsuPad | With InsuPad | P 1 | |
|---|---|---|---|
| Hypoglycemic events | |||
| % of events < 70 mg/dl | 3.5 | 3.6 | .972 |
| % of events < 60 mg/dl | 1.5 | 1.4 | .961 |
| % of events < 50 mg/dl | 0.4 | 0.6 | .271 |
| % of events < 40 mg/dl | 0.1 | 0.1 | .462 |
| Hyperglycemic events | |||
| % of events > 180 mg/dl | 35.2 | 31.6 | .241 |
| % of events > 240 mg/dl | 13.4 | 9.9 | .066 |
| % of events > 300 mg/dl | 4.0 | 2.6 | .098 |
| % of events > 350 mg/dl | 1.0 | 0.6 | .147 |
Controlled for order effects.
There was a reduction of severe high blood glucose values during the use of InsuPad, which failed to reach the significance level. The percentages of severe low blood glucose values were highly comparable between both study phases.
There were no severe device-related adverse events reported. There were also no malfunctions of the InsuPad device reported.
Patient-Reported Outcomes
Figure 3 shows the impact of the InsuPad use on diabetes-related distress, measured according to the PAID questionnaire, and diabetes treatment satisfaction, measured according to the DTSQ. There is no significant change in diabetes-related distress and treatment satisfaction. The level of diabetes-related distress was low at baseline. Results of the variance analysis showed that the use of InsuPad had no significant impact on diabetes-related distress (P = .196). The same was evident for diabetes treatment satisfaction. Treatment satisfaction was high at baseline and remained high during InsuPad use and non–InsuPad use. The variance analysis once again showed no significant impact of the InsuPad use on treatment satisfaction use (P = .647).
Figure 3.
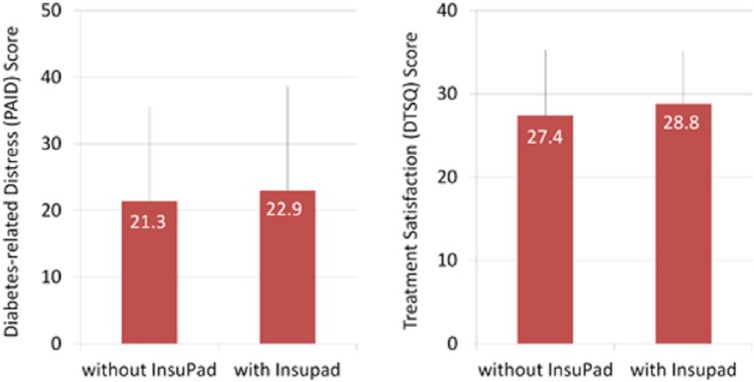
Effects of InsuPad use on diabetes-related distress and diabetes treatment satisfaction.
Discussion
The results of this outpatient study demonstrate that postprandial blood glucose excursions can be statistically significant reduced by the use of the InsuPad device in daily routine in insulin-resistant diabetic patients with multiple daily insulin injection therapy. The results corroborate laboratory findings using standard meals or liquid meals, namely that a beneficial effect on postprandial glucose control is not restricted to artificial experimental conditions, but is also evident in daily life with less standardized meals and timing of insulin injections. It also shows not only that diabetic patients on insulin pump therapy will benefit from local heating of the insulin infusion site, but also that people on MDI treatment can significantly reduce postprandial blood glucose excursions.
The effect of InsuPad on postprandial blood glucose was more pronounced after dinner than after breakfast. This may be due to circadian fluctuations of insulin sensitivity, which normally reaches its nadir in the morning. There was a reduction in high blood glucose values incidence during the use of InsuPad, which failed to reach the significance level. There was no significant impact of the InsuPad use on severe adverse events or biochemically defined hypoglycemic episodes.
Since the use of the InsuPad device requires additional effort from patients, it could not be excluded that the use of InsuPad had an unfavorable effect on treatment satisfaction or may lead to an increase of diabetes-related distress. However, study results indicate no disadvantageous effect of InsuPad on patient-reported outcomes such as self-reported diabetes-related distress or satisfaction with insulin treatment.
The study had some limitations that should be taken into consideration for the interpretation of the study. Patients were selected not according to diabetes type but according to a high insulin demand. This led to a mixed sample of type 1 and type 2 diabetic patients. However demographic data suggest that except for the expected age difference there were no substantial differences between the 2 patient subgroups. Insulin treatment was very similar in both groups. An exploratory adjustment of the statistical model (data not shown) for diabetes type yielded P values (dinner and breakfast P = .009, breakfast alone P = .424 and dinner alone P = .006) similar to the unadjusted variance analysis P values. It is therefore unlikely that diabetes type has specific influence on the effect of the InsuPad device on postprandial glucose control.
The observed postprandial blood glucose excursion was rather low. This might be due to several factors. One possible reason was the choice of postprandial glucose excursion measurement time. The ADA8 recommends considering blood glucose values 2 hours after the start of a meal as postprandial glucose values. The mean time interval in our study was approximately 110 minutes between meal and postprandial glucose measurement, which is close to the ADA definition. Since 1 aim of the study was to test the efficacy of the InsuPad under real-life conditions, a time window between 75 minutes and 135 minutes was allowed to capture postprandial glucose excursions. However this time window makes it unlikely that we have captured the maximal postprandial glucose excursion for each meal. Another possible reason for the low postprandial glucose excursions in this real-life-study compared to previous meal tests with the InsuPad device is that the meals were not standardized, which is unlike liquid meals or standardized meals, previously used to measure the effect of InsuPad. Also meals in this study might have varied with regard to fat and protein content. This might have also influenced magnitude and timing of postprandial glucose excursions unsystematically and may also contribute to the rather low mean postprandial blood glucose excursions. Given these varying amounts of carbohydrate and meal compositions, clearly a continuous glucose monitoring would have had the advantage to capture the maximal postprandial glucose excursions. However, since continuous glucose monitoring is not very common in Germany for this patient group and we wanted to test the impact of the InsuPad under daily life circumstances, we opted to use the self monitored blood glucose values.
Valid pairs of pre- and postprandial blood glucose measurements were available for only 50% of breakfasts and dinners. In this real-life study this might be partially due to flexibility of diet recommendations in multiple insulin injection regimens. However we don’t expect that this has biased the results of the study as the number of valid BGM measurement did not significantly differ between InsuPad use and non–InsuPad use.
Preprandial glucose values were rather high. However, since in this crossover study each patient served as his or her own control subject, it is unlikely that high preprandial glucose values in both study conditions are responsible for the observed study effect.
In summary, the study data indicate that the InsuPad device is efficient and safe for the reduction of postprandial blood glucose excursions without compromising patient satisfaction with diabetes treatment or increasing diabetes-related distress in insulin-resistant people with diabetes on a multiple daily insulin injection treatment. Studies done in rather lean type 1 diabetic populations2-4 suggest that the metabolic beneficial effects of local heating of the injection site are not restricted to insulin-resistant diabetic patients. Of course further studies are needed to clarify the transferability of these results to other diabetic populations.
Footnotes
Abbreviations: ADA, American Diabetes Association; BGM, blood glucose meter; CSII, continuous subcutaneous insulin infusion; DPP-4, dipeptidyl-peptidase-4; DTSQ, Diabetes Treatment Satisfaction Questionnaire; MDI, multiple daily injection; PAID, Problem Areas in Diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NH is a member of the Eli Lilly Global Diabetes Educator Advisory Board. GB is vice president research & diagnostics of Insuline Medical. BK is a member of an advisory board for Roche Diagnostics. TH is a member of the Sanofi-Aventis Global Advisory Board and the Insulin Medical Global Advisory Board.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by an unrestricted grant from Insuline Medical, Israel.
References
- 1. Heinemann L. Time-Action Profiles of Insulin Preparations. Mainz, Germany: Kirchheim; 2004. [Google Scholar]
- 2. Raz I, Weiss R, Yegorchikov Y, et al. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31:980-987. [DOI] [PubMed] [Google Scholar]
- 3. Cengiz E, Tamborlane W, Sherr JL, et al. Effect of a novel warming device on pharmacodynamics (PD) of rapid acting insulin in youth with type 1 diabetes (T1D). Diabetes Technol Ther. 2011;13:173-293. [Google Scholar]
- 4. Cengiz E, Weinzimer SA, Sherr JL, et al. Acceleration of insulin pharmacodynamic profile by a novel insulin infusion site warming device. Pediatr Diabetes. 2013;14:168-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freckmann G, Pleus S, Link M, et al. Performance evaluation of three continuous glucose monitoring systems: comparison of six sensors per subject in parallel. J Diabetes Sci Technol. 2013;7:842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760. [DOI] [PubMed] [Google Scholar]
- 7. Bradley C. Diabetes Treatment Satisfaction Questionnaire (DTQS). In: Bradley C, ed. Handbook of Psychology and Diabetes. Amsterdam, Netherlands: Harwood; 1994:111-132. [Google Scholar]
- 8. American Diabetes Association. Postprandial blood glucose. Consensus statement. Diabetes Care. 2001;24:775-778. [DOI] [PubMed] [Google Scholar]