Abstract
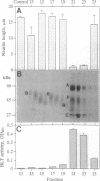
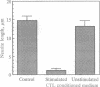
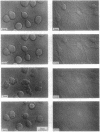
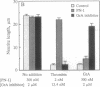
Granzymes are a family of serine proteases that are harbored in cytoplasmic granules of activated T lymphocytes and are released upon target cell interaction. Immediate and complete neurite retraction was induced in a mouse neuronal cell line when total extracts of granule proteins were added. This activity was isolated and identified as granzyme A. This protease not only induced neurite retraction at nanomolar concentrations but also reversed the stellation of astrocytes. Both effects were critically dependent on the esterolytic activity of granzyme A. As neurite retraction is known to be induced by thrombin, possible cleavage and activation of the thrombin receptor were investigated. A synthetic peptide spanning the N-terminal thrombin receptor activation sequence was cleaved by granzyme A at the authentic thrombin cleavage site Leu-Asp-Pro-Arg-Ser. Antibodies to the thrombin receptor inhibited both thrombin and granzyme A-mediated neurite retraction. Thus, T-cell-released granzyme A induces cellular responses by activation of the thrombin receptor. As brain-infiltrating CD4+ lymphocytes are the effector cells in experimental allergic encephalomyelitis, granzyme A released in the brain may contribute to the etiology of autoimmune disorders in the nervous system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Scarpellino L., Hertig S., Dupuis M., Tschopp J. Inhibition of lymphocyte mediated cytotoxicity by perforin antisense oligonucleotides. EMBO J. 1990 Dec;9(12):3815–3819. doi: 10.1002/j.1460-2075.1990.tb07599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G. The cytolytic T lymphocyte and its mode of action. Immunol Lett. 1989 Feb;20(3):169–178. doi: 10.1016/0165-2478(89)90075-8. [DOI] [PubMed] [Google Scholar]
- Bogenberger J., Haas M. cDNA clones from autocrine thymic lymphoma cells encode two mitogenic proteins, a serine protease and a truncated T-cell receptor beta-chain. Oncogene Res. 1988;3(4):301–312. [PubMed] [Google Scholar]
- Brosnan C. F., Cammer W., Norton W. T., Bloom B. R. Proteinase inhibitors suppress the development of experimental allergic encephalomyelitis. Nature. 1980 May 22;285(5762):235–237. doi: 10.1038/285235a0. [DOI] [PubMed] [Google Scholar]
- Cavanaugh K. P., Gurwitz D., Cunningham D. D., Bradshaw R. A. Reciprocal modulation of astrocyte stellation by thrombin and protease nexin-1. J Neurochem. 1990 May;54(5):1735–1743. doi: 10.1111/j.1471-4159.1990.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Kent S. B. Weighing naked proteins: practical, high-accuracy mass measurement of peptides and proteins. Science. 1992 Sep 25;257(5078):1885–1894. doi: 10.1126/science.1411504. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Vu T. K., Hung D. T., Wheaton V. I. Expression cloning and characterization of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Semin Thromb Hemost. 1992;18(2):161–166. doi: 10.1055/s-2007-1002422. [DOI] [PubMed] [Google Scholar]
- Dupuis M., Schaerer E., Krause K. H., Tschopp J. The calcium-binding protein calreticulin is a major constituent of lytic granules in cytolytic T lymphocytes. J Exp Med. 1993 Jan 1;177(1):1–7. doi: 10.1084/jem.177.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K., Chluba-de Tapia J., Hurtenbach U., Kramer M. D., Simon M. M. In vivo primed mouse T cells selectively express T cell-specific serine proteinase-1 and the proteinase-like molecules granzyme B and C. Int Immunol. 1991 Jan;3(1):9–19. doi: 10.1093/intimm/3.1.9. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Regulation of thrombin generation and functions. Semin Thromb Hemost. 1988 Jul;14(3):234–240. doi: 10.1055/s-2007-1002783. [DOI] [PubMed] [Google Scholar]
- Garcia-Sanz J. A., MacDonald H. R., Jenne D. E., Tschopp J., Nabholz M. Cell specificity of granzyme gene expression. J Immunol. 1990 Nov 1;145(9):3111–3118. [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Neurite outgrowth activity of protease nexin-1 on neuroblastoma cells requires thrombin inhibition. J Cell Physiol. 1990 Jan;142(1):155–162. doi: 10.1002/jcp.1041420119. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D., Simon M. M., Fruth U., Cunningham D. D. Protease nexin-1 complexes and inhibits T cell serine proteinase-1. Biochem Biophys Res Commun. 1989 May 30;161(1):300–304. doi: 10.1016/0006-291x(89)91596-9. [DOI] [PubMed] [Google Scholar]
- Haas M., Mally M. I., Bogenberger J. M., Bogart M. H., Buchanan M. A., Augery-Bourget Y., Hyman R., Vogt M. Autocrine growth and progression of murine X-ray-induced T cell lymphomas. EMBO J. 1986 Aug;5(8):1775–1782. doi: 10.1002/j.1460-2075.1986.tb04426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P., Yue C. C. The role of cytoplasmic granules in lymphocyte cytotoxicity. Prog Allergy. 1988;40:82–110. [PubMed] [Google Scholar]
- Jalink K., Moolenaar W. H. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992 Jul;118(2):411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
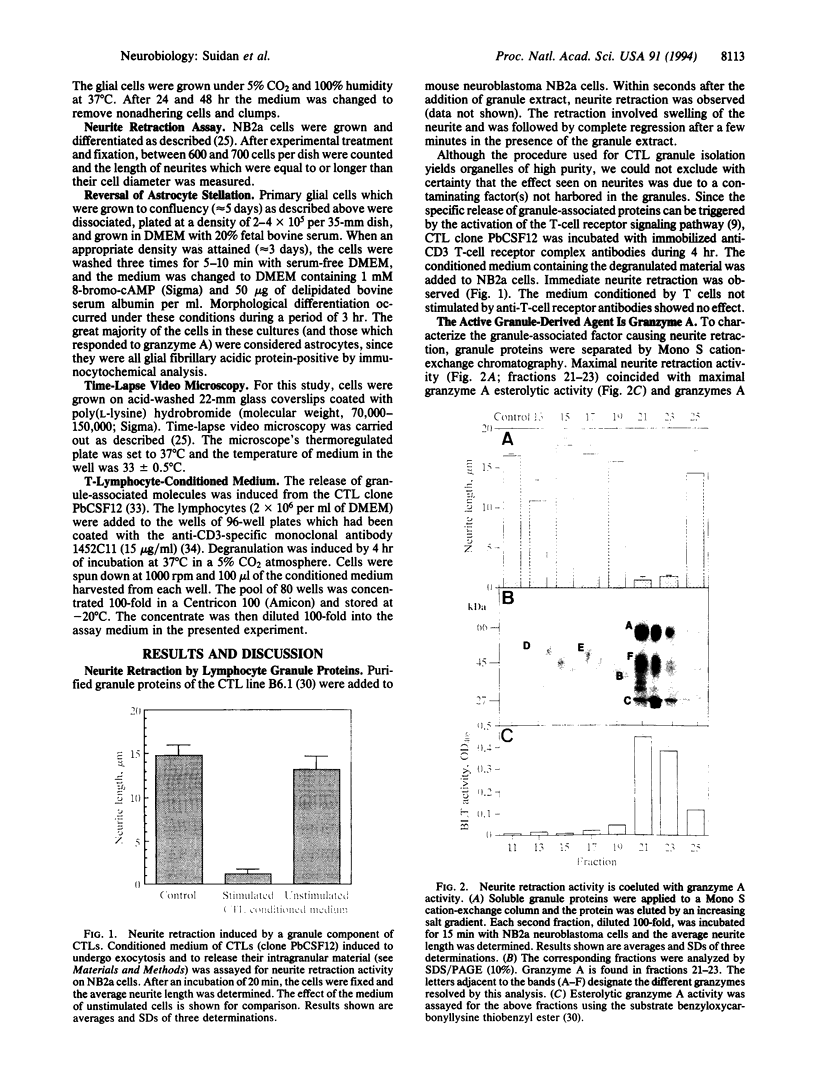
- Jenne D. E., Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Keane R. W., Tallent M. W., Podack E. R. Resistance and susceptibility of neural cells to lysis by cytotoxic lymphocytes and by cytolytic granules. Transplantation. 1992 Sep;54(3):520–526. doi: 10.1097/00007890-199209000-00025. [DOI] [PubMed] [Google Scholar]
- Kramer M. D., Binninger L., Schirrmacher V., Moll H., Prester M., Nerz G., Simon M. M. Characterization and isolation of a trypsin-like serine protease from a long-term culture cytolytic T cell line and its expression by functionally distinct T cells. J Immunol. 1986 Jun 15;136(12):4644–4651. [PubMed] [Google Scholar]
- Krähenbühl O., Rey C., Jenne D., Lanzavecchia A., Groscurth P., Carrel S., Tschopp J. Characterization of granzymes A and B isolated from granules of cloned human cytotoxic T lymphocytes. J Immunol. 1988 Nov 15;141(10):3471–3477. [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Schmidt R. E., Caulfield J. P., Hein A., Bartley G. T., Ritz J., Schlossman S. F., Austen K. F., Stevens R. L. Proteoglycans in cell-mediated cytotoxicity. Identification, localization, and exocytosis of a chondroitin sulfate proteoglycan from human cloned natural killer cells during target cell lysis. J Exp Med. 1985 Dec 1;162(6):1771–1787. doi: 10.1084/jem.162.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Howell M. D., Jaraquemada D., Flerlage M., Richert J., Brostoff S., Long E. O., McFarlin D. E., McFarland H. F. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991 Jan 1;173(1):19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Corthésy P., Nabholz M., Tschopp J. Appearance of cytolytic granules upon induction of cytolytic activity in CTL-hybrids. EMBO J. 1985 Oct;4(10):2533–2538. doi: 10.1002/j.1460-2075.1985.tb03967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. 1987 Jun 5;49(5):679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Masson D., Zamai M., Tschopp J. Identification of granzyme A isolated from cytotoxic T-lymphocyte-granules as one of the proteases encoded by CTL-specific genes. FEBS Lett. 1986 Nov 10;208(1):84–88. doi: 10.1016/0014-5793(86)81537-x. [DOI] [PubMed] [Google Scholar]
- Nelson R. B., Siman R. Thrombin and its inhibitors regulate morphological and biochemical differentiation of astrocytes in vitro. Brain Res Dev Brain Res. 1990 Jun 1;54(1):93–104. doi: 10.1016/0165-3806(90)90069-b. [DOI] [PubMed] [Google Scholar]
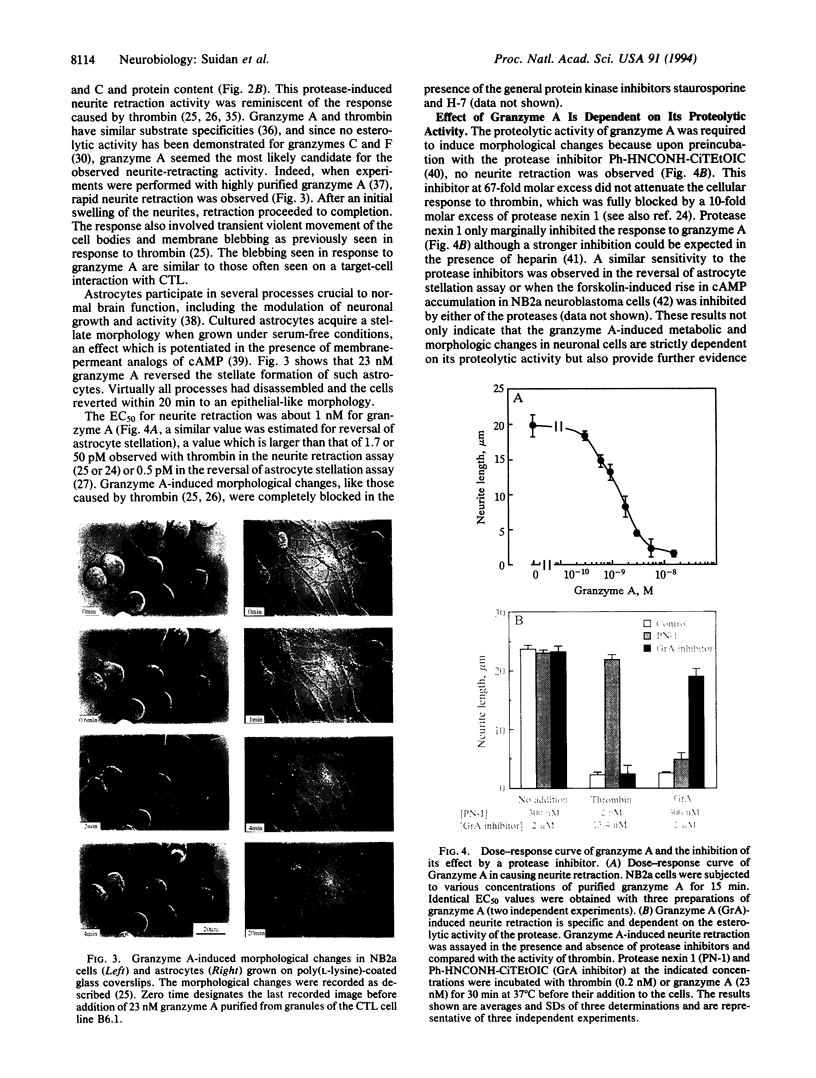
- Odake S., Kam C. M., Narasimhan L., Poe M., Blake J. T., Krahenbuhl O., Tschopp J., Powers J. C. Human and murine cytotoxic T lymphocyte serine proteases: subsite mapping with peptide thioester substrates and inhibition of enzyme activity and cytolysis by isocoumarins. Biochemistry. 1991 Feb 26;30(8):2217–2227. doi: 10.1021/bi00222a027. [DOI] [PubMed] [Google Scholar]
- Perraud F., Besnard F., Sensenbrenner M., Labourdette G. Thrombin is a potent mitogen for rat astroblasts but not for oligodendroblasts and neuroblasts in primary culture. Int J Dev Neurosci. 1987;5(3):181–188. doi: 10.1016/0736-5748(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Olsen K. J., Lowrey D. M., Lichtenheld M. Structure and function of perforin. Curr Top Microbiol Immunol. 1989;140:11–17. doi: 10.1007/978-3-642-73911-8_2. [DOI] [PubMed] [Google Scholar]
- Poe M., Bennett C. D., Biddison W. E., Blake J. T., Norton G. P., Rodkey J. A., Sigal N. H., Turner R. V., Wu J. K., Zweerink H. J. Human cytotoxic lymphocyte tryptase. Its purification from granules and the characterization of inhibitor and substrate specificity. J Biol Chem. 1988 Sep 15;263(26):13215–13222. [PubMed] [Google Scholar]
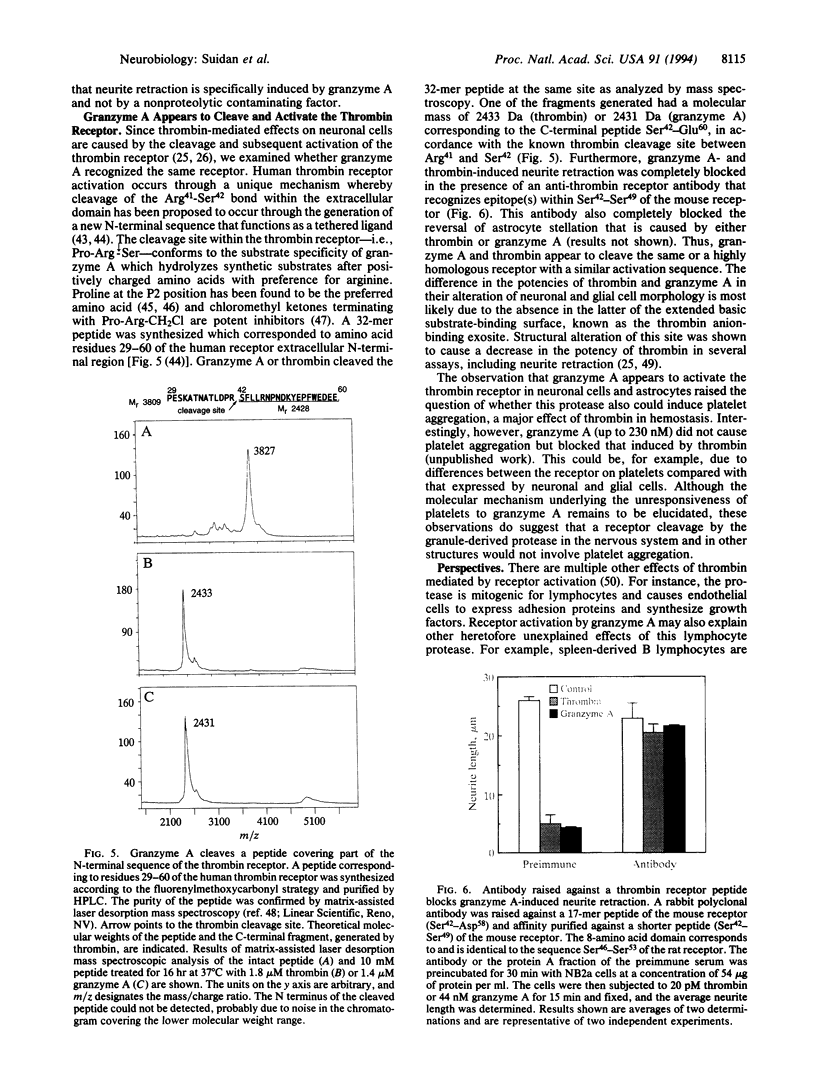
- Romero P., Eberl G., Casanova J. L., Cordey A. S., Widmann C., Luescher I. F., Corradin G., Maryanski J. L. Immunization with synthetic peptides containing a defined malaria epitope induces a highly diverse cytotoxic T lymphocyte response. Evidence that two peptide residues are buried in the MHC molecule. J Immunol. 1992 Mar 15;148(6):1871–1878. [PubMed] [Google Scholar]
- Rovelli G., Stone S. R., Preissner K. T., Monard D. Specific interaction of vitronectin with the cell-secreted protease inhibitor glia-derived nexin and its thrombin complex. Eur J Biochem. 1990 Sep 24;192(3):797–803. doi: 10.1111/j.1432-1033.1990.tb19293.x. [DOI] [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. L. Morphological and biochemical alterations in foetal rat brain cells cultured in the presence of monobutyryl cyclic AMP. Nature. 1973 Jan 19;241(5386):203–204. doi: 10.1038/241203a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Takio K., Okumura K. Homology of perforin to the ninth component of complement (C9). Nature. 1988 Aug 11;334(6182):525–527. doi: 10.1038/334525a0. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Fruth U., Simon H. G., Kramer M. D. Evidence for the involvement of a T-cell-associated serine protease (TSP-1) in cell killing. Ann Inst Pasteur Immunol. 1987 Mar-Apr;138(2):309–314. doi: 10.1016/s0769-2625(87)80085-5. [DOI] [PubMed] [Google Scholar]
- Suidan H. S., Stone S. R., Hemmings B. A., Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992 Feb;8(2):363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Suidan H. S., Tolkovsky A. M. Simultaneous analysis of adenosine 3',5'-cyclic monophosphate accumulation and adenosine 5'-triphosphate metabolism in cultured cells preincubated with [2-3H]adenine. Anal Biochem. 1992 Aug 15;205(1):159–165. doi: 10.1016/0003-2697(92)90593-v. [DOI] [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Takayama H., Sitkovsky M. V. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J Exp Med. 1987 Sep 1;166(3):725–743. doi: 10.1084/jem.166.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplits M. S., Henkart P. A., Hodes R. J. T helper cell cytoplasmic granules. Exocytosis in response to activation via the T cell receptor. J Immunol. 1988 Jul 1;141(1):1–9. [PubMed] [Google Scholar]
- Tas P. W., Koschel K. Thrombin reverts the beta-adrenergic agonist-induced morphological response in rat glioma C6 cells. Exp Cell Res. 1990 Jul;189(1):22–27. doi: 10.1016/0014-4827(90)90251-5. [DOI] [PubMed] [Google Scholar]
- Traugott U., Reinherz E. L., Raine C. S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983 Jan 21;219(4582):308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. The role of cytoplasmic granule components in cytolytic lymphocyte-mediated cytolysis. Ann Inst Pasteur Immunol. 1987 Mar-Apr;138(2):290–295. doi: 10.1016/s0769-2625(87)80081-8. [DOI] [PubMed] [Google Scholar]
- Vanguri P., Lee E., Henkart P., Shin M. L. Hydrolysis of myelin basic protein in myelin membranes by granzymes of large granular lymphocytes. J Immunol. 1993 Mar 15;150(6):2431–2439. [PubMed] [Google Scholar]
- Vernadakis A. Neuron-glia interrelations. Int Rev Neurobiol. 1988;30:149–224. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Wheaton V. I., Hung D. T., Charo I., Coughlin S. R. Domains specifying thrombin-receptor interaction. Nature. 1991 Oct 17;353(6345):674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Cellular and humoral mechanisms of cytotoxicity: structural and functional analogies. Adv Immunol. 1987;41:269–332. doi: 10.1016/s0065-2776(08)60033-4. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]