Abstract
Objectives. We considered changes in the geographic distribution of early stage breast cancer among White and non-White women while secular trends in lifestyle and health care were under way.
Methods. We aggregated tumor registry and census data by age, race, place of residence, and year of diagnosis to evaluate rate variation across Connecticut census tracts between 1985 and 2009. Global and local cluster detection tests were completed.
Results. Age-adjusted incidence rates increased by 2.71% and 0.44% per year for White and non-White women, respectively. Significant global clustering was identified during surveillance of these populations, but the elements of clustering differed between groups. Among White women, fewer local clusters were detected after 1985 to 1989, whereas clustering increased over time among non-White women.
Conclusions. Small-area variation of breast cancer incidence rates across time periods proved to be dynamic and race-specific. Incidence rates might have been affected by secular trends in lifestyle or health care. Single cross-sectional analyses might have confused our understanding of disease occurrence by not accounting for the social context in which patient preferences or provider capacity influence the numbers and locations of diagnosed cases. Serial analyses are recommended to identify “hot spots” where persistent geographic disparities in incidence occur.
Variation from place to place in the occurrence of disease and use of health services is real, although much of the recorded variation has not been fully explained or effectively addressed.1,2 Some variation reflects differences in risk profiles across populations related to biology,3 geography,4 behavioral risks,5 physical environments,6 social ecology,7 or socioeconomic determinants.8 When noted, such differences inform us of etiological forces at play and our potential options for controlling disease burden among high-risk groups. For example, patterns of breast cancer incidence are often attributed to the “geography of affluence” in diet, reproduction, and related behaviors.5,8 Disease control efforts based on that understanding rightfully focus on locales where individuals have appropriate risk profiles.
Variation that is not readily explained by patient or disease factors may be the artifact of a community’s capacity to monitor health events or deliver health services.9 Regional differences in disease occurrence have been repeatedly attributed to variation in the intensity of screening detection efforts.10–13 Likewise, volatility of breast cancer incidence rates has been associated with changes and regional variation in the use of hormone replacement therapies (HRTs).14–16
It is important to distinguish the effects of biological or demographic forces of disease from health system variability in its capacity to deliver care. We illustrated the dynamic temporal and spatial nature of early stage breast cancer incidence within small areas of Connecticut between 1985 and 2009. Because secular trends in lifestyle and health services occurred during this period, changes in breast screening practices13 and menopausal hormone therapy use,16 together with cohort differences in body mass index,17 reproductive health,18 and socioeconomic status,8,19 may well have affected the spatial and temporal presentation of disease within populations. Although not a direct test of the effects of these associations on the geography of breast cancer incidence, we offered insight with regard to the appropriateness of considering context when examining temporal and spatial disparities in disease occurrence.
METHODS
Using 25 years of data from the Connecticut Tumor Registry (CTR),20 we examined the geographic distribution of early stage (i.e., locally invasive) breast cancer (International Classification of Disease codes 0–3; C50.0–50.921) within the state, and hypothesized that rate variation represented, to varying degrees, the composite effects of underlying biological, demographic, and health system factors. Between 1985 and 2009, the CTR identified 40 747 cancers in women in Connecticut, which we aggregated according to 5 surveillance periods (1985–1989, 1990–1994, 1995–1999, 2000–2004, and 2005–2009), 7 age groups (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years), and 827 census tracts of residence (according to geographic boundaries for the 2010 federal census) at time of diagnosis. Because the racial distribution of the state’s population closely paralleled the patterns of income segregation (i.e., the clustering of households according to income levels) that could underscore geographic differences in breast cancer risk profiles and health care use,8,22 we completed separate analyses for the 38 328 White and 2419 non-White (of whom 84% were African American) women.
We produced race-specific and directly age-adjusted statewide incidence rates for each surveillance period by combining aggregate case counts from the CTR with population at-risk estimates interpolated from US Census data files from 1990, 2000, and 2010.23 In turn, race-specific and indirectly age-adjusted rates were produced for each of the state’s 827 census tracts by combining the statewide rates with the estimated populations at-risk.
We examined temporal changes in early stage breast cancer incidence rates using Joinpoint regression analysis24 and the SaTScan by Kulldorff et al.25 Joinpoint analysis uses multisegment least-squares regression to characterize rate changes over time. SaTScan is capable of evaluating significant disparities of observed-to-expected case counts for random durations of time within the overall surveillance period.
We examined the geographic variation of incidence rates using both global (Ipop by Oden26) and local (SaTScan) spatial statistics. The Ipop by Oden (computed using ClusterSeer27) provided an overall assessment of the tendency of data to spatially cluster by estimating the extent to which case counts, in relation to population densities throughout Connecticut, depart in their distribution from randomness. The assessment by Ipop of spatial autocorrelation contrasted observed and expected case counts to estimate the proportion of statewide variability attributed to the co-location of census tracts with comparable case rates (percent variation across tracts).
We used the SaTScan by Kuldorff28 (version 9.1.129) to aggregate the case counts and underlying populations at-risk within census tracts by scanning circles of varying size (encompassing 1%–50% of the at-risk population), independent of existing geopolitical units (e.g., counties or towns) to discern where age-adjusted and race-specific rates significantly departed, whether high or low, from expectation. The spatial scan statistic estimated the risk of cancer incidence among women within a designated area, relative to the risk among women who resided elsewhere around Connecticut. Extensive descriptions and assessments of these and other cluster detection methods are available elsewhere.30,31
RESULTS
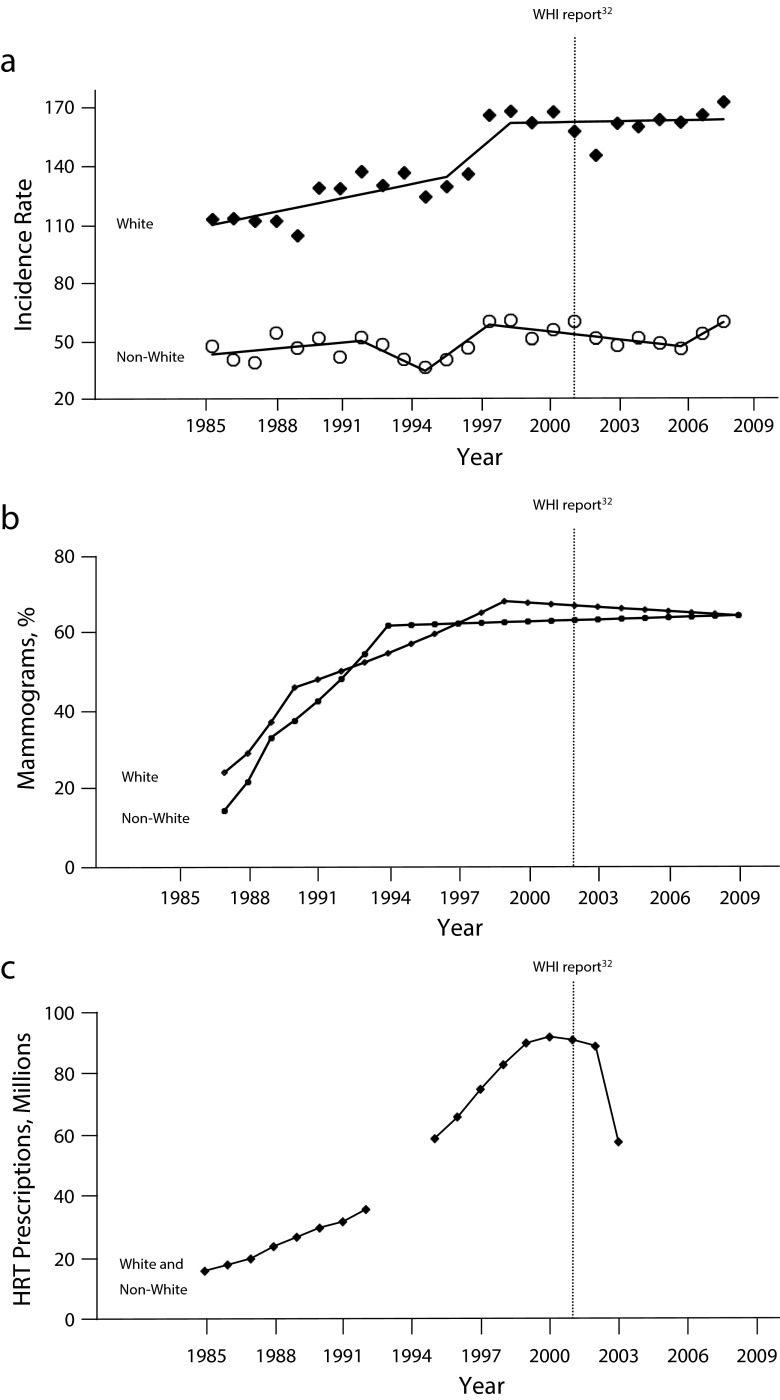
Among White women, an average age-adjusted rate increment of 2.71% per year (P < .001) was observed and attributed by Joinpoint analysis (Figure 1a) to 3 distinct periods of time: 1985 to 1996 (+2.2% per year), 1996 to 1999 (+9.2%), and 1999 to 2009 (+0.02%). The SaTScan of temporal variation identified the final 12 years of surveillance (1998–2009) as a period of significant temporal variation in disease incidence (i.e., the ratio of observed-to-expected cases was 1.12; P < .001).
FIGURE 1—
Trends in (a) age-adjusted early stage breast cancer incidence, (b) percentage of mammography screening in previous 2 years, and (c) hormone replacement therapy (HRT) prescriptions (in millions): Connecticut, 1985–2009.
Note. WHI = Women’s Health Initiative.
Source: (a) CT Tumor Registry,20 (b) Behavioral Risk Factor Surveillance System data,32 and (c) National Prescription Audit data.14,15
For non-White women, the average increment in age-adjusted breast cancer incidence was considerably less (+0.44% per year; P = .02), and attributable to 5 brief time periods: 1985 to 1992 (+1.0% per year), 1992 to 1995 (− 5.1%), 1995–1998 (+7.9%), 1998 to 2007 (− 1.2%), and 2007 to 2009 (+6.3%). The relatively greater rate volatility among non-White women was partially caused by their small numbers within Connecticut (i.e., annual averages of 97 incident cases among 193 675 at-risk women, compared with annual averages of 1533 cases among 1.1 million at-risk White women). Still, similar to findings noted for White women, the SaTScan identified the final 12 years of surveillance (1998–2009) as a period of significant temporal variation in disease incidence among non-White women (i.e., the ratio of observed-to-expected cases was 1.08; P < .001).
The annual breast cancer incidence rates for White women co-varied with nationwide data on annual mammography use32 and HRT prescription volume14,15 (r = 0.97 and 0.90, respectively). Increasing incidence rates between 1985 and 1999 paralleled the expansion of mammography and HRT before 2000, as did the slowing of incidence rates thereafter, following the report of adverse health outcomes associated with HRT use33 (Figure 1b and 1c). By comparison, the annual breast cancer incidence rates for non-White women were less strongly, but still meaningfully, associated with mammography and HRT use over time (r = 0.64 and 0.63, respectively).
Tables 1 and 2 summarize the global and local cluster analyses. The Ipop test identified significant global rate variation across Connecticut (P < .01) for both groups during every surveillance period, but the elements of clustering differed between White and non-White populations. For White women, the Ipop analysis found the proximity of census tracts with comparable incidence rates (identified as percent variation across tracts) decreased between 1985 to 1989 and 2000 to 2004. That is, the co-location of census tracts with similar rates of disease had progressively lesser impact (i.e., reduced geographic disparity) on global rate variation across Connecticut, decreasing by approximately one third from 38.1% to 25.7% during this time. However, that declining trend was reversed for 2005 to 2009 when the Ipop statistic attributed 33.6% of the global rate variability to the proximity of census tracts with comparable disease rates. With regard to the overall distribution of incidence rates among non-White women, the Ipop analysis for 1985 to 1989 found that the proximity of census tracts with comparable incidence rates had minimal influence; 4.9% of the overall variation was found in the statewide non-White rate of disease. However, the proportion of statewide variation attributed to co-location of tracts with similar disease rates increased 5-fold to 26.3% by 2005 to 2009, which signaled increased geographic disparities in breast cancer in non-White women over time.
TABLE 1—
Global and Local Cluster Analysis of Age-Adjusted Early Stage Breast Cancer Incidence Rates Within Census Tracts Among White Women ≥ 20 Years of Age: Connecticut, 1985–2009
| SaTScan26 |
|||||||
| Time Period | IPop,25 % Variation Across Tracts | Locationa | Population at-Riskb | % Affected Population | Age-Adjusted Rate | Relative Riskc | Pd |
| 1985–1989 | 38.1 | Statewide | 1 139 905 | 100.0 | 100.0 | 1.00 | . . . |
| 1 | 155 379 | 13.6 | 74.6 | 0.72 | < .001 | ||
| 2 | 86 084 | 7.6 | 131.5 | 1.35 | < .001 | ||
| 3 | 150 193 | 13.6 | 83.1 | 0.81 | .009 | ||
| 4 | 131 640 | 11.5 | 117.1 | 1.20 | .043 | ||
| 5 | 8 360 | 0.7 | 37.2 | 0.37 | .045 | ||
| 1990–1994 | 35.4 | Statewide | 1 131 106 | 100.0 | 123.7 | 1.00 | . . . |
| 6 | 115 738 | 10.2 | 86.6 | 0.69 | < .001 | ||
| 7 | 33 905 | 3.0 | 176.9 | 1.45 | < .001 | ||
| 8 | 157 763 | 13.9 | 147.3 | 1.23 | < .001 | ||
| 9 | 75 310 | 6.6 | 093.8 | 0.75 | < .001 | ||
| 1995–1999 | 30.0 | Statewide | 1 109 529 | 100.0 | 141.6 | 1.00 | . . . |
| 10 | 25 870 | 2.3 | 198.5 | 1.42 | .001 | ||
| 11 | 62 438 | 5.6 | 172.3 | 1.23 | .047 | ||
| 2000–2004 | 25.7 | Statewide | 1 099 722 | 100.0 | 159.0 | 1.00 | . . . |
| 12 | 13 431 | 1.2 | 86.4 | 0.54 | .004 | ||
| 2005–2009 | 33.6 | Statewide | 1 107 195 | 100.0 | 163.2 | 1.00 | . . . |
| 13 | 197 641 | 17.8 | 140.7 | 0.84 | < .001 | ||
| 14 | 124 448 | 11.2 | 193.2 | 1.21 | < .001 | ||
| 15 | 7 128 | 0.6 | 333.1 | 2.05 | .002 | ||
Area locations illustrated in Figure 2.
Population counts of women ≥ 20 years of age are based on interpolation of 1990, 2000, and 2010 data according to 2010 US Census tract boundaries.
Risk estimates of the likelihood an observed rate within an area was significantly different from the rate observed outside that area.
Estimating the likelihood of rate variation relative to the null hypothesis according to 9999 Monte Carlo simulations.
TABLE 2—
Global and Local Cluster Analysis of Age-Adjusted Early Stage Breast Cancer Incidence Rates Within Census Tracts Among Non-White Women ≥ 20 Years of Age: Connecticut, 1985–2009
| SaTScan26 |
|||||||
| Time Period | IPop,25 % Variation Across Tracts | Locationa | Population at-Riskb | % Affected Population | Age-Adjusted Rate | Relative Riskc | Pd |
| 1985–1989 | 4.9 | Statewide | 144 454 | 100.0 | 37.9 | 1.00 | . . . |
| A | 818 | 0.6 | 202.6 | 5.54 | .022 | ||
| 1990–1994 | 6.5 | Statewide | 157 374 | 100.0 | 44.2 | 1.00 | . . . |
| 1995–1999 | 11.9 | Statewide | 189 055 | 100.0 | 49.0 | 1.00 | . . . |
| 2000–2004 | 14.6 | Statewide | 221 761 | 100.0 | 55.1 | 1.00 | . . . |
| B | 28 191 | 12.7 | 92.4 | 1.86 | < .001 | ||
| C | 18 346 | 8.3 | 23.3 | 0.40 | .047 | ||
| 2005–2009 | 26.3 | Statewide | 255 716 | 100.0 | 56.6 | 1.00 | . . . |
| D | 66 983 | 26.2 | 75.0 | 1.51 | .002 | ||
| E | 34 948 | 13.7 | 33.2 | 0.55 | .034 | ||
Area locations illustrated in Figure 2.
Population counts of non-White women ≥ 20 years of age are based on interpolation of 1990, 2000, and 2010 data according to 2010 US Census tract boundaries.
Risk estimates of the likelihood an observed rate within an area was significantly different from the rate observed outside that area.
Estimating the likelihood of rate variation relative to the null hypothesis according to 9999 Monte Carlo simulations.
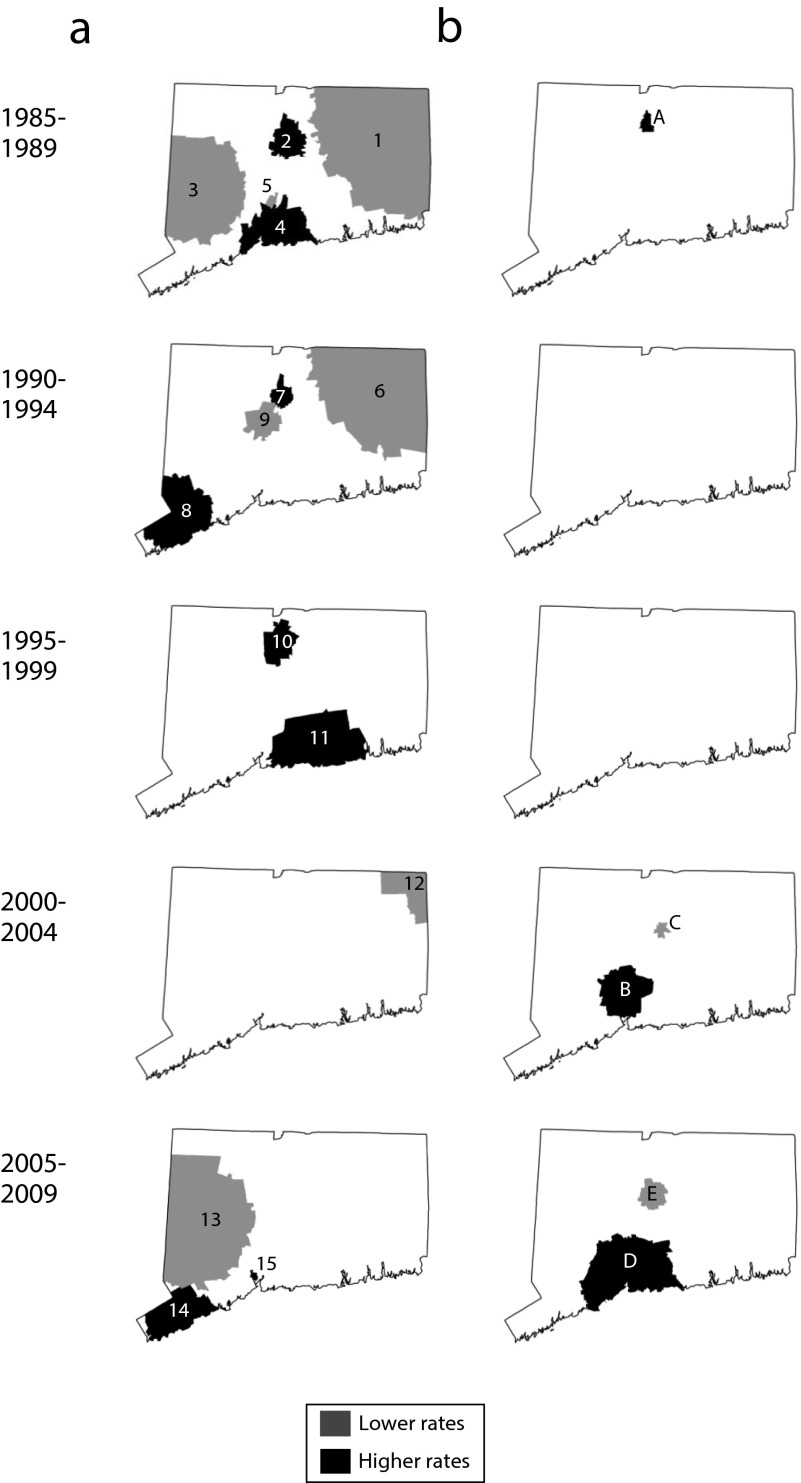
Our findings on the global variation were borne out in the analysis of local event clustering. For the 1985 to 1989 surveillance period, the spatial scan statistic identified 5 locations where average annual age-adjusted early stage breast cancer incidence rates for White women differed significantly from the experiences of at-risk women residing elsewhere around Connecticut (Figure 2a). Areas 1 and 3 on the perimeter of the state had rates approximately 20% lower than other places around the state, whereas Area 5 in central Connecticut had a rate two thirds lower than that of the rest of Connecticut. Areas 2 and 4 along the state’s central corridor, by comparison, were places with rates for White women that exceeded expectations by 35% and 20% respectively. Taken together, these 5 locations involved approximately 45% of diagnosed cases and 47% of the state’s at-risk White population during this period.
FIGURE 2—
SaTScan analysis of significant age-adjusted early-stage breast cancer incidence rates among (a) White women and (b) non-White women: Connecticut: 1985–2009.
Consistent with findings from the Ipop analysis, progressively fewer clusters that involved smaller segments of the at-risk population were identified by the spatial scan statistic between 1985 to 1989 and 2000 to 2004. For 1990 to 1994, the spatial scan statistic identified 4 locations (Areas 6–9; Figure 2a) that pertained to fewer at-risk White women around the state (34% vs 47%) and cases (33% vs 45%) than those detected during 1985 to 1989. Areas 3 to 5 in the initial analysis were found to be no longer significantly different from the statewide rate, whereas a new location (Area 8) reached statistical significance. Areas 6 and 7 were geographically similar to the previously identified Areas 1 and 2.
Geographic smoothing of age-adjusted rates for White women continued through 1995 to 1999. Two locations (Areas 10 and 11; Figure 2a) within the state’s central corridor, which pertained to only 11% of cases and 8% of Connecticut’s at-risk women, reached thresholds for significantly elevated disease rates, whereas no place yielded significantly fewer than expected breast cancer cases. By 2000 to 2004, only a single location in northeastern Connecticut exhibited a lower than expected incidence (Area 12), such that only 99% of at-risk White women around Connecticut lived in locations that had geographically equivalent risks of being diagnosed with early disease. However, this trend toward homogeneity of rates reversed somewhat during 2005 to 2009, where the spatial scan statistic identified 2 locations with significantly high (Areas 13 and 15) and 1 location with significantly low (Area 14) rates of disease that, together, affected approximately one third of at-risk White women within the state.
Local rate variation pertained to non-White women in Connecticut, but in a manner that was dissimilar to the previously described pattern described. Unlike White women, the geography of average annual age-adjusted rates for early breast cancer among non-White women was essentially homogeneous between 1985 and 1999, with progressively more variation noted thereafter. Area A in north central Connecticut (Figure 2b), a place that yielded 12 cases among 818 at-risk non-White women (0.6% of the total), for a local rate 5.54 times greater than that among non-White women residing elsewhere around the state, was the exception. However, by 2000 to 2004 a significant rate variation was identified within 2 settings (Areas B and C) that were home to approximately 20% of the state’s non-White population. By 2005 to 2009, further variation was identified (Areas D and E), which affected approximately 40% of the at-risk, non-White women of Connecticut.
DISCUSSION
Geographic variation in disease incidence is real, and its complex nature is not fully understood. As a consequence, our ability to effectively control (i.e., minimize) geographic disparities in health events is suboptimal. Where breast cancer is diagnosed goes beyond biological, behavioral, environmental, or social exposures of at-risk individuals to include contextual aspects of patient preference, health care delivery, and informatics that influence the likelihood disease is detected and reported. Previously, we described the potential “detection effects” of screenable conditions on the geography of disease.34,35 Such findings contribute to the growing literature on how access health services may indirectly influence the temporal and spatial distributions of disease rates.
We documented the dynamic nature of breast cancer case clusters among White and non-White women over a 25-year period of surveillance. Our findings underscore the importance of cautious assessment before drawing conclusions about causation or control strategies.
We reported findings based on Ipop, which were similar to those generated by SaTScan, and we believe that using multiple cluster detection methods enhances confidence and understanding beyond what is possible by using either alone. We found global variation of breast cancer incidence rates to be evident over time, but that the elements of global clustering differed between White and non-White populations. We further observed that local clustering of cancer rates were dynamic and race-specific across time periods. In the absence of discernable changes in disease etiology or population trends, it could be reasonably concluded that our results might have been affected by secular trends in lifestyle or health care, as well as artifacts of disease detection methods. However, such attribution is beyond the scope of this article and, therefore, untested. Although plausible, several concerns lessen the complete enthusiasm for such a conclusion. At best, our findings are not inconsistent with the expectations that changes in women’s health care could have influenced geographic and temporal differences in disease incidence, but our findings are not sufficient to assert such associations as root causes of observed trends.
Limitations
Our disparate findings on geography of breast cancer among White and non-White women are not easily explained. For White women, our data suggested a trend toward greater homogeneity of rates over much of the surveillance period (fewer, less extensive local clusters and less variation of global clustering that were attributable to proximity of comparable rates around the state), whereas we noted increased heterogeneity of rates for non-White women. Although secular trends in utilization of mammography and HRT therapy might have accounted for overall changes in disease incidence rates for Connecticut, data on race-specific health care use and its effects on clinical outcomes were less abundant and were not directly examined here.
Other secular trends at play, as related to diet and lifestyle, childbearing and child rearing histories, education and employment opportunities, etc., could have accounted for our findings. For example, obesity prevalence that preceded and coincided with this time period17 could have affected incidence and detection patterns of breast cancers to the extent that obesity varied geographically. Likewise, disparate changes to the socioeconomic standing of women and households via education and workplace opportunities might have been relevant.36,37 The capacity of these factors to exert individual, system level, and interactive effects on population health outcomes limit the interpretability of our findings and underscored the need for further detailed analyses.
We do not believe that the demographic changes within Connecticut’s population, alone, were sufficient to produce these results. The comparison of selected census tract data from 1990, 2000, and 2010 regarding population density, the percentage of persons aged 60 years and older, the percentage of non-White persons, the percentage of persons below the poverty level, and per capita income yielded highly correlated results (r = 0.83–0.99), which were indicative of a rather stable population structure within Connecticut. Moreover, the feasibility seemed low; therefore, cyclical population changes could have produced the dynamic results reported here.
Our findings that White incidence rates increased over time, whereas their geographic rate variation diminished, was consistent with a presumption that diffusion of women’s health services over these 25 years served to eliminate disparities in disease occurrence by expanding capacities to detect indolent disease across locales. As health services reached increasing numbers of women during the 1990s and penetrated previously underscreened locales, it could be predicted that the overall rate of disease would have increased through identification of occult cases while relative rate differences between communities decreased. Places with initially low rates would no longer have been so low, whereas places with initially high rates would no longer have appeared as high. If so, the persistence of local variation in disease rates over time might signal genuine inequities in health care delivery where some populations were consistently underserved. Whether the finding of increasing rate variability among non-White women signaled increasing disparities among that population of women deserves attention. Likewise, whether the geographic rate variation we reported here mimics a range of other (unexamined) geographic disparities pertaining to the availability of surgical and adjuvant therapy options or the availability of clinical trials and other ancillary services for women should be considered.
The validity of our findings depends heavily on the tools we selected for analysis. Our measures for global and local cluster detection are well known and routinely used, but they are still not foolproof. The interpretation of no results (i.e., the absence of identified clusters) is challenging because having no results does not necessarily equate with “failure to reject” the null hypothesis. Consensus on how best to use the SaTScan is elusive. Analyses by scanning circles smaller than those of SaTScan’s default setting (i.e., up 50% of at-risk populations), or the aggregation of data into lesser or longer periods of time, might highlight spatial patterns we could not discern. Thorough consideration of these and other issues in cluster analysis are found elsewhere.38,39
Conclusions
In summary, our findings highlight the dynamic conditions of disease surveillance and the importance of ongoing linear panel studies.40 On the one hand, our finding of transitional disparities (i.e., places where extreme rates occurred for limited time periods) offers the potential to increase our understanding of specific factors that might contribute to the under- and overdetection of disease relative to other locales. On the other hand, attention to the results of single cross-sectional analyses might distract attention from the social context in which lifestyle, patient preferences, or provider capacity have longer-term influences on geographic variation that we sought to control. We recommend that serial analyses be used to identify “hot spots” where persistent geographic disparities in incidence occur. We believe this article provides a relevant example of the challenges ahead in addressing the geography of racial disparities that affect many underserved populations. Our understanding of breast cancer in Connecticut and our capacity to control its effects is enhanced by considering locations where both transitional and ongoing disparities can be found.
Human Participant Protection
The institutional review boards of the University of Connecticut and Connecticut State Department of Public Health approved our access to, and analysis of, this information.
References
- 1.Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305(11):1113–1118. doi: 10.1001/jama.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998-1999 to 2000-2001. JAMA. 2003;289(3):305–312. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Cooper RS. Genetic factors in ethnic disparities in health. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press; 2004. pp. 269–309. [PubMed] [Google Scholar]
- 4.Doll R. Geographic variation in cancer incidence: a clue to causation. World J Surg. 1978;2(5):595–602. doi: 10.1007/BF01556055. [DOI] [PubMed] [Google Scholar]
- 5.Vogel VG. Breast cancer risk factors and preventive approaches to breast cancer. In: Kavanagh JJ, Singletary SE, Einhorn N, editors. Cancer in Women. Malden, MA: Blackwell Science; 1998. pp. 58–91. [Google Scholar]
- 6.Gubler DJ. Vector-Borne Diseases: Understanding Environmental, Human Health, and Ecological Connections. Washington, DC: National Academies Press; 2008. The global threat of emergent/re-emergent vector-borne diseases. In: Institute of Medicine Forum on Microbial Threats; pp. 43–64. [PubMed] [Google Scholar]
- 7.National Research Council. Understanding the Changing Planet: Strategic Directions for the Geographical Sciences. Washington, DC: National Academies Press; 2010. [Google Scholar]
- 8.Klassen AC, Smith KC. The enduring and evolving relationship between social class and breast cancer: a review of the literature. Cancer Epidemiol. 2011;35(3):217–234. doi: 10.1016/j.canep.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Wennberg JE. Understanding geographic variations in health care delivery. N Engl J Med. 1999;340(1):52–53. doi: 10.1056/NEJM199901073400111. [DOI] [PubMed] [Google Scholar]
- 10.Mulley AG. Inconvenient truths about supplier induced demand and unwarranted variation in medical practice. BMJ. 2009;339 doi: 10.1136/bmj.b4073. b4073. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R, Boyages J. Estimating risk of breast cancer from population incidence affected by widespread mammographic screening. J Med Screen. 2001;8(2):73–76. doi: 10.1136/jms.8.2.73. [DOI] [PubMed] [Google Scholar]
- 12.Morrell S, Barratt A, Irwig L, Howard K, Bieshauvel C, Armstrong B. Estimates of overdiagnosis of invasive breast cancer associated with screening mammography. Cancer Causes Control. 2010;21(2):275–282. doi: 10.1007/s10552-009-9459-z. [DOI] [PubMed] [Google Scholar]
- 13.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 14.Wysowski DK, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982–1992. Obstet Gynecol. 1995;85(1):6–10. doi: 10.1016/0029-7844(94)00339-f. [DOI] [PubMed] [Google Scholar]
- 15.Robbins AS, Clarke CA. Regional changes in hormone therapy use and breast cancer. J Clin Oncol. 2007;25(23):3437–3439. doi: 10.1200/JCO.2007.11.4132. [DOI] [PubMed] [Google Scholar]
- 16.Krieger N. Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. Int J Epidemiol. 2008;37(3):627–637. doi: 10.1093/ije/dyn055. [DOI] [PubMed] [Google Scholar]
- 17. Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean Body Weight, Height and Body Mass Index, United States 1960–2002. Advance Data From Vital and Health Statistics; No. 347. Hyattsville, MD: National Center for Health Statistics; 2004. [PubMed]
- 18.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):1–68. [PubMed] [Google Scholar]
- 19.Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 20.Heston JF, Kelly JAB, Meigs JWet al. Forty-Five Years of Cancer Incidence in Connecticut 1935–79, Monograph No. 70. Bethesda, MD: National Cancer Institute; 1986. [PubMed] [Google Scholar]
- 21.International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 22.Taylor A, Cheng KK. Social deprivation and breast cancer. J Public Health Med. 2003;25(3):228–233. doi: 10.1093/pubmed/fdg072. [DOI] [PubMed] [Google Scholar]
- 23.Geolytics, Inc. Neighborhood Change Database (NCDB) 1970–2010 Tract Data. East Brunswick, NJ: Geolytics, Inc.; 2013. [Google Scholar]
- 24.National Cancer Institute. Joinpoint Trend Analysis Software. Available at: http://surveillance.cancer.gov/joinpoint. Accessed June 20, 2014.
- 25.Kulldorff M, Athas WF, Feuer EJ, Miller BA, Key CR. Evaluating cluster alarms: a space-time scan statistic and brain cancer in Los Alamos, New Mexico. Am J Public Health. 1998;88(9):1377–1380. doi: 10.2105/ajph.88.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oden N. Adjusting Moran’s I for population density. Stat Med. 1995;14(1):17–26. doi: 10.1002/sim.4780140104. [DOI] [PubMed] [Google Scholar]
- 27.Jacquez GM, Greiling DA, Durbeck H . ClusterSeer™ User Guide 2: Software for Identifying Disease Clusters. Ann Arbor, MI: TerraSeer Press; 2002. [Google Scholar]
- 28.Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26(6):1481–1496. [Google Scholar]
- 29. Kulldorff M, for Information Management Services, Inc. SaTScan™ version 3.1. Software for the Spatial and Space-time Scan Statistics, 2002. Available at: http:/www.satscan.org. Accessed June 20, 2014.
- 30.Rogerson P, Yamada I. Statistical Detection and Surveillance of Geographic Clusters. Boca Raton, FL: Taylor & Francis Group; 2009. [Google Scholar]
- 31.Aldstadt J. Statistical clustering. In: Fischer MM, Getis A, editors. Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications. Berlin, Germany: Springer-Verlag; 2010. [Google Scholar]
- 32.Centers for Disease Control (CDC) Behavioral Risk Factor Surveillance System Prevalence and Trends Data, 2000. Available at: http://apps.nccd.cdc.gov/brfss/page.asp?cat=WH&yr=2000&state= All#WH. Accessed June 20, 2014.
- 33.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 34.Gregorio DI, Samociuk H. Prostate cancer incidence in light of the spatial distribution of another screening-detectable cancer. Spat Spatiotemporal Epidemiol. 2013;6:1–6. doi: 10.1016/j.sste.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Gregorio DI, Kulldorff M, Sheehan TJ, Samociuk H. Geographic distribution of prostate cancer incidence in an era of PSA testing. Urology. 2004;63(1):78–82. doi: 10.1016/j.urology.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Lynch SM. Cohort and life-course patterns in the relationship between education and health. Demography. 2003;40(2):309–331. doi: 10.1353/dem.2003.0016. [DOI] [PubMed] [Google Scholar]
- 37.Fieder M, Huber S, Bookstein FL. Socioeconomic status, marital status and childlessness in men and women: an analysis of census data from six countries. J Biosoc Sci. 2011;43(5):619–635. doi: 10.1017/S002193201100023X. [DOI] [PubMed] [Google Scholar]
- 38.Goovaerts P, Jacques GM. Detection of temporal changes in the spatial distribution of cancer rates using local Moran’s I and geostatistically simulated spatial neutral models. J Geogr Syst. 2005;7(1):137–159. doi: 10.1007/s10109-005-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrienko N, Andrienko G. Exploratory Analysis of Spatial and Temporal Data. Berlin, Germany: Springer-Verlag; 2006. [Google Scholar]
- 40.Kessler RC, Greenberg DF. Linear Panel Analysis, Models of Quantitative Change. New York, NY: Academic Press; 1981. [Google Scholar]