Abstract
We examined the current literature to understand factors that influence endocrine therapy (ET) adherence among racial/ethnic and socioeconomic subpopulations of breast cancer patients. We searched PubMed and PsycINFO databases for studies from January 1, 1978, to June 20, 2014, and January 1, 1991, to June 20, 2014, respectively, and hand-searched articles from relevant literature reviews. We abstracted and synthesized results within a social ecological framework.
Fourteen articles met all inclusion criteria. The majority of included articles reported significant underuse of ET among minority and low-income women. Modifiable intrapersonal, interpersonal, and community-level factors are associated with ET use, and these factors vary across subgroups.
Both race/ethnicity and socioeconomic status are associated with ET use in most settings. Variation in factors associated with ET use across subgroups indicates the need for more nuanced research and targeted interventions among breast cancer patients.
Low medication adherence is common among patients taking oral drugs; an estimated half of all patients are nonadherent to a medication regimen across multiple chronic disease areas.1 This low medication adherence is problematic because it is associated with poorer prognosis for many common conditions.2 Evidence has demonstrated that non-White patients are less likely to adhere to medication regimens than White patients,3,4 suggesting that medication adherence may be an important lever for targeting racial disparities in health care outcomes. Medication adherence has become a particularly important issue in cancer care because the use of oral anticancer drugs in clinical practice has increased.3 Endocrine therapy (ET) for breast cancer is among the most common oral anticancer therapies, and racial variation in ET adherence may play a role in racial disparities in breast cancer care outcomes.
Breast cancer is the most common cancer among women in the United States: of the 232 570 women diagnosed with invasive breast cancer each year, approximately three quarters will have hormone receptor–positive breast cancer.5,6 Typically, women with this type of breast cancer undergo surgery with or without radiation, some will take adjuvant chemotherapy, and nearly all will be eligible for ET.7,8 ET is most commonly given in the adjuvant setting to prevent recurrence of curable cancers.5,9 ET consisting of at least a 5-year course of tamoxifen or an aromatase inhibitor (AI), is the gold standard for adjuvant treatment of these cancers, and it reduces 5-year breast cancer recurrence by 40% and breast cancer mortality by one third.10
However, evidence from observational and patient-reported sources has suggested that many women underuse ET because of noninitiation (i.e., never starting ET), nonadherence (i.e., not taking ET as prescribed), or nonpersistence (i.e., not taking ET for the recommended duration).11–15 ET underuse is associated with shorter time to recurrence, lower quality of life, and increased medical costs.16 Approximately one third of women who initiate adjuvant tamoxifen discontinue the drug before the 5-year, guideline-recommended duration.12,13,15 Of those who continue taking tamoxifen, 16% to 28% do not fully adhere to the therapy.12,13,17–19 Furthermore, adherence and persistence decline over time.19 Thus, by the end of the 5-year course of therapy, only about half of women have taken tamoxifen as prescribed.12,13 AI data have shown similar patterns of underutilization;12 at 5 years, 19% to 25% of women have discontinued their AI,20,21 and 20% to 31% of women have been nonadherent.18,22
Minority populations may be disproportionately affected by ET noninitiation, discontinuation, and nonadherence.12,23–28 Minority and low-income populations are less likely to be integrated into the health care system; thus, they may face unique barriers to care, such as poor access to providers, that influence receipt of ET and other cancer-related treatment.29,30 Among minority women who are also low income or who experience high levels of social stressors, competing social and economic demands may take priority over medication adherence, leading to suboptimal medication use.31 Patterns of ET utilization among minority women are understudied and may contribute to the well-recognized and persistent racial, ethnic, and socioeconomic disparities in outcomes. Despite advances in breast cancer prevention and treatment, breast cancer mortality remains 37% higher among Black women than among White women.32 Biological differences are important but cannot fully explain this racial/ethnic variation in mortality.9,33 Thus, the observed disparities likely arise from a combination of factors, including incomplete or omitted ET treatment.
In several studies of insured women, non-White race11,12,22,24–28 and low socioeconomic status (SES)34 have been associated with lower ET initiation, adherence, and persistence; however, reasons for this variation have not been well described. Although previous literature reviews have described factors that are associated with ET utilization broadly, none have detailed racial variation in the use of ET. We addressed this literature gap by conducting a systematic review of the adjuvant ET literature that is focused on barriers to ET use among low-income and minority populations.
METHODS
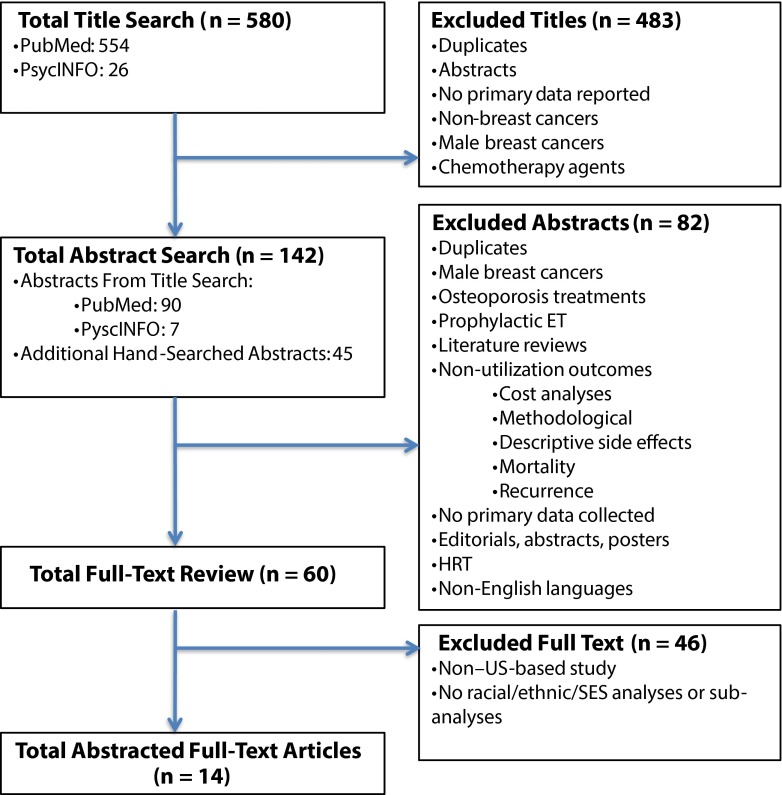
Our review methods followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Figure 1).35
FIGURE 1—
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram depicting the systematic search strategy.
Note. ET = endocrine therapy; HRT = hormone replacement therapy; SES = socioeconomic status.
Data Sources and Searches
We conducted systematic literature searches of the PubMed and PsycINFO databases for articles dated January 1, 1978, to June 20, 2014, and January 1, 1991, to June 20, 2014, respectively. We also hand searched the bibliographies of relevant literature (Figure 1).
The precise search terms used for all searches were as follows: (breast cancer[MeSH Terms]) AND (aromatase inhibitor* OR tamoxifen[MeSH Terms]) AND (adherence OR compliance OR persistence OR maintenance OR discontin* OR initiat*). We chose broad search terms to capture all ET utilization articles, including all types of ET (e.g., tamoxifen and AIs) and utilization terms (e.g., initiat*, persist*, adhere*). To complement these searches, we hand-searched bibliographies of key studies and other relevant review articles to identify additional articles that were not captured in the database searches.9,21,22,34,36–72
Study Selection
Studies with both experimental and nonexperimental study designs, with or without a comparison group, were included. We included studies that explored both (1) racial/ethnic or socioeconomic variation in ET initiation, adherence, or persistence and (2) barriers to ET that varied by race/ethnicity or SES through the use of interaction terms for race, ethnicity, and SES with other factors (e.g., modifiers) or through analyses of ET use among racial/ethnic or socioeconomic subgroup populations. Most of these studies used self-reported race/ethnicity data.
We excluded studies with the following characteristics:
The primary focus was not ET utilization (e.g., efficacy trial data without utilization data presented);
the study was conducted outside of the United States;
ET was delivered as chemoprevention or palliative treatment of metastatic disease;
the article was a literature review, letter to the editor, editorial, or thought piece; and
the article examined diverse patient populations but did not include racial/ethnic or socioeconomic subgroup analyses or interaction terms. We did not exclude studies on the basis of duration of follow-up or clinical setting.
We used EndNote X4 (Thompson Reuters, New York, NY), a citation management software system, to organize and manage our citation database for the review. EndNote enabled us to de-duplicate the individual searches and create a database of unique articles. Using our inclusion and exclusion criteria, we conducted title searches to identify which articles should undergo abstract review.
Next, 1 author (M. C. R.) reviewed abstracts to determine which articles were eligible for full-text review. In this phase, we excluded literature review articles from further analysis; however, we hand searched their bibliographies and added relevant references to the abstract search.9,36–48 We conducted full-text reviews to determine which articles would be abstracted. Study selection and review were conducted by 1 reviewer (M. C. R.). However, if the decision to include an article was unclear after the full-text review, a second reviewer (S. B. W.) assessed the article, and the final inclusion decision was resolved by discussion and consensus between the 2 reviewers.
Data Extraction
Applying the PICOTS framework,73 1 author (M. C. R.) extracted the following data from each article: population, intervention (i.e., types of ET included), comparator group (if applicable), outcomes, timing (duration of follow-up), and setting. We categorized study outcomes into 4 groups: provider discussion, recommendation, and prescribing; initiation; adherence; and persistence.
Definitions of utilization varied from study to study. Thus, we classified ET use outcomes according to our prespecified definitions of initiation, adherence, and persistence. For the purposes of this review, we defined ET initiation, or initial ET use, as whether the patient began ET. ET adherence referred to whether the patient took the prescription at the recommended dose and on the recommended schedule. Typically, studies defined nonadherence as having less than 80% of days covered by prescription fill records. Finally, persistence or continuation referred to whether the patient continued to take the medication for the recommended duration of therapy (regardless of whether the patient took it correctly according to recommended dosing and schedule). We further divided these groups by ET type (i.e., amoxifen, AIs, or both). AIs included letrozole, anastrozole, or exemestane.
Data Synthesis and Analysis
We did not conduct meta-analyses because significant heterogeneity existed among
study populations,
ET type,
outcomes assessed,
independent variable measurement,
duration of follow-up, and
study setting.
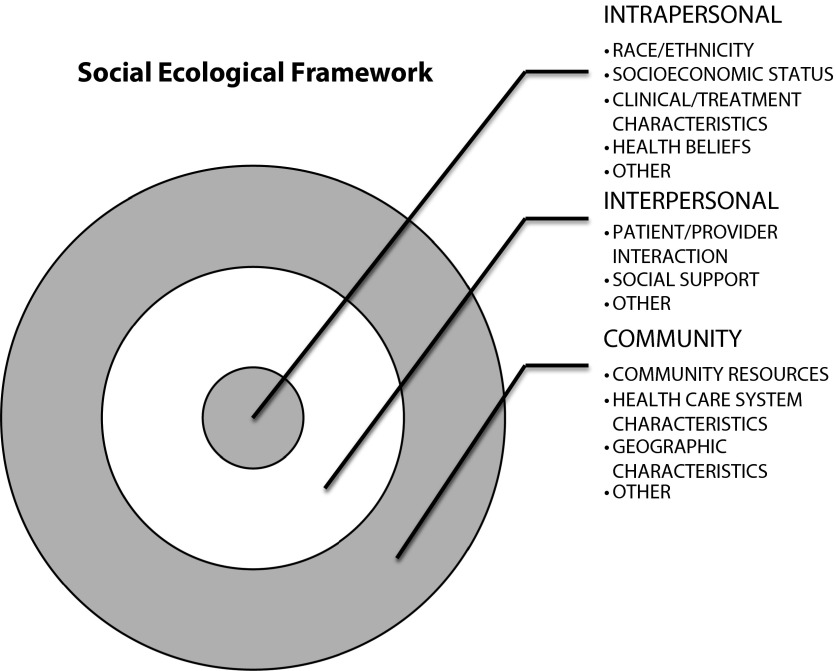
This was a qualitative decision made after data extraction and review. However, we did analyze studies within a well-known theory-driven conceptual framework, the social ecological framework.74
The ecological perspective of the social ecological framework acknowledges that multiple-level factors in the social system (i.e., intrapersonal, interpersonal, and community factors) influence health behaviors such as ET utilization (Figure 2).75,76 Findings from the abstracted articles were organized into intrapersonal, interpersonal, and community levels. We report descriptively on the included studies and their implications. Results from regression analyses and P values were abstracted directly from the included studies.
FIGURE 2—
Social ecological framework guiding data analysis.
RESULTS
Of 142 abstracts, 14 articles met final inclusion criteria (Table 1).19,22,60,77–87 Five of these studies examined socioeconomically disadvantaged populations. These 5 studies included publicly insured patients who were enrolled in state Medicaid programs19,60,81,87 or Medicare’s low-income subsidy program.22 Of all included articles, 677,78,80,81,83,86 examined primary data collected through the use of surveys, interviews, or focus groups; of these, 377,80,83 included self-reported barriers to care (Table 2). Eight studies19,22,60,79,82,83,85,87 used large secondary data sources or medical records to examine racial/ethnic and socioeconomic disparities in ET use; of these, 377,79,86 examined adjuvant breast cancer therapy broadly, including not only ET use but also either chemotherapy, or chemotherapy with radiation therapy. Although these studies evaluated chemotherapy and radiation therapy as dependent variables, some studies included these treatment variables (i.e., surgery, chemotherapy, radiation) as predictor variables of ET use19,22,60,81,82,84,87 and other studies78,80,83, did not include treatment variables in their analyses. One study stratified results by chemotherapy receipt.85 Finally, of all included studies, only 1 used a qualitative approach.77
TABLE 1—
Description of Included Studies
| First Author | Mean Age of Participants, Years | Sample, No. | % White | Cancer Stage | Medicaid or Medicare | Data Source | Type of ET | Dependent Variable | Analysis | Racial Comparators | Years of Data Collection | Setting |
| Ashing-Giwa77 | NR | Patient, 102; provider, 20 | Patient, 12; provider, 20 | 0–IV | Neither | Qualitative: interviews and focus groups | Breast cancer care (including ET) | NA | Qualitative | African American, Asian American, Latina, Caucasian | NR | UCLA community |
| Bhatta78 | 58 | 197 | 69 | I–III | Neither | Survey and medical record review | Tamoxifen, AIs | Adherence, persistence | Multivariable logistic regression | White, Black | NR | University of Chicago Hospital |
| Bickell79 | 60 | 677 | 49 | I–II | Neither | Medical record review | Breast cancer care (including ET) | Initiation | Multivariable logistic regression | White, Black, Hispanic, Asian, unknown | 1999 and 2000 (diagnoses), 2002–2004 (abstraction) | 6 NYC hospitals |
| Friese80 | 59 | 743 | 48 | I–III | Neither | Survey and SEER Registry | Tamoxifen, AIs | Initiation, persistence | Multivariable logistic regression | White, Black, Latina | Time of diagnosis, survey 4 y after diagnosis | Multisite: LA and Detroit sites |
| Kimmick60 | 67 | 1 491 | 59 | I–III | Medicaid | NC Central Tumor Registry and Medicaid claims | Tamoxifen, AIs | Initiation, adherence, persistence | Multivariate logistic regression | White, other | 1998–2002 | NC Medicaid |
| Liu81 | 51 | 303 | 34 | I–III | Medicaid | Survey | Tamoxifen, AIs | Persistence | Multivariate logistic regression | Less acculturated Latina, more acculturated Latina, and other | 2003–2005 enrollment | CA Medicaid |
| Livaudais82 | 59 | 13 753 | 76 | I–III | Neither | SEER Registry, KPNC claims, and US Census | Tamoxifen, AIs | Initiation | Multivariable logistic regression | Hispanic, African American, Chinese, Japanese, Filipino, and South Asian vs WNH | 1996–2007 | Multisite, KPNC |
| Livaudais83 | 69 | 3 575 | 92 | I–III | Neither | WHI Extension Survey | Tamoxifen, AIs | Initiation, persistence | Multivariable logistic regression | Hispanic, African American, Asian/Pacific Islander vs WNH | 2009–2010 | Multisite, parent study (WHI) |
| Livaudais84 | Phase 1 = 49; phase 2 = 50 | Phase 1 = 731; phase 2 = 654 | Phase 1 = 58; phase 2 = NA | I–III | Neither | NCBCF Registry and self-report | Tamoxifen, AIs | Initiation | Multivariable logistic regression | African American, Hispanic, WNH vs Asian American | 1995–1998 (phase 1) or 1998–2003 (phase 2) | Multisite, NCBCF Registry |
| Partridge19 | 75 | 2 378 | 83 | 0–III | Medicaid | NJ cancer registry and Medicaid claims | Tamoxifen | Adherence | Unconditional logistic regression | White, non-White | 1991–1995 | NJ Medicaid and NJ PAAD program |
| Reeder-Hayes85 | NR | 2 640 | 89 | I–III | Neither | NC Central Tumor Registry and Claims: ICISS | Tamoxifen, AIs | Initiation | Generalized estimating equations; Cox proportional hazards | White, Black | 2004–2009 | Multisite: NC Registry |
| Riley22 | NR | 15 542 | 81 | I–IV | Medicare | SEER Registry and Medicare claims | Tamoxifen, AIs | Adherence | Multivariable logistic regression | WNH, WH, Black, other or unknown | 2003–2005 (time of diagnosis), 2006–2007 (time of ET use) | Multisite |
| Shelton86 | NR | 1 145 | 69 | I–III | Neither | Survey and medical record review | Breast cancer care (including hormonal therapy) | Treatment decision-making | χ2 test and analysis of variance | White, Black, Hispanic, Asian, other | 2006–2010 | Multisite: Detroit, NYC, Northern CA (KPNC) |
| Wheeler87 | 49 | 222 | 47 | 0–II | Medicaid | NC Central Tumor Registry and Medicaid claims | Tamoxifen, AIs | Initiation | Multivariable logistic regression | White, Black, other | 2004–2007 | NC Medicaid |
Note. AI = aromatase inhibitor; CA = California; ET = endocrine therapy; ICISS = Integrated Cancer Information Surveillance System; KPNC = Kaiser Permanente Northern California; LA = Los Angeles; NA = not applicable; NC = North Carolina; NCBCF = Northern California Breast Cancer Family Registry; NJ = New Jersey; NR = not reported; NYC = New York City; PAAD = Pharmaceutical Assistance to the Aged and Disabled; SEER = Surveillance, Epidemiology, and End Results; UCLA = University of California, Los Angeles; WH = White Hispanic; WHI = Women’s Health Initiative; WNH = White, non-Hispanic.
TABLE 2—
Reported Barriers to Care and Recommendations From Included Studies
| Barriers and Recommendations | Description |
| Intrapersonal factors | |
| Side effects | Side effects were noted as barriers to care.77,80,83 In particular body image and sexual concerns emerged as common barriers for women across all racial groups during a qualitative analysis.77 Fear of side effects was reported as a reason to not initiate ET among noninitiators (28.8%) and as a reason to discontinue (25%) among discontinuers. Also, some noninitiators (18.8%) chose not to initiate ET despite provider recommendations.80 |
| Cost | Financial burden and job disruption emerged as barriers to care, with participants noting a need for affordable breast cancer care.77 However, in a survey, cost specific to ET was a barrier among only 5% of patients and insurance was a barrier among only 1% of patients.83 Another study reported a small number of women who discontinued ET reported lack of coverage by insurance as a reason for discontinuation (7.1%).80 A small proportion of noninitiators (5%) reported that ET was too expensive.80 However, a larger proportion of women noted cost as a reason for having discontinued ET (18.8%). Furthermore, < 1% of women were concerned about missing work.83 |
| Education | Latina women felt that low education may be a barrier to care.77 |
| Other | Patients infrequently listed inconvenience of use (< 1%) as a barrier to care.83 Some women reported disliking medication (23.2%), being unsure whether it was helping (22.3%), feeling as though they had taken ET long enough (17.9%) and wanting to move on from cancer (16.1%) as reasons for having discontinued ET early. |
| Interpersonal factors | |
| Communication | Lack of a provider recommendation was the most commonly cited barrier across racial groups; however, Black women cited it most often.83 Language was noted as a barrier to communication and breast cancer care.77 “Doctor said I did not need” (33.8%), “doctor left it up to me” (21.3%), and “doctor never discussed” (7.5%) were reported as reasons for noninitiation among a group of noninitiators.80 Patients reported discontinuing ET early because a doctor told them to (25%).80 |
| Social | Discouragement from family (< 1%) and discouragement from friends (< 1%) were given as barriers to care.83 |
| Community factors: recommendations | Recommendations from focus groups in 1 study primarily target a community-level approach so that patients can receive culturally and linguistically appropriate care.77 Furthermore, educating the community and increasing cultural sensitivity were recommended to improve breast cancer care for a diverse patient population.77 |
Note. ET = endocrine therapy.
Intrapersonal Characteristics
Multiple intrapersonal characteristics were associated with ET use: race/ethnicity, medication side effects, patients’ health beliefs, and cost of medications, as well as other person-level characteristics.
Overall effect of race/ethnicity on endocrine therapy use.
The effect of race/ethnicity on ET use varied by study; however, the majority of the studies indicated that there was significant racial/ethnic variation in ET use (Table 3). Several studies indicated that Black women had lower odds of initiating ET77,79,85 and being adherent to ET19,22 than other racial/ethnic groups. One study found no significant racial/ethnic differences in ET persistence by race/ethnicity; however, the authors did note racial/ethnic variation in reported barriers to care.83 For example, the most commonly cited barrier to ET use among minorities was lack of physician recommendation, and this barrier was more commonly reported among Black women (17%) than among Hispanic women (3%; P = .038).83 In another study, although being Black was not associated with ET adherence among women receiving the Medicare low-income subsidy, among those who did not receive this subsidy, Black women had increased odds of being nonadherent to tamoxifen (odds ratio [OR] = 2.60; 95% confidence interval [CI] = 1.39, 4.87) and increased odds of being nonadherent to an AI (OR = 1.86; 95% CI = 1.35, 2.55) compared with White women.22
TABLE 3—
Effect of Race on Endocrine Therapy Use Among Eligible Included Studies
| First Author | Outcome | Disparity | Data Source | Population (% African American) |
| Partridge19 | Adherence | White > non-White | Pharmacy claims | NJ Medicaid and NJ PAAD program (17%a) |
| Riley22 | Adherence | White > African Americanb | Pharmacy claims | Medicare (6%) |
| Kimmick60 | Initiation | No significant association | Pharmacy claims | NC Medicaid (41%a) |
| Persistence | No significant association | |||
| Adherence | No significant association | |||
| Liu81 | Persistence | Less acculturated Hispanic > White | Self-report | CA Medicaid: CA Breast and Cervical Cancer Treatment Program (6%) |
| Livaudais82 | Initiation | NHW > Hispanic, NHW > Chinese | Pharmacy claims | KPNC (6%) |
| Livaudais84 | Initiation | Asian > NHW, African Americanc | Self-report | NCBCF (11%) |
| Livaudais83 | Initiation, persistence | No significant association | Self-report | WHI study (4%) |
| Wheeler87 | Initiation | No significant association | Pharmacy claims | NC Medicaid (53%a) |
| Bhatta78 | Adherence | No significant association | Self-report | University of Chicago Hospital (31.5%) |
| Persistence | Self-report and medical record review | |||
| Compliance | ||||
| Reeder-Hayes85 | Initiation | White > African American | Pharmacy claims | Privately insured (11%) |
| Friese80 | Initiation | African American and Latina > White | Self-report | LA County and metropolitan Detroit SEER regions (14.2%) |
| Persistence | No significant association | |||
| Bickell79 | Initiation | White > Black, Hispanic | Medical record review | 6 NYC hospitals (21%) |
Note. CA = California; LA = Los Angeles; NC = North Carolina; NHW = Non-Hispanic White; NJ = New Jersey; KPNC = Kaiser Permanente of Northern California; NCBCF = Northern California Breast Cancer Family Registry; NYC = New York City; PAAD = Pharmaceutical Assistance to the Aged and Disabled; SEER = Surveillance, Epidemiology, and End Results; WHI = Women’s Health Initiative.
% non-White.
Only among women without the low-income subsidy.
Only among women with suspected hereditary breast cancer.
Among high-risk patients (defined as women with increased genetic susceptibility to breast cancer; e.g., bilateral breast cancer before age 50 years), no racial/ethnic differences emerged in ET initiation; however, the opposite was true for women with sporadic (non–high-risk) breast cancer—Black women had lower odds of initiating ET (OR = 0.20; 95% CI = 0.06, 0.60) and non-Hispanic White women had lower odds of using ET (OR = 0.40; 95% CI = 0.17, 0.94) than Asian women.84 This same study demonstrated that racial/ethnic differences in ET use decreased as the diffusion of ET into clinical practice increased over time.84
Hispanic or Latina ethnicity was also associated with differential ET use in certain studies, but the direction of association varied by study. Compared with non-Hispanic Whites, low-income Latina women participating in the California Breast and Cervical Cancer Treatment Program were more likely to be persistent with ET at 36 months if they were less acculturated (adjusted odds ratio [AOR] = 9.08; P = .001), where acculturation was defined as being more comfortable with the English language.81 Interestingly, this association between ethnicity and persistence was nonsignificant among Latina women who were more acculturated. Other studies, which were not conducted in low-income study populations specifically, found that Hispanic women were less likely to initiate adjuvant therapy than non-Hispanic White women.79,82 Specifically, 1 study82 indicated that Hispanic women had decreased odds of ET initiation compared with White women (AOR = 0.82; CI = 0.71, 0.96). Asian race was also associated with ET use. One quantitative study82 found that Chinese patients had 22% lower odds of initiating ET compared with White patients, and one qualitative study77 also indicated lower ET initiation among Chinese women compared with other racial/ethnic minorities.
Several studies, however, did not find an association between race/ethnicity and ET use. Although a New Jersey Medicaid study indicated lower odds of adherence among non-Whites compared with Whites,19 2 studies conducted within a North Carolina Medicaid population found no association between race/ethnicity and initiation,60,87 adherence,60 or persistence.60 Instead, these studies demonstrated low ET use across the board among low-income women in North Carolina. Another study found no racial differences in ET adherence; however, this was a small study conducted in 1 academic medical center.78 The authors noted that low power and high insurance coverage rates among Black women in the study may explain the nonsignificant findings.78 A study using self-report and Surveillance, Epidemiology, and End Results registry data (Los Angeles, CA, and Detroit, MI, regions) found that race was not associated with persistence; furthermore, the study found that Black and Latina women were more likely to initiate ET than Whites.80 The authors suggested that peer support, patient navigator programs, and other important contextual factors may explain improved ET use among Black and Latina women.80 Overall, variation in the effect of race/ethnicity on ET use likely arises from variation in study designs, populations, ET types, outcomes and measurement of other variables, and settings.
Side effects.
Side effects were strongly associated with ET use across quantitative and qualitative studies. Among low-income women, those who experienced side effects had lower odds of persistence at 36 months (AOR = 0.26; P = .003; Table 4).81 Furthermore, side effects emerged as an important concern during patient focus groups.77 In particular, changes in body image and sexual concerns as a result of ET use were noted as common concerns across all racial/ethnic groups.77 In 1 study, fear of side effects was reported as a barrier among 28.8% of noninitiators: 40% of women who discontinued therapy reported side effects a reason for discontinuation, and 25% of women who discontinued therapy reported being worried about risks associated with ET.80
TABLE 4—
Correlates of Endocrine Therapy Initiation, Persistence, and Adherence Among Minority and Low-Income Populations and Subpopulations in Included Studies
| Correlate | Initiation | Adherence | Persistence |
| Age | |||
| Older | Positive,60,82 NS87,80 | Negative,19 NS60 | Negative,80 NS81 |
| Younger | Negative19 | ||
| Married | Positive,82 negative60 | Negative,60 NS22 | Negative,60 NS81 |
| Education | NS81 | ||
| Financial adequacy | NS81 | ||
| Blind or disabled | NS87 | ||
| Comorbidity | Negative,82 NS60,85,87 | Positive,19 NS60 | Positive60,81 |
| High hierarchical condition category (insurance risk) | Positive,22 NS22 | ||
| Preexisting depression | NS85 | ||
| History of estrogen replacement therapy | NS19 | ||
| No. of prescription medications | Positive60 | NS60 | Positive,89 NS60 |
| Hormone receptor positive status | Positive60 | NS60 | NS60 |
| Stage | Negative,85 NS80,87 | NS,22 positive22 | NS80,81 |
| Grade 2 (vs 1) | Positive80,82 | NS80 | |
| Grade 3 (vs 1) | NS80,82 | NS80 | |
| Well differentiated (vs poorly) | Positive85 | ||
| Moderately differentiated (vs poorly) | Positive85 | ||
| Unknown differentiation (vs poorly) | NS85 | ||
| Lobular (vs ductal) | Positive82 | ||
| Other nonlobular histology (vs ductal) | Negative82 | ||
| Regional (direct extension or lymph node) vs local | Positive60,82 | NS60 | Positive60 |
| Regional (direct extension and lymph node) vs local | NS82 | ||
| Mastectomy (vs BCS and/or no surgery) | Positive,82 NS60,87 | Negative,19 NS22,60 | NS60,81 |
| BCS no radiation (vs BCS with radiation) | Negative85 | ||
| Mastectomy, no radiation (vs mastectomy with radiation) | NS85 | ||
| Adjuvant chemotherapy | Negative,60,85 NS82,87 | NS19,60 | NS60,81 |
| Radiation | Positive,60 NS87 | NS19,60 | NS60,81 |
| Perceived importance of ET | Positive78 | ||
| Value provider’s opinion | Positive78 | ||
| Concern about side effects | NS78 | ||
| Worry about recurrence | Positive80 | NS80 | |
| Perceived efficacy in patient-provider interactions | Positive81 | ||
| ET side effects | Negative81 | ||
| Out-of-pocket costs | Negative22 | ||
| No insurance | Negative81 | ||
| Insurance plan type (public employee versus other) | NS85 | NS19,22 | |
| Age at Part D enrollment | NS22 | ||
| Breast Cancer Cervical Cancer Control Program (vs Medicaid only) | Positive87 | ||
| Oncology visit within year | Positive19 | ||
| Primary oncology provider: medical oncology (vs surgeon) | Positive80 | NS80 | |
| Patient-centered care | Positive81 | ||
| Discussion about ET | NS81 | ||
| Received enough information about ET | Positive80 | ||
| Use of other prescriptions (nonbaseline) | NS19 | ||
| No. of outpatient visits | NS19 | ||
| Nursing home use | NS19 | ||
| Days of acute hospitalization in prior y | NS19 | ||
| Urban | NS60,87 | NS22,60 | NS60 |
| Small hospital (vs large) | Positive60 | NS60 | NS60 |
| Zip code income ($30 000–$40 000 vs < $30 000) | Positive,22 NS22 | ||
| % county poverty | |||
| Lowest quartile vs high mid | NS85 | ||
| Lowest quartile vs highest | NS85 | ||
| Lowest quartile vs low mid | Negative85 | ||
| No. hospitals with oncology services in county | |||
| Lowest quartile vs high mid | NS85 | ||
| Lowest quartile vs highest | NS85 | ||
| Lowest quartile vs low mid | Negative85 | ||
| Calendar year | Positive,82 negative,87 NS85 | Negative,22 NS19,22 |
Note. BCS = Breast-conserving surgery; ET = endocrine therapy; negative = negative association with outcome (P ≤ .05); NS = nonsignificant association with outcome (P > .05); positive = positive association with outcome (P ≤ .05). Studies that looked at adjuvant breast cancer treatment broadly are not included. For studies that looked at racial/ethnic and socioeconomic subpopulations, only multivariable regression results for racial/ethnic minorities or low-income populations are included in this table.
Racial/ethnic variation existed in the reporting of side effects as a barrier to ET use. For example, in 1 study, the most commonly cited barrier to ET use among Hispanic patients was side effects, whereas side effects were the least commonly cited barrier among Black women (16% vs 8%; nonsignificant).83 Regardless of racial/ethnic variation in reporting, however, side effects were among the top reported barriers to ET.83 Interestingly, 1 small prospective study did not find an association between concerns about side effects and ET adherence; this study instead found a positive association with ET adherence when women reported increased value in their provider’s opinion and when women had a higher perceived importance of ET, suggesting these factors may be the drivers for ET adherence, not concerns about side effects.78
Health beliefs.
Several health beliefs were associated with ET use. Higher perceived efficacy of patient–physician interactions was associated with increased ET persistence among low-income women (OR = 1.04; P = .04).81 Worry about recurrence was associated with increased odds of ET initiation; however, this association was not found with ET persistence,80 suggesting that different factors influence different types of ET behavior.
A dislike for medication (23.2%), being unsure whether ET was helping (22.3%), feeling as though they had taken ET long enough (17.9%), and wanting to move on from cancer (16.1%) were all reported reasons for discontinuing ET by 4 years.80
Endocrine therapy–related costs.
Costs were reported as a barrier to ET use across racial/ethnic groups in both qualitative and quantitative studies. Out-of-pocket costs among Medicare beneficiaries influenced ET use regardless of ET type and SES (including both patients who received low-income subsidies and those who did not across racial/ethnic groups).22 In a qualitative study, financial burden and access to affordable breast cancer care were recurring themes among both key informants (i.e., community health workers and advocates in diverse breast cancer populations) and breast cancer survivors.77
In particular, Latinas noted job disruptions and financial hardships as barriers to ET initiation and adherence.77 However, another study found that cost was a barrier to ET use among only 5% of women.83 In yet another study, costs were rarely reported as a reason for noninitiation (5%); however, cost was reported more often as a reason for discontinuation among women who stopped ET before 4 years of therapy (18.8%), and a small proportion stopped for insurance-related reasons (7%).80 Thus, it is unclear to what extent costs are a barrier specific to minorities in the use of ET across settings and populations. Variation in results regarding cost may be explained by changes in generic availability for tamoxifen and AIs over time during the different study periods.
Other person-level characteristics.
Associations between other person-level characteristics and ET use were also observed. Education was not associated with ET use among a diverse, low-income population.81 However, in a qualitative study, Latinas reported that, broadly, low education and language presented barriers to breast cancer care.77 These differences may be explained by the inclusion of provider–patient communication factors, potentially suggesting that good communication, not education level, influences ET use.
Mixed evidence was found regarding associations between ET use and age, income, health care utilization (e.g., number of office visits), prescription use (e.g., number of other prescriptions), insurance status, clinical characteristics, tumor characteristics, and treatment characteristics. This variation is likely the result of variation in study designs and analysis methods, patient populations and settings, and ET use measures.
Interpersonal Characteristics
Several interpersonal characteristics were associated with ET use. In particular, studies reported that provider referral, patient–provider communication, and social support played a role in the use of ET.
Provider referral and recommendation.
One study investigated the relationship between receiving a referral to a medical oncologist, race/ethnicity, and ET use.79 Race/ethnicity was not associated with receiving a medical oncologist referral.79 Furthermore, among women who saw a medical oncologist, race/ethnicity was not associated with receipt of adjuvant therapy (including radiation, chemotherapy, or ET). However, among women who did not see a medical oncologist, racial/ethnic differences in receipt of adjuvant therapies persisted, in that non-Hispanic White women were more likely to use ET than non-Hispanic Black or Hispanic White women.
Overall, these results suggest that referral to a medical oncologist may ameliorate disparities in the use of appropriate breast cancer care, perhaps by bridging knowledge gaps or provider network gaps through medical oncology consultation.79 Another study indicated that women whose primary oncology provider was a medical oncologist had a higher likelihood of ET initiation than those whose primary provider was a surgeon; this association did not hold for ET persistence.80 The authors suggested that patients who see a medical oncology provider may have clearer indications for ET use than those who see a surgeon or other provider, which may explain why there were differences in ET initiation by provider type, but not in persistence.80
Among ET-eligible women who did not initiate ET, 33.8% reported not taking ET because their provider said they did not need to, because the doctor left the decision up to them (21.3%), or because the doctor never discussed ET (7.5%).80 However, some women reported not initiating ET despite a doctor’s recommendation (18.8%).80 Finally, of women who discontinued ET, 25% who stopped within 4 years after ET initiation reported doing so because of a doctor’s recommendation.80
Patient–provider communication quality.
The quality of communication between provider and patient appears to influence ET use across qualitative and quantitative studies. Patient-centered communication increased ET use among low-income Latina women, where patient-centered communication was defined as communication that explores “patients’ ideas and concerns, and assesses and responds to their emotions and understanding” (AOR = 1.22; P = .006).81(p830) The effect of patient-centered communication on ET use did not vary by ethnicity in this low-income population.81 Although quality of communication was important to patients, this study found that provider–patient discussion specifically about the hormonal activity of ET and how ET works biologically was not associated with ET use.81
Results from another survey indicated that communication about ET was rated lower among Black patients than among White patients (P ≤ .001).86 Quality of provider communication, extent of provider’s involvement, and level of trust in the medical system were all rated lowest among Black patients.86 Emergent themes from qualitative interviews and focus groups showed that patients and key informants believed there was “an urgent need for health care providers to become more culturally sensitive” during patient–provider interactions with respect to adjuvant treatment discussions.77(p425) Women who felt they received adequate information about ET were more likely to initiate ET than those who did not.80
Social support.
In our review, we found that social support was not strongly associated with ET use. Hispanics (32%) were significantly more likely than were Whites (18%) and Asians (13%) to report being helped by parents, children, or grandchildren during ET-related decision-making, whereas Asians (38%) were more likely than were Blacks (22%) to be helped by a husband or partner.86 This information may be important for the small minority of patients who indicated that discouragement from family (< 1%) and friends (< 1%) was a barrier to ET use.83
We should note that although marital status was associated with increased initiation in 1 study,82 it was not associated with ET adherence among participants receiving a low-income subsidy through Medicare,22 and not being married was associated with improved adherence (OR = 1.90; P = .006) and persistence (OR = 1.74; P = .031) among North Carolina Medicaid participants.60 The authors suggested that this association reflects a different pattern of social support among the North Carolina Medicaid population than among other populations.60 Variation in findings may reflect not only differences in patient populations but also differences in measures of social support. Marital status has been used as a proxy for social support; however, it may reflect only a fraction of the social support construct.
Community Factors
Community factors may also be associated with ET use. During interviews in a qualitative study, key informants indicated that “communities must be educated about breast cancer to maximize their use of available resources.”77(p412) Also noted was the need for more diversity in staff and more partnerships with psychosocial services in the health care system. Finally, culturally and linguistically appropriate programs, such as community-based support groups and targeted public health programs, were identified as potential interventions that may improve quality of care for breast cancer patients.77
In support of this qualitative work, 1 study found that participating in the North Carolina Breast and Cervical Cancer Control Program was associated with increased odds of initiating ET.87 This program provides free and low-cost breast cancer screening and follow-up to low-income women. Services are provided at local health departments, community health centers, hospitals, and practices across North Carolina. Thus, increasing access to public health resources may improve ET use. Other health system–level factors, such as hospital size,60 urban versus rural residence,87 and census tract–level income,22 were not significantly related to ET use among low-income populations, suggesting that provider- and patient-level factors may play a greater role in ET use. However, in 1 North Carolina Medicaid study, women who were seen at a small hospital (< 100 beds) had greater odds of using any ET than women who were seen at a larger hospital (> 100 beds; OR = 1.49; P = .024).60 Reasons for this difference were not discussed.
DISCUSSION
Medication initiation, adherence, and persistence remain a challenge for women taking adjuvant ET. Generally, medication adherence decreases as the longevity of a drug regimen increases.2 Thus, issues surrounding adherence to ET have become even more important because evidence has demonstrated the additional benefit of taking ET for as long as 10 years after hormone receptor–positive breast cancer diagnosis.88 Patterns of nonadherence mirror those of other long-term oral medications, with only approximately half of women completing ET as prescribed.
Although the evidence is mixed, the vast majority of studies included in this review suggested that ET is less optimally used by minorities and that barriers and facilitators to use also vary by race/ethnicity and SES. Studies examining adherence and persistence across multipayer populations will provide more insight into racial disparities in ET use. Although some barriers to care are relevant and cut across all racial/ethnic and socioeconomic subgroups (e.g., patient-centered communication, community factors), other barriers seem to vary in importance by subgroup and even within subgroups. For example, side effects, less education, and lack of physician recommendation were reported as potential barriers to ET use at different rates across racial/ethnic groups. At a more granular level, variation in ET use existed within racial/ethnic subgroups, such as Latina women with different levels of acculturation.
Results also indicated potential interactions between SES and race. In 1 study, the effect of cost on ET use did not vary by race among women receiving Medicare low-income subsidies; however, this was not true for women without the subsidy.22 SES has long been recognized as a confounding factor for racial/ethnic disparities.31 Competing social and economic demands may take priority over medication adherence, resulting in lower adherence among those in lower SES groups. Two of the 4 included Medicaid studies found no association between ET use and race, which contrasts with findings with more socioeconomically diverse populations.
Although race/ethnicity and SES are associated with medication behaviors, the current literature suggests that modifiable targets for improving ET exist. These targets include intrapersonal characteristics (such as side effect management, health beliefs, and costs), interpersonal characteristics (such as provider referral and provider communication), and community factors (such as community-based support groups, education, and resources). The multidimensional mechanisms behind nonadherence to medication remain complex and uncertain; however, this literature review homes in on modifiable barriers to ET use among racial/ethnic minority and low-SES subgroups and suggests that interventions to improve ET adherence should target these patient-specific modifiable barriers. Discussions in broader reviews of medication adherence suggest that the majority of current interventions to improve medication adherence have reported relatively small gains.1–3 Thus, there remains a need for more innovative, multidimensional, patient-centered, and methodologically sound interventions.1–3 The results of this literature review indicate that tailoring interventions to racial/ethnic and socioeconomic subgroups may improve ET use.
Looking forward, further disentangling the independent and interactive effects of race/ethnicity and SES on ET use will be important. Drawing a clear conclusion about their effects on ET use remains difficult because the current literature has used heterogeneous study designs, populations, and measures. Longitudinal cohort studies and qualitative work with providers and patients are needed to assess the role of race/ethnicity in ET initiation, adherence, and persistence, as well as to identify unique, multilevel barriers and facilitators across racial/ethnic and low-income groups.
This literature review has several limitations. First, we did not rate the quality of each included article. The quality of included studies varies, thus individual results should be interpreted with caution. Our literature review narrowly focused on racial/ethnic minority and low-SES patient populations in the United States; thus, results may not be applicable to broader breast cancer patient populations. ET is commonly used among women with metastatic breast cancer. We did not examine ET use in this setting; however, to our knowledge no such studies have been conducted. Finally, although we conducted a thorough systematic literature search in 2 large databases, the possibility remains that our review could have missed relevant articles.
To our knowledge, this literature review is the first to examine racial/ethnic and socioeconomic disparities in ET initiation, adherence, and persistence. Although other literature reviews have examined the broad use of ET,36–48 we have taken a deeper look at studies that examined variations in and barriers to ET use among specific racial/ethnic minority and low-income patient populations. These results raise awareness of the need for (1) more nuanced information on how to overcome barriers to ET use across racial/ethnic and socioeconomic subgroups and (2) development of tailored interventions to improve ET use in targeted subpopulations. By further developing knowledge about barriers to ET use among racial/ethnic and low-SES subgroups, we can build the evidence required to help ameliorate disparities in breast cancer outcomes.
Acknowledgments
This work was supported by several funding mechanisms. M. C. Roberts was supported by the UNC Lineberger Cancer Control Education Program (R25 CA57726). S. B. Wheeler was supported by an Agency for Healthcare Research and Quality Comparative Effectiveness Research Career Development Award (1-K-12 HS019468-01; PI: Weinberger), an American Cancer Society Mentored Research Scholar Award (MRSG-13-17-01-CPPB; PI: S. B. W.), and a University Cancer Research Fund and Lineberger Comprehensive Cancer Center Developmental Award in Population Sciences (“Linking Cohort Study Data with Insurance Claims to Understand Endocrine Therapy Initiation Among Breast Cancer Survivors”; PI: S. B. W.). K. Reeder-Hayes was supported by the National Institutes of Health, Building Interdisciplinary Careers in Women’s Health (BIRCWH) Career Development Program (5K12HD001441-12; PI: Orringer, Gene).
Human Participant Protection
This systematic review used secondary data with no individual identifiers; therefore, human participant protection was not necessary.
References
- 1.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD000011.pub3. CD000011. [DOI] [PubMed] [Google Scholar]
- 2.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 3.Mathes T, Antoine SL, Pieper D, Eikermann M. Adherence enhancing interventions for oral anticancer agents: a systematic review. Cancer Treat Rev. 2014;40(1):102–108. doi: 10.1016/j.ctrv.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Gerber BS, Cho YI, Arozullah AM, Lee SY. Racial differences in medication adherence: a cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother. 2010;8(2):136–145. doi: 10.1016/j.amjopharm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Breast cancer overview. Available at: http://www.cancer.org/cancer/breastcancer/overviewguide/breast-cancer-overview-key-statistics. Accessed September 1, 2013.
- 6.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Temin S, Anderson H et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eifel P, Axelson JA, Costa J et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93(13):979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien KM, Cole SR, Tse CK et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Neugut AI, Subar M, Wilde ET et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershman DL, Kushi LH, Shao T et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCowan C, Shearer J, Donnan PT et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman DL, Shao T, Kushi LH et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 16.McCowan C, Wang S, Thompson AM, Makubate B, Petrie DJ. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109(5):1172–1180. doi: 10.1038/bjc.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dezentjé VO, van Blijderveen NJ, Gelderblom H et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28(14):2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 18.Ziller V, Kalder M, Albert US et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20(3):431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 19.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 20.Huiart L, Dell’Aniello S, Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer. 2011;104(10):1558–1563. doi: 10.1038/bjc.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittmer C, Roeder K, Hoellen F, Salehin D, Thill M, Fischer D. Compliance to adjuvant therapy in breast cancer patients. Eur J Gynaecol Oncol. 2011;32(3):280–282. [PubMed] [Google Scholar]
- 22.Riley GF, Warren JL, Harlan LC, Blackwell SA. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1(4) doi: 10.5600/mmrr.001.04.a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Short LJ, Fisher MD, Wahl PM et al. Disparities in medical care among commercially insured patients with newly diagnosed breast cancer: opportunities for intervention. Cancer. 2010;116(1):193–202. doi: 10.1002/cncr.24691. [DOI] [PubMed] [Google Scholar]
- 25.Prehn AW, Topol B, Stewart S, Glaser SL, O’Connor L, West DW. Differences in treatment patterns for localized breast carcinoma among Asian/Pacific Islander women. Cancer. 2002;95(11):2268–2275. doi: 10.1002/cncr.10965. [DOI] [PubMed] [Google Scholar]
- 26.Wu XC, Lund MJ, Kimmick GG et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 27.Freedman RA, Virgo KS, He Y et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110(10):2169–2177. doi: 10.1002/cncr.23026. [DOI] [PubMed] [Google Scholar]
- 29.Urban Institute. Vulnerable populations. Available at: http://www.urban.org/health_policy/vulnerable_populations/index.cfm. Accessed February 1, 2014.
- 30.Agency for Health Care Research and Quality. Health and healthcare disparities among vulnerable populations. Available at: http://innovations.ahrq.gov/issue.aspx?id=28. Accessed February 1, 2014.
- 31.Dressler WW, Oths KS, Gravlee CC. Race and ethnicity in public health research: models to explain health disparities. Annu Rev Anthropol. 2005;34:231–252. [Google Scholar]
- 32.Jemal A, Thun MJ, Ries LA et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund MJ, Trivers KF, Porter PL et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 34.Yen TW, Hunt KK, Mirza NQ et al. Physician recommendations regarding tamoxifen and patient utilization of tamoxifen after surgery for ductal carcinoma in situ. Cancer. 2004;100(5):942–949. doi: 10.1002/cncr.20085. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 36.Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care (Engl) 2012;21(1):10–19. doi: 10.1111/j.1365-2354.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 37.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1–2):1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 38.Doggrell SA. Adherence to oral endocrine treatments in women with breast cancer: can it be improved? Breast Cancer Res Treat. 2011;129(2):299–308. doi: 10.1007/s10549-011-1578-z. [DOI] [PubMed] [Google Scholar]
- 39.Gotay C, Dunn J. Adherence to long-term adjuvant hormonal therapy for breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2011;11(6):709–715. doi: 10.1586/erp.11.80. [DOI] [PubMed] [Google Scholar]
- 40.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73(2):156–166. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Kelly A, Agius CR. Improving adherence to endocrine therapies: the role of advanced practice nurses. Oncology (Williston Park) 2006;20(10 suppl Nurse Ed) 50–54. [PubMed] [Google Scholar]
- 42.Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res (Phila) 2011;4(9):1360–1365. doi: 10.1158/1940-6207.CAPR-11-0380. [DOI] [PubMed] [Google Scholar]
- 43.Miaskowski C, Shockney L, Chlebowski RT. Adherence to oral endocrine therapy for breast cancer: a nursing perspective. Clin J Oncol Nurs. 2008;12(2):213–221. doi: 10.1188/08.CJON.213-221. [DOI] [PubMed] [Google Scholar]
- 44.Moore S. Nonadherence in patients with breast cancer receiving oral therapies. Clin J Oncol Nurs. 2010;14(1):41–47. doi: 10.1188/10.CJON.41-47. [DOI] [PubMed] [Google Scholar]
- 45.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Partridge AH, Ades T, Spicer P, Englander L, Wickerham DL. Helping breast cancer patients adhere to oral adjuvant hormonal therapy regimens. Community Oncol. 2007;4(12):725–731. [Google Scholar]
- 47.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 48.Verma S, Madarnas Y, Sehdev S, Martin G, Bajcar J. Patient adherence to aromatase inhibitor treatment in the adjuvant setting. Curr Oncol. 2011;18(suppl 1):S3–S9. doi: 10.3747/co.v18i0.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albert US, Zemlin C, Hadji P et al. The impact of breast care nurses on patients’ satisfaction, understanding of the disease, and adherence to adjuvant endocrine therapy. Breast Care (Basel) 2011;6(3):221–226. doi: 10.1159/000329006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer. 2006;42(14):2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22(24):4951–4957. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 52.Davidson B, Vogel V, Wickerham L. Oncologist-patient discussion of adjuvant hormonal therapy in breast cancer: results of a linguistic study focusing on adherence and persistence to therapy. J Support Oncol. 2007;5(3):139–143. [PubMed] [Google Scholar]
- 53.Files JA, Ko MG, Pruthi S. Managing aromatase inhibitors in breast cancer survivors: not just for oncologists. Mayo Clin Proc. 2010;85(6):560–566. doi: 10.4065/mcp.2010.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg. 2006;192(4):496–498. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Gold DT, McClung B. Approaches to patient education: emphasizing the long-term value of compliance and persistence. Am J Med. 2006;119(4 suppl 1):S32–S37. doi: 10.1016/j.amjmed.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 56.Hadji P, Blettner M, Harbeck N et al. The Patient’s Anastrozole Compliance to Therapy (PACT) Program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013;24(6):1505–1512. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 57.Hadji P, Blettner R, Haidinger N et al. Patient’s Anastrozole Compliance to Therapy Programme (PACT) influence of the addition of a standardized information and reminder service on compliance in comparison to standard clinical care alone in women with early breast cancer. Eur J Cancer Suppl. 2008;6(7):127. [Google Scholar]
- 58.Lipkus IM, Kimmick GG, Chui S, Fifield DL, Werner LA, Marcom PK. Relationship between numeracy and breast cancer patients’ estimates of adjuvant treatment benefit. J Clin Oncol. 2006;24(18S):586. [Google Scholar]
- 59.Karam AK. Breast cancer posttreatment surveillance: diagnosis and management of recurrent disease. Clin Obstet Gynecol. 2011;54(1):157–163. doi: 10.1097/GRF.0b013e318208393b. [DOI] [PubMed] [Google Scholar]
- 60.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirk MC, Hudis CA. Insight into barriers against optimal adherence to oral hormonal therapy in women with breast cancer. Clin Breast Cancer. 2008;8(2):155–161. doi: 10.3816/CBC.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 62.Lash TL, Gurwitz JH, Silliman RA. Physicians’ assessments of adjuvant tamoxifen’s effectiveness in older patients with primary breast cancer. J Am Geriatr Soc. 2005;53(11):1889–1896. doi: 10.1111/j.1532-5415.2005.53562.x. [DOI] [PubMed] [Google Scholar]
- 63.Neugut AI, Hillyer GC, Kushi LH et al. Non-initiation of adjuvant hormonal therapy in women with hormone receptor-positive breast cancer: the Breast Cancer Quality of Care Study (BQUAL) Breast Cancer Res Treat. 2012;134(1):419–428. doi: 10.1007/s10549-012-2066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 65.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94(9):652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 66.Rosenzweig M, Brufsky A, Rastogi P, Puhalla S, Simon J, Underwood S. The attitudes, communication, treatment, and support intervention to reduce breast cancer treatment disparity. Oncol Nurs Forum. 2011;38(1):85–89. doi: 10.1188/11.ONF.85-89. [DOI] [PubMed] [Google Scholar]
- 67.Crean SM, Reynolds M, Motabar S, Barghout V, Mody-Patel N. Discontinuation rates of adjuvant letrozole or anastrozole in breast cancer patients in an electronic medical record database observational study. Paper presented at: ASCO Breast Cancer Symposium; September 7–8, 2007; San Francisco, CA.
- 68.Schwartzberg LS, Cobb P, Senecal F et al. Initial treatment and changes in adjuvant endocrine therapy for early stage breast cancer. Breast. 2009;18(2):78–83. doi: 10.1016/j.breast.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Silliman RA, Guadagnoli E, Rakowski W et al. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20(11):2680–2688. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 70.Smith SL, Wai ES, Alexander C, Singh-Carlson S. Caring for survivors of breast cancer: perspective of the primary care physician. Curr Oncol. 2011;18(5):e218–e226. doi: 10.3747/co.v18i5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vogel VG, Costantino JP, Wickerham DL et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila) 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wengström Y, Aapro M, Leto di Priolo S, Cannon H, Georgiou V. Patients’ knowledge and experience of adjuvant endocrine therapy for early breast cancer: a European study. Breast. 2007;16(5):462–468. doi: 10.1016/j.breast.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Liberati A, Altman DG, Tetzlaff et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 74.Sallis JF, Owen N, Fisher EB. Ecological models of health behavior. In: Glanz K, Rimer B, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. San Francisco, CA: Jossey-Bass; 2008. pp. 465–486. [Google Scholar]
- 75.Bronfenbrenner U. Ecological Models of Human Development. Readings on the Development of Children. 5th ed. New York, NY: Worth; 1997. [Google Scholar]
- 76.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 77.Ashing-Giwa KT, Padilla G, Tejero J et al. Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psychooncology. 2004;13(6):408–428. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhatta SS, Hou N, Moton ZN et al. Factors associated with compliance to adjuvant hormone therapy in Black and White women with breast cancer. Springerplus. 2013;2:356. doi: 10.1186/2193-1801-2-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bickell NA, Wang JJ, Oluwole S et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 80.Friese CR, Pini TM, Li Y et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Malin JL, Diamant AL, Thind A, Maly RC. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat. 2013;137(3):829–836. doi: 10.1007/s10549-012-2387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Livaudais JC, Hershman DL, Habel L et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Livaudais JC, Lacroix A, Chlebowski RT et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev. 2013;22(3):365–373. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Livaudais JC, Li C, John EM et al. Racial and ethnic differences in adjuvant hormonal therapy use. J Womens Health (Larchmt) 2012;21(9):950–958. doi: 10.1089/jwh.2011.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, Wheeler SB. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145(3):743–751. doi: 10.1007/s10549-014-2957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shelton RC, Clarke Hillyer G, Hershman DL et al. Interpersonal influences and attitudes about adjuvant therapy treatment decisions among non-metastatic breast cancer patients: an examination of differences by age and race/ethnicity in the BQUAL study. Breast Cancer Res Treat. 2013;137(3):817–828. doi: 10.1007/s10549-012-2370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wheeler SB, Kohler RE, Reeder-Hayes KE et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv. 2014;8(4):603–610. doi: 10.1007/s11764-014-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davies C, Pan H, Godwin J et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]