Abstract
Background
Hepatic resection of liver metastases of non-colorectal, non-neuroendocrine, and non-sarcoma (NCNNNS) primary malignancies seems to improve survival in selected patients. The aims of the current review were to describe long-term results of surgery and to evaluate prognostic factors for survival in patients who underwent resection of NCNNNS liver metastases.
Methods
We identified 30 full texts (25 single-center and 5 multicenter studies) published after year 1995 and published in English with a total of 3849 patients. For NCNNNS liver metastases, 83.4 % of these subjects were resected.
Results
No prior systematic reviews or meta-analyses on this topic were identified. All studies were case series without matching control groups. The most common primary sites were breast (23.8 %), genito-urinary (21.8 %), and gastrointestinal tract (19.8 %). The median 5- and 10-year overall survival were 32.3 % (range 19–42 %) and 24 % (indicated only in two studies, range 23–25 %), respectively, with 71 % of R0 resections.
Conclusions
There is evidence suggesting that surgery of NCNNNS metastases is safe, feasible, and effective if treatment is part of a multidisciplinary approach and if indication is based on the prognostic factors underlined in literature analysis.
Keywords: Liver metastases, Non-colorectal, Non-neuroendocrine, Non-sarcoma, Liver resection, Prognostic factors
Review
Due to its filter role in the portal circulation, the liver is often the first organ involved in metastatic dissemination of gastrointestinal neoplasms. Hepatic resection is an established and recognized procedure for the treatment of colorectal liver metastases, and it is associated with higher survival rates than more conservative therapies. In fact, patients who undergo complete resections (R0) have a 5-year survival rate of approximately 40 % and a 10-year overall survival rate of 25 % [1–10]. The role of surgery for metastases from neuroendocrine neoplasms on long-term outcome is also well-documented [11, 12]. Recently, some authors analyzing liver metastases from sarcomas reported similar results to colorectal and neuroendocrine cancer with a 5-year survival ranging from 26 to 36 % [13, 14]. The increasing attention has led to a better standard of care. A multidisciplinary approach, surgical techniques, perioperative management, and technological advances have all contributed to the improvement of long-term survival after liver resections over the last two decades.
On the other hand, several other primary sites develop metastases in hepatic parenchyma. With regard to this third group of cancers (non-colorectal, non-neuroendocrine, and non-sarcomas (NCNNNS)), there is a paucity of data in medical literature. In fact, studies either had small number of patients likely due to the relative rarity and the lack of centralization in high volume centers, or they investigated diseases from primary tumors with different prognoses, including metastasis from cancer of the colorectum, neuroendocrine tissues, and sarcoma.
The aims of the current review were to describe long-term results of surgery and identify prognostic factors for survival in a heterogeneous group of liver metastases from NCNNNS primaries malignancies.
Material and methods
Literature search strategy
The original published studies were searched via PubMed and Medline databases, between 1995 and 2014. The following keywords were utilized: “liver” and “metastases”, “non-colorectal”, “non-neuroendocrine”, “non-sarcoma”, “hepatectomy” (or “resection”), and “prognostic factors”. More than 5000 references were identified. The reference lists of all retrieved articles were reviewed to further identify potentially relevant studies.
The purpose of data extraction has been to identify those items in which the percentage of patients who were resected for NCNNNS liver metastases was higher than 50 %.
Selection criteria
Observational clinical studies that used hepatic resection as a therapeutic option for NCNNNS malignancies were identified for inclusion. All relevant prospective and retrospective series were also included. Specific inclusion criteria were studies published after the year 1995, human articles, and papers published in the English language.
Abstracts, reviews, letters, editorials, case reports, expert opinions, and articles contain short reviews were excluded. The final result is the analysis of 30 full texts: 25 single-center studies and 5 multicenter studies [15–44].
Data extraction
Two reviewers independently appraised each article using similar protocols. Data extracted were: methodology, patient number and characteristics, outcomes, length of follow-up, overall survival or progression-free survival, mortality, morbidity, and prognostic factors. Median values and percentages were determined after tabulation of the results from the included studies. All the studies included in the present review aimed to demonstrate the efficacy of hepatic resection for liver metastases, although a minority evaluated also other concomitant ablative techniques.
Results
The 30 studies included a total of 3849 patients (Fig. 1). All studies were case series without matching control groups: 11 studies described more than 100 patients [16, 24, 26, 29, 34–37, 41, 43, 44], 6 studies between 50 and 100 patients [15, 17, 31, 38, 39, 42], and 13 studies less than 50 patients [18–23, 25, 27, 28, 30, 32, 33, 40]. No prior systematic reviews or meta-analyses on this topic were identified. The largest series was published by Adam et al. in 2006 [37] including 1452 patients who underwent surgical procedures. In 22 studies, patients candidates to hepatic resection of liver metastases (LM) were the target population, but subjects undergoing alternative, additional surgical procedures (mainly radiofrequency ablation or cryoablation in addition to partial hepatectomy) or palliative treatment was included for the purpose of comparison [15, 20–31, 34–37, 39–43]. Study objectives and inclusion criteria were clearly described in all the studies.
Fig. 1.
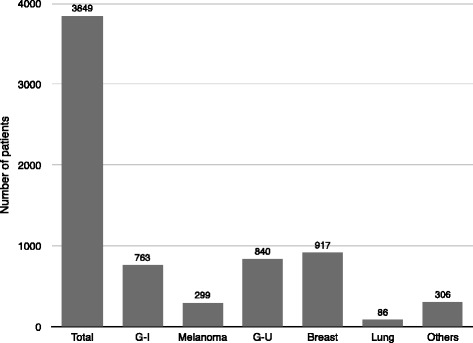
Total number of patients and origins of metastases
Twenty-eight studies reported overall survival results (Table 1), which were unclear in one article [23]. Twenty-one studies reported data on progression-free survival [16–20, 24, 25, 29, 31–33, 35–44]. Each article reported length of follow-up of at least 5 years. The 5- and 10-year overall survival ranges were 19–42 % and 23–25 %, respectively, with a mean 5-year overall survival of 32.3 % (Table 1).
Table 1.
Overall and disease-free survival
| First author | Overall survival | Disease-free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (months) | 1 year (%) | 2 years (%) | 3 years (%) | 5 years (%) | 10 years (%) | Median (months) | 1 year (%) | 2 years (%) | 3 years (%) | 5 years (%) | 10 years (%) | |
| Harrison [38] | 32 | 80 | – | 45 | 37 | – | – | – | – | – | – | 18.7 |
| Lindell [27] | 32 | 75 | – | – | 36 | 25 | – | – | – | – | – | – |
| Elias [24] | – | – | – | – | 36 | – | – | – | – | – | 28 | – |
| Berney [19] | 19 | 61 | 43 | – | 27 | – | 36a | – | – | – | – | – |
| Hamy [22] | 19.6 | 54 ± 8 | 42 ± 8 | – | 27 ± 8 | – | – | – | – | – | – | – |
| Benevento [25] | 38 ± 11 | 54 | 42 | – | 21 | – | – | – | – | – | – | – |
| Hemming [40] | 46 | 85 | – | 55 | 45 | – | 28a | – | – | – | – | – |
| Takada [23] | 0–53 | – | – | – | – | – | – | – | – | – | – | – |
| Van Ruth [18] | 21 | – | – | – | 35 | – | 12 | – | – | – | 20 | – |
| Laurent [30] | – | 81 | – | 40 | 35 | – | – | – | – | – | – | – |
| Goering [20] | 45 | 82 | – | 55 | 39 | – | – | 43 | – | 21 | – | – |
| Karavias [28] | – | – | – | 78 | – | – | – | – | – | – | – | – |
| Torras [33] | – | 86 | 48 | – | – | – | 53 ± 38 | 50 | 25 | – | – | – |
| Yedibela [16] | 23 | – | 49 | – | 26 | – | 25 | – | – | – | – | – |
| Weitz [35] | 42 | – | – | 57 | – | – | 17 | – | – | 30 | – | – |
| Cordera [31] | 28.8 | 81.1 | – | 43 | 30.2 | – | 13.2 | 64.7 | – | – | 15.8 | – |
| Earle [17] | 36 | 88.5 | – | 49.1 | 34.9 | – | 21.5 | – | – | – | – | – |
| Adam [37] | 35 | – | – | – | 36 | 23 | 13 | – | – | – | 21 | 15 |
| Verhoef [21] | 37 | – | – | – | 42 | – | – | – | – | – | – | – |
| Lendoire [34] | 27 | 67 | – | 34 | 19 | – | – | – | – | – | – | – |
| O’Rourke [29] | 42 | – | – | 56.1 | 38.5 | – | 18 | – | – | 37.2 | 26.5 | – |
| Pais Costa [32] | – | – | – | 50 | – | – | – | – | – | 40 | – | – |
| Ercolani [41] | 35.5 ± 6.4 | 83.6 | – | 56.5 | 40 | – | 26.6 ± 4.1 | 75 | – | 44 | 30 | – |
| Duan [26] | 38.8 ± 26.7 | 84.8 | – | 44.7 | 29.5 | – | – | – | – | – | – | – |
| Bresadola [39] | 20 | 71.9 | – | 42.8 | 28.9 | – | 19–44 | 68–85 | – | 29–63 | 19–52 | – |
| Marudanayagam [42] | 19 | 72.9 | – | 47.9 | 25.6 | – | 19 | – | – | – | – | – |
| Treska [15] | – | 88.6 | – | 72.5 | 36.9 | – | – | – | – | – | – | – |
| Groeschl [36] | 49 | 73 | – | 50 | 31 | – | 23 | – | – | – | – | – |
| Slotta [43] | 20.5 | 66 | – | 43 | 30 | – | – | – | – | – | 39 | – |
| Takemura [44] | 41.8 | 83.9 | – | 55.4 | 41 | – | 10 | 43.7 | – | 21.1 | 18.1 | – |
| Range | 0–53 | 19–42 | 23–25 | 10–53 | 15.8–52 | 15–18.7 | ||||||
| Average | 32.3 | 32.3 | 24 | 23.1 | 26 | 16.9 | ||||||
aOnly metachronous group
Figures 1 and 2 present the results of the primary sites and systemic spread of metastases. The most common primary sites were breast (23.8 %), genito-urinary (21.8 %), and gastrointestinal tract (19.8 %). Sixteen studies included patients with extrahepatic metastases [16–18, 24, 25, 27–31, 33, 36, 37, 42–44], whereas fourteen studies included patients with isolated liver disease only, or the presence of extrahepatic metastases at the time of hepatic resection was not specified [15, 19–23, 25, 26, 32, 34, 38–41]. The presence of extrahepatic disease wildly ranged between 0 and 53.1 % because several authors considered it an exclusion criterion for surgery, while others did not deem extrahepatic disease a contraindication or a negative prognostic factor. Therefore, liver resection was performed with the intent to achieve a radical resection (R0). Metastases affected two hepatic lobes in 0–37.5 % of patients. The presence of bilobar metastases or extrahepatic disease may not be considered as a contraindication for surgery [16, 17, 29–31, 33, 34, 38, 40, 42, 44]; 18 % of authors resected bilobar diseases, otherwise considering this criteria a prognostic factor that impact prognosis. Figure 3 also shows the results regarding the status of the surgical margin: although an R0 resection is always the primary objective of the surgeon, it is obtained in 2736 of the 3849 patients, although it is specified only in 21 studies.
Fig. 2.
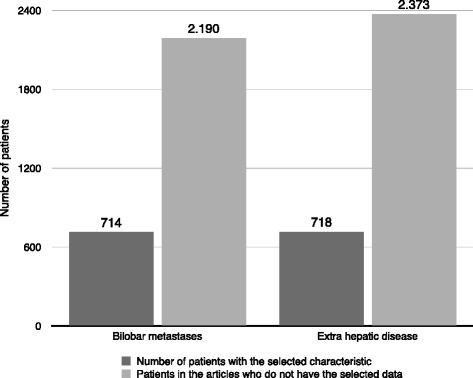
Metastatic involvement
Fig. 3.
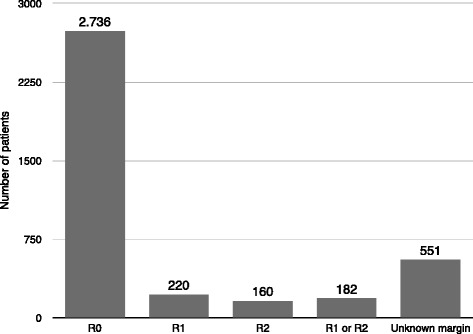
Resection margin
A detailed evaluation of significant and non-significant prognostic factors for overall survival and progression-free survival is presented in Table 2. By the univariate analysis, the most common factors (reported in ≥3 studies) associated with poor survival analysis were the following: age, synchronous metastatic disease, site of the primary tumor, presence of symptoms, type and extension of hepatectomy, macroscopic (R2) residual disease and distance of free-surgical margin, adjuvant treatment, presence of extrahepatic disease, number of hepatic lesions and the size of the greatest one, and bilobar disease.
Table 2.
Prognostic factors
| Prognostic factor | Variables that influence overall survival | Variables that influence progression-free survival |
|---|---|---|
| Sex | 39 | 35 |
| Preoperative treatment of liver | 15 | |
| Metastatic situation: synchronous/metachronous | 17, 23, 27, 34, 38 | 44 |
| Primary site and histological subtype | 15–26, 33, 34, 37–41, 43 | 31, 35 |
| Symptomatic at the time of resection | 41a | |
| Hepatic involvement | 25, 26 | |
| Type of the intervention performed | 17, 37, 39–41 | |
| Macroscopically incomplete resection (R2) | 17, 19, 38, 40 | 29 |
| Surgical margin status | 16, 17, 19, 32, 34, 37, 38, 40, 42 | 35 |
| Adjuvant treatment | 15, 17, 18, 20, 21, 24–26, 37 | 44 |
| Presence of extrahepatic disease | 15, 16, 20, 27, 28, 30, 37 | |
| Surgery timing | 19, 22, 26, 31 | 31 |
| Postoperative complications | 17, 44 | 44 |
| Number of metastases | 17, 19, 26, 27, 33, 39, 44 | 44 |
| Size of metastases | 26, 29, 36, 41, 42 | 29, 44 |
| Presence of vascular invasion | 36 | |
| Lymph node metastases | 29, 36, 39 | 29 |
| Disease-free survival | 26, 30, 37, 38, 41 | 17, 31, 35, 44 |
| Blood transfusion | 44 | 44 |
aSymptoms within 1 year
In particular, we analyzed the five main prognostic factors as follows:
primary site and histological subtype (22 articles, Table 2); liver metastases from breast kidney, uterus, ovary, testicle, ampulla of Vater, and adrenal gland cancer had a 5-year overall survival greater than 30 %, while metastases from gastric and duodenum cancer had a survival between 30 and 15 %; liver metastases from pancreas, anus, esophagus, cardia, and lung cancers had a 5-year overall survival less than 15 % [37, 43].
size and number of metastases (11 articles, Table 2); the prognosis worsened especially when the number of metastases was higher than 3–4 and the size greater than 5–6 cm.
surgical margin status (10 articles, Table 2); to perform surgery with curative intent, it is necessary to accomplish disease-free resection margins (R0). Nevertheless, R0 resection was reported with a range between 55.9 [19] and 100 % of cases [28, 30, 32], showing that a complete excision of the lesion is not always achievable suggesting that the margin of resection could be an important prognostic factor, on overall survival.
type of the intervention performed (5 articles, Table 2); the extent of hepatectomy was also an independent negative prognostic factor, possibly reflecting the magnitude of tumor burden [37].
time of metastasis appearance (6 articles, Table 2). Despite the presence of synchronous metastases might represent the disease aggressiveness, the 5-year overall survival in patients with metachronous disease or synchronous disease were 31–37 % and 31–36 %, respectively [16, 37].
Discussion
The role of surgery in the management of liver metastases from colorectum (CR) or neuroendocrine tumors has been well described in the literature; several studies have focused their attention on the surgical indications of these patients and the respective outcomes, showing the need for an aggressive surgical therapy. In particular, surgical treatment of metastatic CR cancer has improved long-term outcome. A 5-year survival rate was 25 % in the 80s, progressively increased up to 47 % in 2008 [7, 45–51]. In contrast, the treatment of NCNNNS liver metastases does not have a clearly defined role, mostly because of discrepant characteristics of patients, difficulty in their selection, and lack of high volume series.
The aim of this review is to help shedding light on this controversial topic not adequately outlined so far.
Several multicenter studies and reviews report a survival rate ranging from 27.9 to 49.3 % in patients with metastases from non-CR cancer, depending on the tumor histology [5]. Although the set of “non-CR metastases” is extremely heterogeneous, there is a general agreement on the surgical treatment of some homogeneous groups of tumors such as metastases from neuroendocrine tumors and sarcoma. In these cases, the resection has been proved safe and able to prolong survival compared to non-invasive treatments, obtaining an overall survival rate at 5-year of 20–33 % and of 46–86 % for sarcoma and neuroendocrine tissues, respectively [11–14, 24, 52].
The results of different studies suggest that the technological and cultural evolutions in surgery has improved the prognosis in patients treated from 1978 to 2014, especially in terms of 5-year overall survival. The most recent data on the treatment of metastatic non-CR cancer are comparable to those reported in the literature in studies on the treatment of metastatic CR cancer dated 15–20 years ago [53–55].
Beyond the heterogeneity in terms of histology of the primary disease and the stage of cancer, the improvement in the survival rates of patients undergoing surgery strengthens and demonstrates the utility of resection with a 19–42 % 5-year survival rates.
The theory defining tumor spreading to the liver as a “systemic” and not a “regional” disease is obsolete and justifies why liver metastases were not surgically removed until some decades ago. Despite the presence of metastases representing an advance disease and that hematogenous dissemination may be a contraindication for surgery, the most recent results suggest that surgery, combined by chemotherapy, is useful in this type of systemic diseases by improving overall.
The treatment of liver non-CR metastases remained a debated topic until about 15 years ago, when improved survival was reported among patients operated on for hepatic metastases from testicular, kidney, and breast cancers [19, 24].
The surgical approach to non-CR liver metastases is essentially based on two fundamental issues that have become increasingly important in the last two decades:
Hepatic resection has become safer thanks to improved surgical techniques and an accurate pre-and intraoperative imaging allowing parenchyma spearing.
The complementary role of new and effective chemotherapy agents and surgery for some non-CR tumors
Therefore, it appears that the role of surgery in the treatment of liver metastases may be a relevant hope for patients when they meet the criteria of resectability, regardless the number of metastases; further efforts should be pursued to better evaluate its clinical impact and standardize management protocols.
Regarding surgical margin, it is considered safe when >0.5–1 cm since shorter margins are associated with higher rates of recurrence. However, as already shown for colorectal cancer, the overall survival rate seems to be not significantly influenced by the margin width but more by the presence of residual disease (R1-R2 versus R0) [17, 19, 34, 37, 38, 40, 42]. As such, it might be that 1 mm is sufficient to improve survival. In addition, the more patients with advanced diseases (multiple metastases, bilobar, or large) are resected, the more clearly liver parenchyma must be preserved, obtaining less wide margins. Debulking surgery is a fairly debated topic, because up to date, it has not been demonstrated to impact survival results [14, 56, 57], unless an R0 resection is unachievable with adjuvant/neoadjuvant [13] and medical therapy has failed.
Contraindications to debulking surgery seem to apply in particular to rapidly progressive metastatic disease not controlled by systemic treatments and to synchronous liver metastases, except for breast [37] and genito-urinary tract [43] neoplasms because the efficacy of medical treatments allow to improve survival and to stretch the limit of surgery.
Up-front hepatic resection seems contraindicated in patients with major comorbidities or for tumors with advanced invasion of major vessels and in those with an expected residual liver volume less than 30–40 % after resection. In the latest cases, neoadjuvant chemotherapy or other cytoreductive approaches may allow delayed reexamination and new judgment of operability and resectability. However, in some chemoresistant tumors (melanomas, kidney), surgery should be considered, when feasible, as the only possible therapy with curative intent and should not be delayed.
The presence of extrahepatic disease seems to be a relative contraindication to liver surgery.
An half of the studies analyzed deem the presence of an extrahepatic an absolute contraindication to surgical resection, while the remnant studies push the limit of surgical approach with the aim to obtain an R0 resection.
In our univariate analysis, the most common prognostic factors related to the 5-year overall survival rate was the site of the primitive tumor and the histological subtype. In particular, the worse prognosis was for hepatic metastases from gastrointestinal tumors, except for well-selected patients with metachronous metastases from gastric cancer.
The low rate of complication after elective liver surgery and the survival benefit observed in association with hepatectomy with more than one third of patients alive at 5 years and subsequently a quarter to 10 years support the inclusion of surgery in a multidisciplinary set of care for these patients.
Conclusions
Liver resection for NCNNNS metastases seems to be safe and feasible. Long-term outcome is deeply affected by some clinical and pathological features, such as the histology of the primary tumor. Statistical models based on correct patient prognostic factors can help to predict long-term survival.
Currently, there is evidence that the surgery of NCNNNS metastases is effective if the indication is based on the evaluation of precise risk factors and if surgery is associated with complementary therapies. The major benefits are accomplished for genito-urinary and breast cancer, for size of mestastases less than 5 cm, a curative resection is achieved and when the appearance of hepatic lesions is longer than 12 months from primary tumors.
Acknowledgements
Sources of funding for research and publication, Grant from Milano-Bicocca University.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FR and FU conceived of the study. FR, FU, and LG participated in the design of the study. PAR, PG, and MG performed the acquisition, analysis, and interpretation of the data. LD and LN drafted the manuscript. LG revised critically the manuscript. All authors read and approved the final manuscript.
Contributor Information
Fabio Uggeri, Phone: +39 3474311545, Email: fabio.uggeri@unimib.it.
Paolo Alessandro Ronchi, Email: broska87@gmail.com.
Paolo Goffredo, Email: goffredo.paolo@gmail.com.
Mattia Garancini, Email: mattia_garancini@yahoo.it.
Luca Degrate, Email: degluc@inwind.it.
Luca Nespoli, Email: luca.nespoli@unimib.it.
Luca Gianotti, Email: luca.gianotti@unimib.it.
Fabrizio Romano, Email: fabrizio.romano@unimib.it.
References
- 1.Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, et al. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001;96(11):3178–84. doi: 10.1111/j.1572-0241.2001.05278.x. [DOI] [PubMed] [Google Scholar]
- 2.Osada S, Imai H, Sasaki Y, Tanaka Y, Tokuyama Y, Okumura N, et al. Strategy for synchronous and multiple liver metastasis. Hepatogastroenterology. 2012;59:198–203. doi: 10.5754/hge10080. [DOI] [PubMed] [Google Scholar]
- 3.Vladov N, Vasilevski I, Takorov I, Mutafchiyski V, Sergeev S, Odiseeva E, et al. Rational surgical aggression in multimodal treatment of liver colorectal metastases. Hepatogastroenterology. 2012;59:241–4. doi: 10.5754/hge10158. [DOI] [PubMed] [Google Scholar]
- 4.Quan D, Gallinger S, Nhan C, Auer RA, Biagi JJ, Fletcher GG, et al. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: a systematic review. Surgery. 2013;153(3):438. doi: 10.1016/j.surg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Spelt L, Andersson B, Nilsson J, Andersson R. Prognostic models for outcome following liver resection for colorectal cancer metastases: a systematic review. Eur J Surg Oncol. 2012;38(1):16–24. doi: 10.1016/j.ejso.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan MC, Castaldo ET, Gao F, Chari RS, Linehan DC, Wright JK, et al. A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by positron emission tomography with [18F]fluoro-2-deoxy- D-glucose: role of primary tumor variables. J Am Coll Surg. 2008;206(5):857–68. doi: 10.1016/j.jamcollsurg.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Shimada H, Fujii Y, Endo I, Sekido H, Togo S, et al. Pre-hepatectomy prognostic staging to determine treatment strategy for colorectal cancer metastases to the liver. Langenbecks Arch Surg. 2004;389(5):371–9. doi: 10.1007/s00423-004-0490-y. [DOI] [PubMed] [Google Scholar]
- 9.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246(5):806–14. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 10.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29(1):89–102. doi: 10.1111/j.1478-3231.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath P, Chhabra D, Shrikhande S, Shah R. Surgical treatment of liver metastases in neuroendocrine neoplasms. Int J Hepatol. 2012;2012:782672. doi: 10.1155/2012/782672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SY, Cheow PC, Teo JY, Ooi LL. Surgical treatment of neuroendocrine liver metastases. Int J Hepatol. 2012;2012:146590. doi: 10.1155/2012/146590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stavrou GA, Flemming P, Oldhafer KJ. Liver resection for metastasis due to malignant mesenchymal tumours. HPB. 2006;8:110–3. doi: 10.1080/13651820500472143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang H, Nussbaum KT, Kaudel P, Fruhauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: a single-center experience with 34 liver resections during a 15-year period. Ann Surg. 2000;231:500–5. doi: 10.1097/00000658-200004000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treska V, Liska V, Skalicky T, Sutnar A, Treskova I, Narsanska A, et al. Non-colorectal liver metastases: surgical treatment options. Hepatogastroenterology. 2012;59:245–8. doi: 10.5754/hge10292. [DOI] [PubMed] [Google Scholar]
- 16.Yedibela S, Gohl J, Graz V, Pfaffenberger MK, Merkel S, Hohenberger W, et al. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumors: a single-institutional review. Ann Surg Oncol. 2005;12(10):778–85. doi: 10.1245/ASO.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Earle SA, Perez EA, Gutierrez JC, Sleeman D, Livingstone AS, Franceschi D, et al. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. J Am Coll Surg. 2006;203:436–46. doi: 10.1016/j.jamcollsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Van Ruth S, Mutsaerts E, Zoetmulder FAN, Van Coevorden F. Metastasectomy for liver metastases of noncolorectal primaries. Eur J Surg Oncol. 2001;27(7):662–7. doi: 10.1053/ejso.2001.1210. [DOI] [PubMed] [Google Scholar]
- 19.Berney T, Mentha G, Roth AD, Morel P. Results of surgical resection of liver metastases from non-colorectal primaries. British Journal of Surgery. 1998;85:1423–7. doi: 10.1046/j.1365-2168.1998.00856.x. [DOI] [PubMed] [Google Scholar]
- 20.Goering JD, Mahvi DM, Niederhuber JE, Chicks D, Rikkers LF. Cryoablation and liver resection for noncolorectal liver metastases. Am J Surg. 2002;183:384–9. doi: 10.1016/S0002-9610(02)00806-1. [DOI] [PubMed] [Google Scholar]
- 21.Verhoef C, Kuiken BW, IJzermans JN, De Wilt JH. Partial hepatic resection for liver metastases of non-colorectal origin, is it justified? Hepatogastroenterology. 2007;54(77):1517–21. [PubMed] [Google Scholar]
- 22.Hamy AP, Paineau JR, Mirallie EC, Bizouarn P, Visset JP. Hepatic resections for noncolorectal metastases: forty resections in 35 patients. Hepatogastroenterology. 2000;47:1090–4. [PubMed] [Google Scholar]
- 23.Takada Y, Otsuka M, Seino K, Taniguchi H, Koike N, Kawamoto T, et al. Hepatic resection for metastatic tumors from noncolorectal carcinoma. Hepatogastroenterology. 2001;48(37):83–6. [PubMed] [Google Scholar]
- 24.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, Plaud B, Ducreux M, Spielmann M, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187(5):487–93. doi: 10.1016/S1072-7515(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 25.Benevento A, Boni L, Frediani L, Ferrari A, Dionigi R. Result of liver resection as treatment for metastases from noncolorectal cancer. J Surg Oncol. 2000;74(1):24–9. doi: 10.1002/1096-9098(200005)74:1<24::AID-JSO6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Duan XF, Dong NN, Zhang T, Li Q. Comparison of surgical outcomes in patients with colorectal liver metastases versus noncolorectal liver metastases: a Chinese experience. Hepatol Res. 2012;42(3):296–303. doi: 10.1111/j.1872-034X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindell G, Ohlsson B, Saarela A, Andersson R, Tranberg KG. Liver resection of noncolorectal secondaries. J Surg Oncol. 1998;69(2):66–70. doi: 10.1002/(SICI)1096-9098(199810)69:2<66::AID-JSO4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Karavias DD, Tepetes K, Karatzas T, Felekouras E, Androulakis J. Liver resection for metastatic noncolorectal non-neuroendocrine hepatic neoplasms. Eur J Surg Oncol. 2002;28(2):135–9. doi: 10.1053/ejso.2001.1221. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke TR, Tekkis P, Yeung S, Fawcett J, Lynch S, Strong R, et al. Long-term results of liver resection for non-colorectal, non-neuroendocrine metastases. Ann Surg Oncol. 2007;15(1):207–18. doi: 10.1245/s10434-007-9649-4. [DOI] [PubMed] [Google Scholar]
- 30.Laurent C, Rullier E, Feyler A, Masson B, Saric J. Resection of noncolorectal and nonneuroendocrine liver metastases: late metastases are the only chance of cure. World J Surg. 2001;25:1532–6. doi: 10.1007/s00268-001-0164-7. [DOI] [PubMed] [Google Scholar]
- 31.Cordera F, Rea DJ, Rodriguez-Davalos M, Hoskin TL, Nagorney DM, Que FG. Hepatic resection for noncolorectal, nonneuroendocrine metastases. J Gastrointest Surg. 2005;9:1361–70. doi: 10.1016/j.gassur.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 32.Pais Costa SR, Horta SH, Miotto MJ, Costas MC, Henriques AC, Speranzini MB. Hepatic resection for non-colorectal and non-neuroendocrine metastatic cancer: indications and results in ten resectable cases. Einstein. 2008;6(1):56–62. [Google Scholar]
- 33.Torras J, Ramos E, Figueras J. Surgical treatment of hepatic metastases from non-colorectal non-neuroendocrine tumours. Rev Oncol. 2004;6(2):86–89. [Google Scholar]
- 34.Lendoire J, Moro M, Andriani O, Grondona J, Gil O, Raffin G, et al. Liver resection for noncolorectal, non-neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB (Oxford) 2007;9(6):435–9. doi: 10.1080/13651820701769701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D’Angelica M, Harrison LE, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine Carcinoma. Ann Surg. 2005;241:269–76. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groeschl RT, Nachmany I, Steel JL, Reddy SK, Glazer ES, De Jong MC, et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. 2012;214:769–77. doi: 10.1016/j.jamcollsurg.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 37.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. doi: 10.1097/01.sla.0000246847.02058.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison LE, Brennan MF, Newman E, Fortner JG, Picardo A, Blumgart LH, et al. Hepatic resection for non colorectal non neuroendocrine metastases: a fifteen-year experience with ninetysix patients. Surgery. 1997;121(6):625–32. doi: 10.1016/S0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 39.Bresadola V, Rossetto A, Adani GL, Baccarani U, Lorenzin D, Favero A, et al. Liver resection for noncolorectal and nonneuroendocrine metastases: results of a study on 56 patients at a single institution. Tumori. 2011;97(3):316–22. doi: 10.1177/030089161109700310. [DOI] [PubMed] [Google Scholar]
- 40.Hemming AW, Sielaff TD, Gallinger S, Cattral MS, Taylor BR, Greig PD, et al. Hepatic resection of noncolorectal nonneuroendocrine metastases. Liver Transpl. 2000;6(1):97–101. doi: 10.1016/S1527-6465(00)80040-4. [DOI] [PubMed] [Google Scholar]
- 41.Ercolani G, Vetrone G, Grazi GL, Cescon M, Di Gioia P, Ravaioli M, et al. The role of liver surgery in the treatment of non-colorectal non-neuroendocrine metastases (NCRNNE). Analysis of 134 resected patients. Minerva Chir. 2009;64(6):551–8. [PubMed] [Google Scholar]
- 42.Marudanayagam R, Sandhu B, Perera MTPR, Taniere P, Coldham C, Bramhall S, et al. Hepatic resection for non-colorectal, non-neuroendocrine, non-sarcoma metastasis: a single-centre experience. HPB (Oxford) 2011;13(4):286–92. doi: 10.1111/j.1477-2574.2010.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slotta JE, Schuld J, Distler S, Richter S, Schilling MK, Kollmar O. Hepatic resection of noncolorectal and non-neuroendocrine liver metastases—survival benefit for patients with nongastrointestinal primary cancers—a case-controlled study. Int J Surg. 2014;12:163–8. doi: 10.1016/j.ijsu.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, et al. Long-term results of hepatic resection for non-colorectal, non-neuroendocrine liver metastases. Hepatogastroenterology. 2013;60(127):1705–12. doi: 10.5754/hge13078. [DOI] [PubMed] [Google Scholar]
- 45.Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. The natural history of hepatic metastases from colorectal cancer. Ann Surg. 1984;199(5):502–8. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adson MA, Van Heerden JA, Adson MH, Wagner JS, Ilstrup DM. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119(6):647–51. doi: 10.1001/archsurg.1984.01390180015003. [DOI] [PubMed] [Google Scholar]
- 47.August DA, Sugarbaker PH, Ottow RT, Gianola FJ, Schneider PD. Hepatic resection of colorectal metastases. Influence of clinical factors and adjuvant intraperitoneal 5-fluorouracil via Tenckhoff catheter on survival. Ann Surg. 1985;201(2):210–8. doi: 10.1097/00000658-198502000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bengtsson G, Carlsson G, Hafström L, Jönsson PE. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg. 1981;141(5):586–9. doi: 10.1016/0002-9610(81)90057-X. [DOI] [PubMed] [Google Scholar]
- 49.Lim CN, McPherson TA. Surgery as an alternative to chemotherapy for hepatic metastases from colorectal cancer. Can J Surg. 1983;26(5):458–9. [PubMed] [Google Scholar]
- 50.Mancini R, Ettorre G, Vennarecci G, Sperduti I, Garufi C, Esposito A, et al. Personal experience on treatment of colorectal liver metastases: a multidisciplinary approach. J Exp Clin Cancer Res. 2003;22(4 Suppl):213–7. [PubMed] [Google Scholar]
- 51.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19(1):59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 52.De Matteo RP, Fong Y. Result for hepatic resection for sarcoma metastatic to liver. Ann Surg. 2003;10:1007–11. doi: 10.1245/ASO.2003.09.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, et al. Long-term survival following resection of colorectal hepatic metastases: association Francaise de Chirurgie. Br J Surg. 1997;84(7):977–80. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 54.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238(6):871–83. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Matteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–7. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutkowski P, Nyckowski P, Grzesiakowska U, Nowecki ZI, Nasierowska-Guttmejer A, Pienkowski A, et al. The clinical characteristics and the role of surgery and imatinib treatment in patients with liver metastases from c-Kit positive GastroIntestinal Stromal Tumors (GIST) Neoplasma. 2003;50(6):438–42. [PubMed] [Google Scholar]


