Abstract
Pain management in people with dementia is a critical problem. Recently, psychophysical and neuroimaging techniques have been used to extend our understanding of pain processing in the brain as well as to identify structural and functional changes in Alzheimer disease (AD). But interpreting the complex relationship between AD pathology, brain activation, and pain reports is challenging. This review proposes a conceptual framework for designing and interpreting psychophysical and neuroimaging studies of pain processing in people with AD. Previous human studies describe the lateral (sensory) and medial (affective) pain networks. Although the majority of the literature on pain supports the lateral and medial networks, some evidence supports an additional rostral pain network, which is believed to function in the production of pain behaviors. The sensory perception of pain as assessed through verbal report and behavioral display may be altered in AD. In addition, neural circuits mediating pain perception and behavioral expression may be hyperactive or underactive, depending on the brain region involved, stage of the disease, and type of pain (acute experimental stimuli or chronic medical conditions). People with worsening AD may therefore experience pain but be unable to indicate pain through verbal or behavioral reports, leaving them at great risk of experiencing untreated pain. Psychophysical (verbal or behavioral) and neurophysiological (brain activation) approaches can potentially address gaps in our knowledge of pain processing in AD by revealing the relationship between neural processes and verbal and behavioral outcomes in the presence of acute or chronic pain.
Keywords: dementia, lateral pain network, medial pain network, rostral pain network, pain behaviors, pain processing in people with dementia, neurobiology of pain in dementia
Introduction
Pain management in people with dementia is a common condition that challenges the skills of health care providers. The prevalence of Alzheimer disease (AD)1 and pain2 both increase with advancing age. AD is the most common cause of dementia.3 Worldwide, 26 million people are living with AD and it is projected that 106 million people will live with AD by 2050.4 The prevalence of chronic painful conditions increases with advancing age and negatively impacts quality of life.5,6 Given the high prevalence of both dementia and chronic pain, it is likely that many older adults with AD have chronic or persistent pain.
Pain in people with AD poses assessment challenges for clinicians6 because brain changes in AD may impair the sensory and affective responses to pain.7,8 In mild to moderate stages of AD, people with AD may be unable to consistently report pain9–11; as AD progresses to more severe stages, people lose the ability to communicate verbally. Discerning behaviors that indicate the presence of pain12 also become increasingly difficult to observe as dementia progresses because pain behaviors diminish in people with severe dementia.13 All these factors place individuals with AD at risk of underdetection and undertreatment of pain,14 negatively impacting the remaining quality of life. People with AD receive fewer analgesics when compared to people without AD of similar age and with similar painful conditions.15–18 It is plausible that people with severe AD may also experience pain.
In recent years, new neuroimaging techniques have been used to extend our understanding of (1) pain processing in the brain and (2) structural and functional changes in AD. Neuroimaging research can provide unique opportunities to advance pain management practices in people with dementia. Indeed, functional magnetic resonance imaging (fMRI) has been shown to detect the presence of a signature activation pattern in brain regions known to be associated with pain processing in communicative people with mild to moderate AD.19
Over the last 2 decades, neuroimaging studies have described interconnected brain regions that mediate pain processing. The majority of these studies describe brain activation in networks of structures comprising the lateral and medial pain networks.20–22 Additionally, a rostral pain network may be important in the development of behaviors in response to pain.23 Pain is typically described in sensory-discriminative, affective-motivational, and cognitive-evaluative dimensions.24 Definitive evidence is not available to determine whether the pain processing in the brains of people with AD is altered in one or more of these dimensions19,25 and this must be addressed to inform future research endeavors that seek to develop evidence-based pain management in AD.26
Aim
The aim of this article is to present a brief review of the pain network literature (Figures 1 and 2) and to describe a conceptual framework that can be used for designing and interpreting neuroimaging and psychophysical studies of pain processing in people with AD (Figure 3). To accomplish this, we outline neuroimaging studies of pain processing in healthy people (Table 1) followed by a review of neuroimaging, electrophysiological, and psychophysical studies of pain processing in people with AD. We conclude with recommendations for future studies.
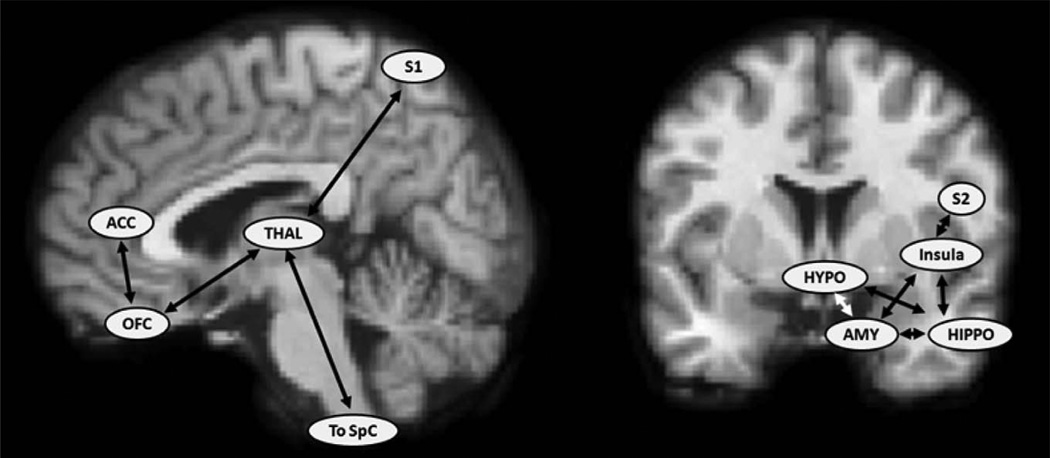
Figure 1.
Select cortical and subcortical regions involved in pain processing. Regions associated with pain processing (Treede et al27; Scherder et al28; Apkarian et al29) are listed on sagittal (left) and coronal (right) anatomic magnetic resonance imaging (MRI) images: anterior cingulate cortex (ACC), primary somatosensory cortex (S1), secondary somatosensory cortex (S2), orbital frontal cortex (OFC), thalamus (THAL), spinal cord (SpC), hypothalamus (HYPO), amygdala (AMY), and hippocampus (HIPPO).
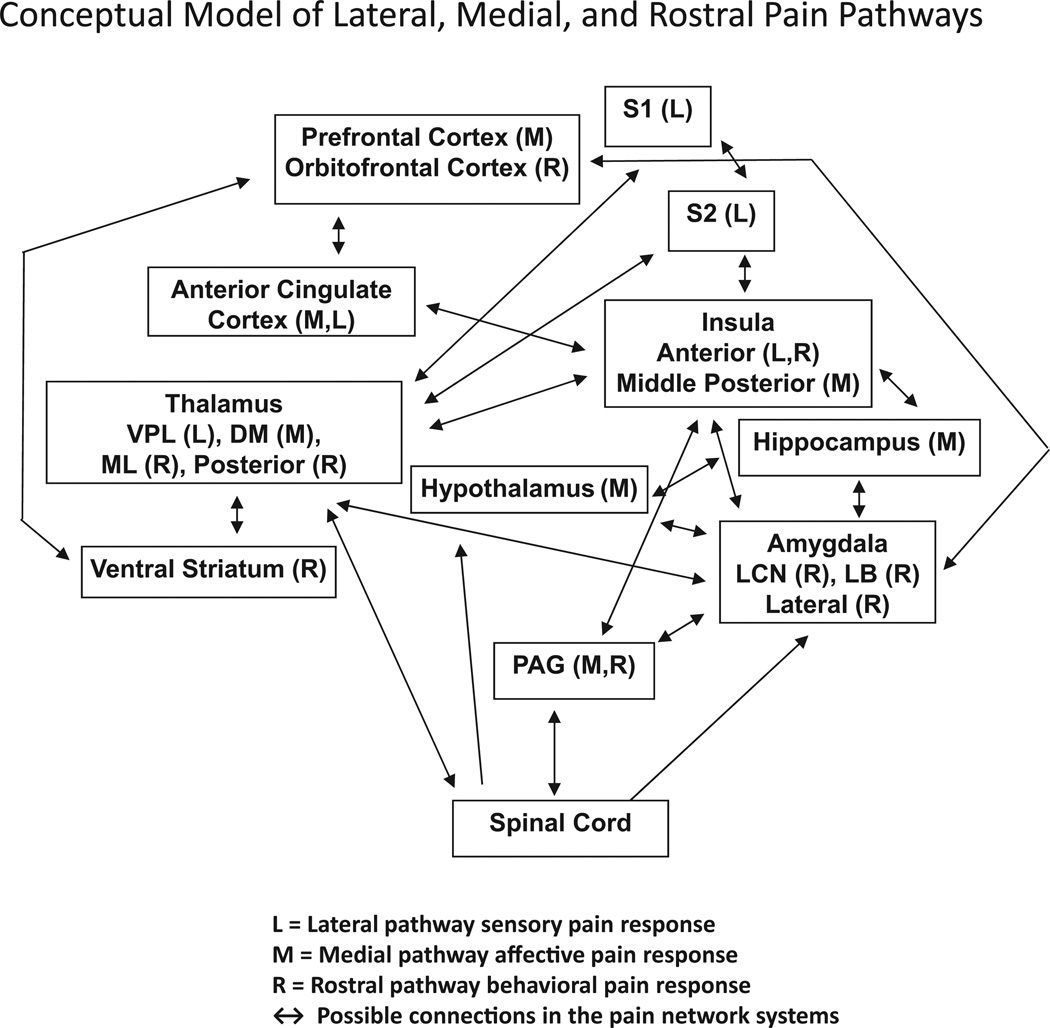
Figure 2.
Pain processing in the lateral (L) and medial (M) network based on previous reviews (Treede et al27; Price30; Borsook and Becera31; Scherder et al32; Apkarian et al29; Chen20). Based on the current review, the rostral (R) network is further integrated into the model. The black arrows show possible connections in different areas of the pain network systems. These networks have been described as “possible functional connections” (Chen20) and as areas with increased BOLD responses in acute pain studies (Kupers and Kehlet33).
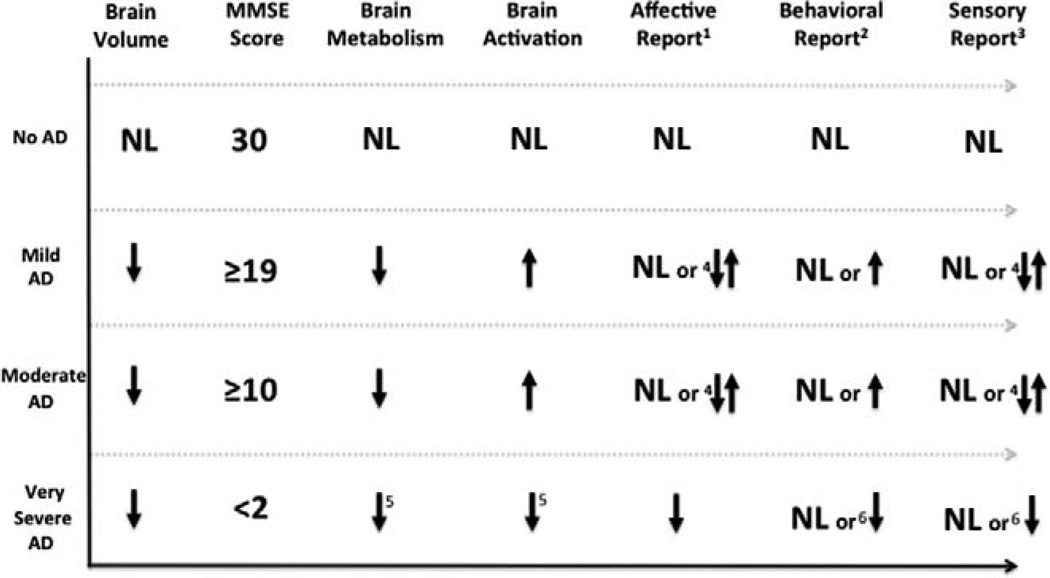
Figure 3.
The values 1, 2, and 3 are associated with 1 medial, 2rostral, and 3lateral pain systems. 4 = on average, when compared to controls, people with AD reported an increased affective (unpleasantness) and an increased sensory (intensity) response to acute pain. Conversely, people with AD reported a decreased affective and sensory response to chronic pain. 5 = hypothesized response. 6 = behavioral and sensory response to acute severe pain may be preserved or decreased. AD indicates Alzheimer disease; NL, normal; MMSE, Mini-Mental State Examination.
Table 1.
Human Brain Regions, Location, and Function in Pain Processing
| Brain Region | Pathway | Function in Pain Processing |
|---|---|---|
| Primary somatosensory cortex (S1)27–29,31,33–38 | Lateral | Sensory-discriminative |
| Secondary somatosensory cortex (S2)19,23,27,28,33,34,39,40 | Lateral | Sensory-discriminative |
| Thalamus19,23,27,28,31,34,40 | Lateral | Sensory-discriminative |
| Medial | Affective-motivational | |
| Rostral | Cognitive-evaluative (behavioral) | |
| Prefrontal (orbitofrontal) cortex33,37,41–44 | Medial | Affective-motivational |
| Rostral | Cognitive-evaluative (behavioral) | |
| Amygdala28,23,31,37,41,44–46 | Medial | Affective-motivational |
| Rostral | Cognitive-evaluative (behavioral) | |
| Insular cortex23,27,28,31,33,34,47 | Lateral | Sensory-discriminative |
| Medial | Affective-motivational | |
| Rostral | Cognitive-evaluative (behavioral) | |
| Anterior cingulate cortex29,31,33–38,41 | Medial | Affective-motivational |
| Rostral | Cognitive-evaluative (behavioral) | |
| Periaqueductal Gray Matter28,31,33–38,41 | Medial | Affective-motivational |
| Rostral | Cognitive-evaluative (behavioral) | |
| Hippocampus28,33 | Medial | Affective-motivational |
| Hypothalamus28,30,31,34 | Medial | Autonomic-endogenous (heart rate, blood pressure, endogenous opioid release) |
| Ventral striatum23 | Rostral | Cognitive-evaluative (behavioral) |
Methods and Literature Search
An overview of the studies which describe the effects of AD pathology on brain volume, activation, and metabolism is presented followed by a discussion of pain networks in the brain. A literature search for studies reporting pain processing in people with AD was performed in PubMed, Google Scholar, and PsychINFO using the search terms “imaging or EEG or fMRI or functional connectivity and pain and Alzheimer’s disease or pain processing in Alzheimer’s disease.”
Eligible studies for data extraction were any study examining the neurobiology of pain in people with AD, including electroencephalogram (EEG), fMRI, and positron emission tomography (PET). Moreover, we included seminal studies and case reports examining the sensory, affective, and/or the behavioral reporting of pain in people with AD or probable AD. Exclusion criteria were studies in a non-English language. This search methodology resulted in 78 articles. Search results were complemented by examining each abstract and reference list for mention of sensory and/or affective pain processing in people with AD. After removing citations that did not meet inclusion criteria, 28 studies remained describing sensory and/or affective pain processing in people with AD (Table 2).
Table 2.
Psychophysical and Neurophysiological Studies of Pain Processing in People With Alzheimer Disease
| Pain Outcome Measures |
Cognitive Measures | Pain Stimulus | Key Findings | Reference |
|---|---|---|---|---|
| Behavioral report | Cognitive Performance Scale (CPS) score where 0 is cognitively intact and 6 is very severe cognitive impairment. CPS scores of 0, 1, 2, 3,4, 5, and 6 are associated with MMSE scores of 25,22, 19, 15,7,5, and 1, respectively. | Terminal cancer | (1) People with equivalent MMSE scores less than 2 had very few behavioral signs of pain recorded in the medical record. (2) People with average equivalent MMSE scores of 19 had the highest behavioral indicators of pain recorded in the medical record. |
Monroe et al48 |
| Behavioral report | MMSE scores (exact scores not reported). | Not reported | Could not determine whether level of cognitive impairment had an effect on display of pain. | Husebo et al123 |
| Verbal report | Modified Mini-Mental State Examination (3 MS) scored from 0 to 100. Cognitively intact ≥77. | Noncancer pain | (1) More cognitively intact people verbally reported noncancer pain. (2) Of those who reported pain, moderate, and severe pain were reported equally between the cognitively intact and cognitively impaired. |
Shega et al49 |
| Functional connectivity | MMSE (13–25) | Mechanical pressure to the thumb nail of right hand | Relative to healthy controls, interregional functional connectivity during experimental pain was increased between the right-DLPFC, hypothalamus, and PAG in people with AD. | Cole et al50 |
| Behavioral report (case study) | End stage AD (could not complete a sentence) | Acute abdominal pain. Diagnosis of perforated bowel. | (1) Preserved sensory-discriminative component of pain. Patient moaned loudly when enema given for presumed fecal impaction. (2) Preserved cognitive-behavioral component of pain. Patient consistently pointed to her stomach and back while moaning. |
Craft121 |
| Behavioral report | CPS score where 0 is cognitively intact and 6 is very severe cognitive impairment. CPS scores of 0, 1, 2, 3, 4, 5, and 6 are associated with MMSE scores of 25, 22, 19, 15, 7, 5, and 1, respectively. | Terminal cancer | In the presence of similar cognitive impairments and opioid intake, African Americans displayed significantly more behavioral displays of pain when compared to Caucasian Americans. | Monroe and Carter52 |
| Verbal report; behavioral report | MMSE (16.4 ± 5.3 SD) | Electrical shock | (1) Sensory-discriminative component preserved, yet ability to provide self-report of pain diminishes in people with dementia. (2) Affective component altered. Facial responses to noxious stimulation were significantly increased in demented patients. |
Kunz et al53 |
| Verbal report | MMSE (17–24) | Existing diagnosis of arthrosis or arthritis | (1) Sensory-discriminative component altered. The level and pain intensity reported by patients with AD was less than controls. (2) Affective component altered. The level and pain affect reported by patients with AD was less than controls. |
Scherder et al54 |
| Verbal report; behavioral report | MMSE(16.3 ± 5.5 SD) | Mechanical pressure | (1) Some people with dementia were unable to provide self-report of pain. However, in those who could self-report, stimuli were rated as painful as controls. (2) Affective component altered. Facial responses to noxious stimulation were significantly increased in demented patients. |
Kunz et al55 |
| Verbal report; behavioral report | N/A (included nursing home residents with a diagnosis of dementia) | Diagnoses known to be associated with pain | (1) The presence of pain significantly decreased with age. (2) People with dementia had lower odds of having “substantial daily pain.” |
Sawyer et al56 |
| Verbal report; behavioral report | Abbreviated Mental Test (AMT) and MMSE. Severe dementia MMSE M = 9 range (8–14), moderate dementia MMSE M = 13 range (7–21) | Not reported | (1) Those with impaired cognition verbally reported more frequent and more severe pain. (2) Among noncommunicative participants, behavioral display of pain decreased with worsening cognitive impairment. |
Leong and Nuo57 |
| fMRI; verbal report | MMSE (13–25) | Mechanical pressure to the thumb nail of right hand | (1) Sensory-discriminative component is maintained; how-ever, people with AD required greater pain stimulus to report “just noticeable pain.” (2) Affective-motivational component is maintained; how-ever, people with AD reported the pain stimulus as more unpleasant. (3) Brain activation in both the sensory (lateral) and affective (medial) pain pathways showed increased brain activation in people with AD. |
Cole et al19 |
| EEG; verbal report | MMSE (10–20) | IV sticks to the hand with and without lidocaine | (1) Decreased placebo response to analgesic medication in people with AD and altered prefrontal cortex connectivity with the rest of the brain. | Benedetti et al58 |
| Behavioral report | CPS score where 0 is cognitively intact and 6 is very severe cognitive impairment. CPS scores of 0, 1, 2, 3, 4, 5, and 6 are associated with MMSE scores of 25, 22, 19, 15, 7, 5, and 1, respectively. | Not reported | (1) People with very severe dementia had fewer pain behaviors than people with severe or moderately severe dementia. | Stevenson et al13 |
| Verbal report (proxy); behavioral report (proxy) | MMSE(<21) | Acutely painful diagnoses or procedure versus chronic painful diagnosis | (1) Sensory-discriminative preserved. Acute pain consumption of opioid was nearly identical between people with AD and controls. (2) Affective component possibly altered. Chronic pain consumption of opioid was significantly lower in people with AD. |
Pickering et al59 |
| Verbal report (proxy); behavioral report (proxy) | CPS scores | Percent with painful diagnoses | As severity of cognitive impairment increased pain recorded in the medical record decreased. | Wu et al51 |
| EEG; verbal report | MMSE (8–24) | Electrical shock to wrist | (1) People with worsening cognitive impairment experienced more severe EEG changes. (2) Sensory-discriminative components are preserved. (3) Cognitive and affective components are severely affected. |
Benedetti et al60 |
| Verbal report; caregiver report | Based on DSM-IV and lll-R criteria (specific measures not reported). | Documentation of a chronic disease associated with pain | When compared to people without dementia, people with dementia had lower prevalence rates for any pain, any daily pain, interfering daily pain, and daily pain at rest. | Mantyselkä et al61 |
| EEG; verbal report | MMSE (2–19) | Carbon dioxide laser detection and heat pain thresholds | (1) EEG measures suggest that pain sensation is intact, yet a slower cortical processing of the painful stimulus occurs in people with AD. (2) Sensory-discriminative component altered. Detection threshold (amount of stimulus to just notice pain) was higher in people with AD, yet pain threshold was similar between people with AD and controls. (3) Affective component preserved. |
Gibson et al7 |
| Verbal report | MMSE (8–18) | Electrical shock to wrist | (1) Sensory-discriminative components altered. Strong noxious stimulation produced significantly decreased pain perception response (lower MMSE scores reported lower pain intensity). Mild noxious stimulus produced normal pain perception response. | Rainero, et al |
| Verbal report | MMSE (18–24) | Chronic painful conditions | (1) Sensory-discriminative component altered. AD group reported less pain intensity. (2) Affective-motivational component altered. AD group reported less pain affect. |
Scherder et al134 (2001) |
| EEG; verbal report | MMSE (10–19) | Electrical shock to wrist and tourniquet technique | (1) More severe EEG changes noted with increasing cognitive impairment. (2) Sensory-discriminative component was maintained; however, the more severe the cognitive impairment, the higher the pain tolerance. |
Benedetti et al63 |
| Verbal report | (1) Dutch Cognitive Screening Test (CST). Scores less than 14 are considered cognitively impaired. CST score range 0 = completely cognitively impaired to 20 = completely cognitively intact. CST for AD group M = 9.39 (8.5–13); control group M = 17.5 (14–20). | Frequency and number of painful conditions | (1) People with AD report less pain intensity. (2) People with AD report less pain affect. |
Scherder et al25 |
| Verbal report (proxy); behavioral report (proxy) | Washington University Clinical Dementia Rating Scale (0–3; 0 being no cognitive impairment and 3 being very severe cognitive impairment) | Frequency and number of painful conditions | (1) People with dementia received less pain medications and this was not due to a change in the affective component of pain. | Scherder and Bouma64 |
| Verbal report; behavioral report (case study) | Functional Staging of Dementia Scale (stage 4) | Number and frequency of painful diagnoses and during procedures known to be painful in people who are cognitively intact | (1) Altered sensory-discriminative component of pain. Communicative people with senile AD did not verbally report pain. (2) Altered cognitive-behavioral component of pain. Communicative people with senile AD did not behaviorally report pain. |
Fisher-Morris and Gellafly120 |
| Verbal report; behavioral report | Washington University Clinical Dementia Rating Scale (0–3; 0 being no cognitive impairment and 3 being very severe cognitive impairment) | Standard venipuncture (IV) | (1) AD severity interfered with the ability to self-report pain. (2) Facial expression of pain was increased in people with dementia. (3) Independent of age, increased severity of dementia was associated with blunting of physiologic response to pain (decreased heart rate). |
Porter et al66 |
| Verbal report | MMSE (average 12.1 ± 7.9). | Not reported | (1) 62% reported pain. (2) 83% of cognitively impaired participants with pain could complete at least 1 pain scale. |
Ferrell et al10 |
Abbreviations: AD, Alzheimer disease; DLPFC, dorsolateral prefrontal cortex; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised); DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; IV, intravenous; MMSE, Mini-Mental State Examination; N/A, not applicable; NS, not significant; PAG, periaqueductal gray; SD, standard deviation.
Brief Overview of Pain Processing in Healthy People: The Lateral and Medial Pain Networks
Both the lateral and the medial pathways begin with primary peripheral afferent neurons (nociceptors) that generally respond to unimodal or polymodal mechanical, thermal, chemical,67,68 or electrical69 stimuli. Nociception can be initiated in the skin, internal organs, bone, or muscle.67,68 In response to painful stimuli on the skin, action potentials generated by nociceptors transmit pain information in ascending pathways through lamina I, II, and IV70 of the anterolateral spinal cord—and then to the brain.67,70 Two main types of primary afferent fibers—A-fibers and C-fibers—transmit sensory pain information from the periphery to the central nervous system.67,68 Although all nociceptive fibers may be described as slowly conducting, because the conduction velocity of A-fibers is faster than that in C-fibers, A-fibers are often described as “fast” and C-fibers as “slow.”68,71 Several subtypes of A-fibers exist67; here, we are referring to A-δ fibers, which are thinly myelinated and fast (14–25 m/s)68 conductors of pain that encode noxious sensations. In contrast, polymodal C-fibers are unmyelinated slow (1.2 m/s)72 conductors of pain that encode noxious sensations,71 while unimodal C-warm fibers encode the innocuous sensation of warmth.73,74 The lateral pathway mediates the sensory-discriminative components22,33—location, intensity, and quality—of pain,20,27,29 while the medial pathway mediates the affective-motivational and cognitive-evaluative components of pain19,22,27,33,30,40 including the memory,33 emotion, arousal, attention,19 and the unpleasant aspect of pain.75 A critical point is that, in general, healthy older adults have increased pain thresholds resulting in decreased pain sensitivity.76 This observation likely results from an increased concentration of peripheral C-fibers and decreased concentration of A-δ fibers. These age-related changes in fiber numbers are postulated to have a direct effect in pain processing in the primary somatosensory cortex.76
The Behavioral Aspect of Pain: Evidence of a Rostral Pain Network?
In addition to research which supports the role of the lateral and medial pain networks in sensory, cognitive, and affective processing, some researchers suggest that an additional rostral (limbic) network may be responsible for the behavioral expression of pain.23 The rostral pain system overlaps with several components of the medial pain network and consists of specific nuclei in the amygdala, periaqueductal gray (PAG) orbitofrontal, anterior cingulate (ACC), and anterior insular cortices23 striatum,23,77 thalamus, and hypothalamus.75 The striatum is a key structure in the rostral pain network that is not generally associated with either the lateral or the medial pain network.27,29
Encoding pain in the rostral pathway begins when nociceptive information from the spinal cord enters the intralaminar thalamic nuclei, which projects to the ACC75 or the central lateral nucleus (CLN) of the amygdala via the spino-parabrachio-amygdaloid pathway to the cortex.78 The ACC has been described as functioning in reward, cognition, emotion, motivation, and motor control79 and a possible nociceptive circuit that connects the ACC with the striatum.77 Top-down modulation or influence occurs via the midline and posterior thalamic nuclei that convey sensory information from the cortex to the lateral and basolateral (BL) amygdaloid nuclei.78 Pain behaviors may then be modulated via the CLN and BL nuclei which project to the ventral striatum,75,77,80 PAG, brainstem,75,77,78 and premotor cortex.75 Specifically, nociceptive projections from the lateral nuclei of the PAG to the basal ganglia (striatum) are associated with orientation to pain, autonomic arousal (eg, hypertension and tachycardia), escape,77 or defensive behaviors in response to pain,81 while nociceptive projections to the ventral striatum may be associated with the avoidance of pain.80
Many structures that have been identified in pain processing contribute to more than 1 pathway (Figure 2). Table 1 outlines the basic pain functions thought to be associated with specific regions in the lateral, medial, and rostral pain networks. Figure 1 shows select cortical and subcortical regions involved in pain processing (see Apkarian,29 Borsook,31 Chen,20 Price,30 and Treede27) for comprehensive reviews of pain imaging studies.
The Neurobiology of Pain and AD
Pain Processing in People With AD
Brain neuropathological changes that occur in AD82–85 may impair the memory,15 experience,32 and the verbal15 or behavioral13 reporting of pain. Findings summarized in the current review of AD individuals’ ability to verbally or behaviorally report pain are mixed (Figure 3). People with AD reported diminished, increased, or normal sensory, affective, and behavioral responses to painful stimuli.7,19,53–55,59,60 Factors contributing to mixed findings in psychophysical and neurophysiological studies of pain in people with dementia include: study design, cognitive ability of participants, and acute versus chronic pain conditions. Despite these mixed findings, no studies described an absence of pain report in people with AD (Figure 3).
Pain Assessment in People With AD
Although the subjective self-report of pain is considered the gold standard for pain assessment in cognitively intact individuals, self-report is not possible in individuals with advanced AD who are noncommunicative. Examining brain activation in regions associated with pain processing during delivery of experimental pain stimuli in the laboratory may serve as a surrogate marker or indicator of intact pain processing in people who cannot reliably report their pain and may therefore inform or shape clinical practice and clinical assumptions about pain in AD. However, demonstrated nociceptive pathway activity does not necessarily indicate pain. Because pain is a psychological state, the perceptual experience of pain can occur in the absence of activation in the peripheral nociceptive pathways.86 Thus, brain regions that are generally associated with pain could show activation in the absence of pain reports. Likewise, pain reports could exist without demonstrated brain activation in the regions associated with pain. Depending solely on neuroimaging to recognize pain in someone with limited ability to verbally or behaviorally report pain is not without potential limitations.
Brain Volume, Activation, and Metabolism in People With AD
Alzheimer disease and advancing age generally involve progressive loss of brain volume. The most pronounced brain volume loss with normal aging is seen in the hippocampus and prefrontal cortex.76 These structures are further compromised by the volume loss occurring in AD, which begins in the entorhinal cortex and hippocampus87,88 progressing to the lateral temporal lobe and other neocortex.89 The amount of volume loss in AD is associated with cognitive decline.90 The Mini-Mental State Examination (MMSE)91 is a commonly used tool to quantify cognitive abilities in AD that allows tracking the progression of AD and response to treatments. Ridha and colleagues found that MMSE scores in individuals with AD were strongly correlated with brain volume loss.92 A cross-sectional study found that people with moderate AD (MMSE = 13.8 +3.0) had significantly decreased brain volume compared to those with mild AD (MMSE = 24 + 1.8).89 Notably, the neuropathological alterations93 and volume loss89 in AD seem to spare the primary somatosensory and motor cortex.
The apolipoprotein E4 (APOE-4) allele is a genetic marker indicating a risk for the development of AD94 and people with the APOE-4 allele are also at risk for brain volume loss associated with AD.95,96 Interestingly, in the presence of decreased brain volume, people with AD or those with the APOE-4 allele may exhibit increased brain activation. One study examining cerebral atrophy relative to fMRI activation found that brain volume loss in mild AD was associated with increased brain activation.97 Moreover, several fMRI studies demonstrated that when compared to healthy controls, people at risk for AD secondary to carrying the APOE-4 allele had a greater magnitude and extent of brain activation in multiple regions including structures that are involved in pain processing; that is hippocampus,98–100 orbitofrontal cortex,98,99 and prefrontal cortex.101–103 Consistent with studies showing increased activation, a single fMRI study of pain processing in people with mild and moderate AD found increased activation, relative to controls, in the lateral and medial pain networks.19
In addition to decreasing brain volume and increased brain activation, people with AD tend to show decreased resting state functional connectivity (fcMRI) and metabolism. The fcMRI is a measure of brain activation patterns at rest while overall brain metabolism using PET or single-photon emission computed tomography (SPECT) are measures of synaptic activity. The fcMRI in people with AD shows decreased resting state connectivity between the posterior cingulate,104 hippocampus,104,105 and fusiform gyri105 while overall brain hypometabolism in AD is well established.94,106,107 Similar to the volume loss and increased activation identified in APOE-4 carriers, PET studies have shown that people with the APOE-4 gene without cognitive decline show decreased brain glucose metabolism in the posterior cingulate, parietal, temporal, and prefrontal regions.94 Using SPECT in people with mild to moderate AD revealed reduced cerebral perfusion in the parietal and posterior temporal brain regions.108 Moreover, brain metabolism tends to decrease as both cognitive decline and AD pathology progress.109
Although not an exhaustive list, these studies demonstrate that, in general, people with AD or APOE-4 allele have predictable brain volume loss, exhibit greater task-related increases in overall brain activation, and conversely have decreased resting state metabolism when compared to controls.
Damage to the Lateral (Sensory) and Medial (Affective) Pain Networks in AD
The time course of damage to the lateral and medial pain network in AD is well established. As discussed above, the location, intensity, and quality of pain are modulated by the lateral pain network, which mediates acute or fast pain sensations. Reviews suggest that the lateral network is less affected in the course of AD.28,110 Conversely, the medial pain network mediates the unpleasant, affective response to noxious stimuli and the neurodegenerative changes in AD affect the medial pain network earlier in the course of illness.64,111,112
Behavioral Display of Pain in AD
Because assessment of pain-related behaviors is currently recommended as part of a comprehensive pain assessment in nonverbal or cognitively impaired older adults,12,14 neuroimaging studies examining the function of the rostral pain structures, of which many overlap with the medial pain system, may potentially offer new insight into the area of behavioral assessment of pain in people with AD. AD pathology studies show that structures in the rostral pain network such as the amygdala,113 the orbitofrontal cortex,114 insula,114 PAG,115 and striatum,116 each develop neurofibrillary tangles and neuritic plaques. Damage in these areas is associated with altered behavioral responses. For example, neurofibrillary tangles in the orbitofrontal cortex are associated with atypical motor behaviors and neuritic plaques in the anterior insula result in apathy.114 Additionally, the striatum is severely affected by AD pathology,117 so older adults with severe AD may be at increased risk for diminished behavioral response to pain. When compared to a healthy young cohort (mean age = 26), a recent fMRI study found decreased activation among cognitively intact (MMSE > 25) older adults (mean age = 79) in the striatum (dorsal portion) in response to experimental pain.118 Thus, it may be possible that, relative to healthy older adults, the striatum may show increased or decreased activation in people with AD who exhibit few motor (behavioral) displays of pain. Studies examining behavioral display of pain in people with AD found that while facial responses to acute pain may be increased in people with mild to moderate AD,53,76,119 behaviors associated with chronic pain may significantly diminish in people with severe cognitive impairments13,48 or AD.120
Proposed Conceptual Framework of Pain in People With AD
Based upon the lateral, medial, and rostral pain networks (Figure 1) and current evidence regarding volume loss, brain activation, and brain metabolism, we present a framework for designing and interpreting studies in people with AD (Figure 2). First, the y-axis represents stages of dementia severity (no AD, mild, moderate, and very severe) that were identified in the current review. From the left, the first column indicates the predictable progressive brain volume loss. The second column lists MMSE scores identified in the review that were used as a proxy for dementia severity, no dementia = MMSE of 30,48 mild dementia = MMSE ≥ 18,48,54,57,89 moderate dementia = MMSE ≥ 10,53,55,57,89 and very severe dementia = MMSE <248,120,121 (Note: Few studies included participants with MMSE scores from 3 to 10). The third column represents the predictable course of AD brain hypometabolism93,106,107 and the fourth column represents an overall increased task-related brain activation that occurs in people with mild and moderate AD or in those with the APOE-4 allele. Notably, increased activation in mild and moderate AD seems to occur throughout the brain despite the presence of gray matter brain volume loss. One theory is that in people at risk of AD, or perhaps in those with AD-related brain damage, a compensatory recruitment of neurons is needed to sustain cortical function. Another possible explanation is that the patients with AD have reduced basal cerebral blood flow122 and/or different coupling of flow to neural activity and metabolism.66 Because no studies to date have examined the brain activation in severe AD, we hypothesize that compensatory mechanisms fail in severe AD resulting in decreased activation. The fifth, sixth, and seventh columns represent the medial (affective), rostral (behavioral), and lateral (sensory) pain pathways, respectively. The AD pathological and autopsy studies have consistently demonstrated that the lateral (sensory) pathway function is spared until late in the illness, 85,93 while the medial (affective) 85,93, and rostral (behavioral)114 pathways are damaged earlier in the disease process. Depending on the severity of AD, affective, behavioral, and sensory reports can be normal, increased, or decreased relative to experimental, acute, or chronic pain, respectively 7,10,13,19,25,28,48–53,56–61,66,120–124 (see Figure 3).
Ethical Considerations in Imaging Pain Research in People With Dementia
All human research must address the ethical principles of autonomy, beneficence, and justice. In the case of vulnerable individuals, such as those with dementia, there are a number of considerations the investigator must address regarding the informed consent, decisional capacity, and surrogate decision making. The United States,125 European,126 and Australian 127 governments require informed consent from the participants. But the ability to understand a study’s purpose, its procedures, potential risks, and benefits declines as dementia progresses. Thus, determining decisional capacity is essential. There is no universal definition of “lacking capacity”128 or a standard assessment tool. One approach is to combine objective data, based on a standardized screening tool, with subjective data, based on the clinical judgment of the investigator.129 The individual’s capacity to make decisions may vary depending on the situation or task.135 Thus, if the individual is found to lack decisional capacity for informed consent, he may still be able to have the capacity to appoint a surrogate decision maker135 and to provide assent. The individual who is designated as the surrogate decision maker varies by state, country, or territory. Regardless of the state regulations and any additional institutional requirements, the surrogate decision maker is ideally the one who knows the values and wishes of the individual. All studies have potential risks and benefits. Mechanistic studies of pain will not only cause pain sensations, but are also unlikely to have direct benefit on the individual with dementia. Study procedures other than the pain stimulus, such as MRIs, may be uncomfortable or frightening. Studies of pain in individuals with dementia may well require the presence of the surrogate decision maker, adding further risk of loss of work time, travel costs, and so on. Explaining the degree of pain induced by the stimulus can be accomplished by comparing the pain sensation with common life experiences. For example, a thermal pain paradigm requiring a cold sensation could be described as holding an ice cube for 15 seconds. Review of the pain stimulus procedures is required to ensure the pain is relieved upon removal of the stimulus and causes no tissue damage. Although brain processing studies of pain in people with dementia have no direct benefit to the individual, they are necessary to inform future research endeavors to guide evidence-based pain management.19,25
Discussion
Pain is a common and poorly managed condition in people with dementia. Because people with advanced dementia lose the ability to verbally or behaviorally communicate pain, clinicians have difficulty judging its presence or severity. Current guidelines exist for pain assessment in people with dementia, but they rely on verbal, nonverbal, and physiologic external signs.130 These assessment guidelines are excellent for people who can verbally and behaviorally report pain, but may provide limited data on people with very severe dementia. For this group of people, alternative pain assessment strategies are urgently needed to help clinicians provide better care.8 Noninvasive neuroimaging approaches have the potential to provide critical information about the neurobiology of pain processing in people with AD—or similar medical conditions—who may eventually lose the ability to verbally or behaviorally report pain.
The persistent vegetative state (PVS) is one medical condition with severe brain damage that has been described as preserved wakefulness with absent voluntary movement.131,132 Although pathologically different from AD, the PVS is mentioned here because of its conceptual similarities to severe AD. Namely, people with PVS are unable to verbally or behaviorally report pain. In a PET study of brain metabolism in response to noxious stimuli,131 people with PVS had 40% of the brain metabolism of healthy volunteers. Yet, in every person with PVS, the midbrain, contralateral thalamus, and primary somatosensory cortex were metabolically activated.131 However, there was no metabolic activation in the secondary somatosensory cortex or higher order associative cortices.131 The authors concluded that the primary and secondary somatosensory cortices were disconnected from the thalamus. These findings in the PVS lead to the question of consciousness and awareness. A central question emerges for conceptualizing pain in the individual with very severe cognitive impairments and the concept of “awareness.” Preliminary studies of pain in people with mild and moderate AD have shown that many higher order associative areas required for conscious pain processing are activated in response to experimental pain.19,63
Although, using imaging methods supports the role of the lateral and medial pain networks in sensory and affective pain processing, few imaging studies have examined the neurobiology of pain in people with AD. Notably, the physiology of AD seems to alter pain processing in the lateral and medial pain network.28 More research is needed targeting both the neurobiology and assessment of pain in people with AD. Because the behavioral assessment of pain is currently the accepted standard in people in AD, we present a conceptual framework of the pain networks in the brain. This framework can potentially be used for designing and interpreting neuroimaging and psychophysical studies of pain in people with AD.
The current evidence regarding pain in people with dementia is mixed. Reasons for these mixed findings include small sample sizes,19,76,48,120,121 requiring people with dementia to report pain based heavily on the memory of painful experiences,25,61 nonhomogenous samples,53,55 and examining the response to acute experimental pain,53,55 while others examined chronic nonmalignant13,52 and malignant48,120,52 pain. Moreover, stimuli used in acute pain studies included electrical shock,63,53,55 mechanical pressure,19,118 venipuncture or intravenous stick,58 and CO2 laser.7
Recommendations for Future Research
Continued study of pain networks in people with all forms of dementia while enrolling homogenous cohorts. Since the MMSE is a simple and fast tool that is widely used as a proxy for severity of cognitive impairment, we recommend that all investigators report MMSE scores so that findings between studies can be more easily interpreted and compared.
Use imaging methods to study the rostral pain network, which may help to validate acute versus chronic pain behaviors in people with AD.8 Considering the magnitude of literature supporting the development of behavioral indicators to assess for pain, future studies should be aimed at exploring the association between signal intensity in brain regions comprising the rostral pain system and behavioral display of pain. An important step is to specifically examine the role of the striatum in the behavioral response to pain in people with all forms of dementia.
Determine how to interpret increased or decreased brain activation in response to experimental pain in AD and other imaging studies of people with dementia. Considering the relationship between AD pathology and its predicable contributions to increasing signal intensity on brain activation patterns, determining methods to account for this increase in future studies is warranted.
Examine the association of verbal reports, behavioral reports, AD pathology, and pain networks—given the range of mixed findings to date. For example, one method may be to use Pittsburg compound B133 to image the amount and dispersion of amyloid plaque deposition in the pain network system—relative to an individual’s verbal and behavioral pain reports.
Pain receptor numbers and function are infrequently examined in people with AD. Using PET to study specific pain receptor ligands may provide important information about the endogenous and exogenous pain systems in people with AD. These findings could be used to design and implement drug intervention studies targeted at the lateral, medial, and rostral pain networks in people with AD.
In summary, older adults with severe AD are likely at risk for undertreatment of pain because many have lost the ability to verbally or behaviorally report their pain. Despite mixed behavioral findings, neuroimaging methods—such as PET, EEG, and fMRI—may provide researchers the ability to assess experimental pain in people who are unable to speak or unable to display recognized pain behaviors. Using imaging methods to learn more about the pain networks in people with all forms of dementia may provide critical knowledge to improve pain treatment. Few imaging studies have examined pain in people with dementia and more research is urgently needed in this area. Ultimately, psychophysical and neuroimaging research findings may one day translate into improved clinical practice providing a better quality of life for people with dementia and pain.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding or this research was supported by the John A. Hartford Foundation & Atlantic Philanthropies, the Mayday Fund, and the Vanderbilt Institute for Clinical and Translational Research - Vanderbilt CTSA grant UL1 RR024975 from NCRR/NIH.
Footnotes
Author’s Note
T. Monroe, J. Gore, and R. Cowan conceptualized the review; T. Monroe and R. Cowan prepared the initial draft; L. Chen, J. Gore, L. Mion and R. Cowan were responsible for critical revisions for important intellectual content.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Alzheimer’s Disease International. [Accessed Janurary 30, 2011];World Alzheimer’s Report 2010. Alzheimer’s Disease International. http://www.alz.co.uk/research/statistics.html.
- 2.Català E, Reig E, Artés M, Aliaga L, López JS, Segú JL. Prevalence of pain in the Spanish population telephone survey in 5000 homes. Eur J Pain. 2002;6(2):133–140. doi: 10.1053/eujp.2001.0310. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association. [Accessed October 17, 2009];Alzheimer’s disease facts and figures. http://www.alz.org/national/documents/report_alzfactsfigures2009.pdf.
- 4.Brookmeyer R. [Accessed February 26, 2011]; http://www.jhsph.edu/publichealthnews/press_releases/2007/brookmeyer_alzheimers_2050.html.
- 5.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110(1–2):361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Helme D, Gibson S. The Epidemiology of Pain in Elderly People. New York, NY: Elsevier; 2001. [DOI] [PubMed] [Google Scholar]
- 7.Gibson S, Voukelatos X, Ames D, Flicker L, Helme RD. An examination of pain perception and cerebral event-related potentials following carbon dioxide laser stimulation in patients with Alzheimer’s disease and aged-matched control volunteers. Pain Res Manag. 2001;6(3):126–132. doi: 10.1155/2001/814374. [DOI] [PubMed] [Google Scholar]
- 8.Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain. 2009;145(3):276–278. doi: 10.1016/j.pain.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Feldt KS, Ryden MB, Miles S. Treatment of pain in cognitively impaired compared with cognitively intact older patients with hip-fracture. J Am Geriatr Soc. 1998;46(9):1079–1085. doi: 10.1111/j.1532-5415.1998.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10(8):591–598. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 11.Tsai PF, Beck C, Richards KC, Phillips L, Roberson PK, Evans J. The pain behaviors for osteoarthritis instrument for cognitively impaired elders (PBOICIE) Res Gerontol Nurs. 2008;1(2):116–122. doi: 10.3928/19404921-20080401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Geriatrics Society Panel on persistent pain in older persons. Clinical practice guidelines: the management of persistent pain in older persons. J Am Geriatr Soc. 2002;50(6 suppl):S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson KM, Brown RL, Dahl JL, Ward SE, Brown MS. The discomfort behavior scale: a measure of discomfort in the cognitively impaired based on the minimum data set 2.0. Res Nurs Health. 2006;29(6):576–587. doi: 10.1002/nur.20168. [DOI] [PubMed] [Google Scholar]
- 14.Herr K, Coyne P, Key T, et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manage Nurs. 2006;7(2):44–52. doi: 10.1016/j.pmn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Farrell MJ, Katz B, Helme RD. The impact of dementia on the pain experience. Pain. 1996;67(1):7–15. doi: 10.1016/0304-3959(96)03041-2. [DOI] [PubMed] [Google Scholar]
- 16.Cook AK, Niven CA, Downs MG. Assessing the pain of people with cognitive impairment. Int J Geriatr Psychiatry. 1999;14(6):421–425. doi: 10.1002/(sici)1099-1166(199906)14:6<421::aid-gps932>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Frampton M. Experience assessment and management of pain in people with dementia. Age Ageing. 2003;32(3):248–251. doi: 10.1093/ageing/32.3.248. [DOI] [PubMed] [Google Scholar]
- 18.Proctor WR, Hirdes JP. Pain and cognitive status among nursing home residents in Canada. Pain Res Manag. 2001;6(3):119–125. doi: 10.1155/2001/978130. [DOI] [PubMed] [Google Scholar]
- 19.Cole L, Farrell M, Duff E, Barber J, Egan G, Gibson S. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain. 2006;129(pt 11):2957–2965. doi: 10.1093/brain/awl228. [DOI] [PubMed] [Google Scholar]
- 20.Chen LM. Imaging of pain. Int Anesthesiol Clin. 2007;45(2):39–57. doi: 10.1097/AIA.0b013e31803419d3. [DOI] [PubMed] [Google Scholar]
- 21.Willis W, Westlund K. Neuroanatamy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14(1):2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albe-Fessard D, Berkley K, Kruger L, Ralston H, Willis W. Diencephalic mechanisims of pain sensation. Brain Res. 1985;356(3):217–296. doi: 10.1016/0165-0173(85)90013-x. [DOI] [PubMed] [Google Scholar]
- 23.Devinsky O, Morrell M, Vogt B. Contributions of the anterior cingulate cortex to behavior. Brain. 1995;118(pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 24.Melzack R, Casey K. Sensory, motivational, and central control determinants of pain. In: Kenshalo D, Thomas CC, editors. The Skin Senses. Springfield, IL: Thomas; 1968. pp. 423–443. [Google Scholar]
- 25.Scherder E, Bouma A, Borkent M, Rahman O. Alzheimer patients report less pain intensity and pain affect than non-demented elderly. Psychiatry. 1999;62(3):265–272. doi: 10.1080/00332747.1999.11024871. [DOI] [PubMed] [Google Scholar]
- 26.Harvath TA, Beck C, Flaherty-Robb M, et al. Best practice initiatives in geriatric nursing: experiences from the John A. Hartford Foundation Centers of geriatric nursing excellence. Nurs Outlook. 2006;54(4):212–218. doi: 10.1016/j.outlook.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79(2–3):105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 28.Scherder EJA, Sergeant JA, Swaab DF. Pain processing in dementia and its relation to neuropathology. Lancet Neurol. 2003;2(11):677–686. doi: 10.1016/s1474-4422(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 29.Apkarian A, Bushnell M, Treede Z. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 31.Borsook D, Becera L. Pain imaging: future applications to integrative clinical and basic neurobiology. Adv Drug Deliv Rev. 2003;55(8):967–986. doi: 10.1016/s0169-409x(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 32.Scherder E, Knol D, van Someren E, et al. Effects of low-frequency cranial electrostimulation on the rest-activity rhythm and salivary cortisol in Alzheimer’s disease. Neurorehabil Neural Repair. 2003;17(2):101–108. doi: 10.1177/0888439003017002004. [DOI] [PubMed] [Google Scholar]
- 33.Kupers R, Kehlet H. Brain imaging of clinical pain states: a critical review and stratagies for future studies. Lancet Neurol. 2006;5(12):1033–1044. doi: 10.1016/S1474-4422(06)70624-X. [DOI] [PubMed] [Google Scholar]
- 34.Coghill R, Sang C, Maisog J, Idarola M. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophyisol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 35.Buffington A, Hanlon C, McKeown M. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113(1–2):172–184. doi: 10.1016/j.pain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Bingel U, Quante M, Knab R, Bromm B, Weiler C, Buchel C. Single trial fMRI reaveals significant contralateral bias in responses to laser pain within the thalamus and somatosensory cortices. Neuroimage. 2003;18(3):740–748. doi: 10.1016/s1053-8119(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 37.Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from singletrial fMRI. Pain. 2002;99(1–2):313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 38.Bingel U, Lorenz J, Glauche V, et al. Somatototopic organization of uman somatosensory cortices for pain: a single trial fMRI study. Neuroimage. 2004;23(1):224–232. doi: 10.1016/j.neuroimage.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Maihofner C, Herzner B, Handwerker H. Secondary somatosensory cortexis important for the sensory-discriminative dimension of pain: a functional MRI study. Eur J Neurosci. 2006;23(5):1377–1383. doi: 10.1111/j.1460-9568.2006.04632.x. [DOI] [PubMed] [Google Scholar]
- 40.Bingel U, Schoell E, Buchel C. Imaging pain modulation in health and disease. Curr Opin Neurol. 2007;20(4):424–431. doi: 10.1097/WCO.0b013e328259c34d. [DOI] [PubMed] [Google Scholar]
- 41.Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somato-sensory cortex? Proc Natl Acad Sci U S A. 1999;96(14):7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis K, Kwan CL, Crawley AP, Mikulis DJ. Event-related fMRI of pain: entering a new era in imaging pain. Neuroreport. 1998;9(13):3019–3023. doi: 10.1097/00001756-199809140-00018. [DOI] [PubMed] [Google Scholar]
- 43.Porro CA, Cettolo V, Francescato MP, Baraldi P. Temporal and intensity coding of pain in human cortex. J Neurophysiol. 1998;80(6):3312–3320. doi: 10.1152/jn.1998.80.6.3312. [DOI] [PubMed] [Google Scholar]
- 44.Morris J, Frith C, Perrett D, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 45.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a singletrial fMRI study. Brain. 2002;125(pt 6):1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 46.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 47.Craig A, Chen K, Bandy D, Reiman E. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3(2):184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 48.Monroe T, Carter M, Feldt K, Tolley B, Cowan R. Assessing advanced cancer pain in older adults with dementia at the end-of-life. J Adv Nurs. 2012;68(9):2070–2078. doi: 10.1111/j.1365-2648.2011.05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shega JW, Paice JA, Rockwood K, Dale W. Is the presence of mild to moderate cognitive impairment associated with self-report of non-cancer pain? A cross-sectional analysis of a large population-based study. J Pain Symptom Manage. 2010;39(4):734–742. doi: 10.1016/j.jpainsymman.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole L, Gavrilescu M, Johnston L, Gibson S, Farrell M, Egan G. The impact of Alzheimer’s disease on the functional connectivity between brain regions underlying pain perception. Eur J Pain. 2011;15(6) doi: 10.1016/j.ejpain.2010.10.010. 568.e1–568.e11. [DOI] [PubMed] [Google Scholar]
- 51.Wu N, Miller SC, Lapane K, Roy J, Mor V. The quality of the quality indicator of pain derived from the minimum data set. Health Serv Res. 2005;40(4):1197–1216. doi: 10.1111/j.1475-6773.2005.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monroe T, Carter M. A retrospective pilot study of African-American and caucasian nursing home residents with dementia who died from cancer. J Pain Symptom Manage. 2010;40(4):e1–e3. doi: 10.1016/j.jpainsymman.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunz M, Mylius V, Scharmann S, Schepelman K, Lautenbacher S. Influence of dementia on multiple components of pain. Eur J Pain. 2009;13(3):317–325. doi: 10.1016/j.ejpain.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Scherder E, Eggermont L, Plooij B, et al. Relationship between chronic pain and cognition in cognitively intact older persons and in patients with Alzheimer’s disease. The need to control for mood. Gerontology. 2008;54(1):50–58. doi: 10.1159/000113216. [DOI] [PubMed] [Google Scholar]
- 55.Kunz M, Scharmann S, Hemmeter K, Schepelmann K, Lautenbacher S. The facial expression of pain in patients with dementia. Pain. 2007;133(1–3):221–228. doi: 10.1016/j.pain.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Sawyer P, Lillis JP, Bodner EV, Allman RM. Substantial daily pain among nursing home residents. J Am Med Dir Assoc. 2007;8(3):158–165. doi: 10.1016/j.jamda.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Leong IY-O, Nuo TH. Prevalence of pain in nursing home residents with different cognitive and communicative abilities. Clin J Pain. 2007;23(2):119–127. doi: 10.1097/01.ajp.0000210951.01503.3b. [DOI] [PubMed] [Google Scholar]
- 58.Benedetti F, Ardunio C, Costa S, et al. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less efective. Pain. 2006;121(1–2):133–144. doi: 10.1016/j.pain.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Pickering G, Jourdan D, Dubray C. Acute verses chronic pain treatment in Alzheimer’s disease. Eur J Pain. 2006;10(4):379–384. doi: 10.1016/j.ejpain.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Benedetti F, Arduino C, Vighetti S, Asteggiano G, Tarenzi L, Rainero I. Pain reactivity in Alzheimer’s patients with different degrees of cognitive impairment and brain electrical activity deterioration. Pain. 2004;111(1–2):22–29. doi: 10.1016/j.pain.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Mäntyselkä P, Hartikainen S, Louhivuori-Laako K, Sulkava R. Effects of dementia on perceived daily pain in home-dwelling elderly people: a population-based study. Age Ageing. 2004;33(5):496–499. doi: 10.1093/ageing/afh165. [DOI] [PubMed] [Google Scholar]
- 62.Rainero I, Vighetti S, Bergamasco B, Pinessi L, Benedetti F. Autonomic responses and pain perception in Alzheimer’s disease. Eur J Pain. 2000;4(3):267–274. doi: 10.1053/eujp.2000.0185. [DOI] [PubMed] [Google Scholar]
- 63.Benedetti F, Vighetti S, Ricco C, et al. Pain threshold and tolerance in Alzheimer’s disease. Pain. 1999;80(1–2):377–382. doi: 10.1016/s0304-3959(98)00228-0. [DOI] [PubMed] [Google Scholar]
- 64.Scherder E, Bouma A. Is decreased use of analgesics in Alzheimer disease due to a change in the affective component of pain? Alzheimer Dis Assoc Disord. 1997;11(3):171–174. doi: 10.1097/00002093-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100(1):328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 66.Porter F, Malhotra K, Wolf C, Morris J, Miller J, Smith M. Dementia and response to pain in the elderly. Pain. 1996;68(2–3):413–421. doi: 10.1016/s0304-3959(96)03210-1. [DOI] [PubMed] [Google Scholar]
- 67.Caterina M, Gold M, Meyer R. Molecular biology of nociceptors. In: Koltzenburg SHM, editor. The Neurobiology of Pain. London, UK: Oxford University Press; 2005. pp. 1–35. [Google Scholar]
- 68.Caterina M, Gold M, Meyer R. Molecular biology of nociceptors. In: Hunt S, editor. The Neurobiology of Pain. Oxford, UK: Oxford University Press; 2009. pp. 1–33. [Google Scholar]
- 69.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 70.Hunt S, Bester H. The ascending pain pathways. In: Hunt S, editor. The Neurobiology of Pain. London, UK: Oxford University Press; 2005. pp. 165–184. [Google Scholar]
- 71.Yarnitsky D, Ochoa J. Differential effect of compression-ischaemia block on warm senation and heat-induced pain. Brain. 1991;114(2):907–913. doi: 10.1093/brain/114.2.907. [DOI] [PubMed] [Google Scholar]
- 72.Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J Neurophysiol. 1979;42(5):1297–1315. doi: 10.1152/jn.1979.42.5.1297. [DOI] [PubMed] [Google Scholar]
- 73.Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry. 1975;38(9):865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hallin RG, Torebjo¨rk HE, Wiesenfeld Z. Nociceptors and warm receptors innervated by C fibres in human skin. J Neurol Neurosurg Psychiatry. 1982;45(4):313–319. doi: 10.1136/jnnp.45.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sewards TV, Sewards MA. The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull. 2002;59(3):163–180. doi: 10.1016/s0361-9230(02)00864-x. [DOI] [PubMed] [Google Scholar]
- 76.Farrell MJ. Age-related changes in the structure and function of brain regions involved in pain processing. Pain Med. 2012;13(suppl 2):S37–S43. doi: 10.1111/j.1526-4637.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 77.Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60(1):3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- 78.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 79.Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 81.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 82.Herrup K. Reimagining Alzheimer’s disease-an age-based hypothesis. J Neurosci. 2010;30(50):16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulugeta E, Molina-Holgado F, Elliott MS, et al. Inflammatory mediators in the frontal lobe of patients with mixed and vascular dementia. Dement Geriatr Cogn Disord. 2008;25(3):278–286. doi: 10.1159/000118633. [DOI] [PubMed] [Google Scholar]
- 84.Schuler M, Njoo N, Hestermann M, Oster P, Hauer K. Acute and chronic pain in geriatrics: clinical characteristics of pain and the influence of cognition. Pain Med. 2004;5(3):253–262. doi: 10.1111/j.1526-4637.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 85.Rub U, Del Tredici K, Del Turco D, Braak H. The intralaminar nuclei assigned to the medial pain system and other components of this system are early and progressively affected by the Alzheimer’s disease-related cytoskeletal pathology. J Chem Neuroanat. 2002;23(4):279–290. doi: 10.1016/s0891-0618(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 86.International Association for the Study of Pain. [Accessed March 12, 2012];IASP Taxonomy. http://www.iasp-pain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/default.htm.
- 87.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal hippocampal atrophy in incipient and very mild Alzheimer’s disease. This research was supported by grants P01 AG09466 and P30 AG10161 from the National Institute on Aging, National Institutes of Health. Neurobiol Aging. 2001;22(5):747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 88.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58(8):1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 89.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002;99(7):4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cardenas VA, Chao LL, Studholme C, et al. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging. 2011;32(4):572–580. doi: 10.1016/j.neurobiolaging.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Folstein M, Folsten S, McHugh P. Mini-mental state: a practical method for grading the cogntive state of patietns for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 92.Ridha B, Anderson V, Barnes J, et al. Volumetric MRI and cognitive measures in Alzheimer disease. J Neurol. 2008;255(4):567–574. doi: 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- 93.Braak E, Griffing K, Arai J, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl 3):14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- 94.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the Œm4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 95.Baron JC, ChÈtelat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxelbased morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 96.Chetelat G, Baron JC. Early diagnosis of alzheimer’s disease: contribution of structural neuroimaging. Neuroimage. 2003;18(2):525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 97.Johnson SC, Saykin AJ, Baxter LC, et al. The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11(3):179–187. doi: 10.1006/nimg.1999.0530. [DOI] [PubMed] [Google Scholar]
- 98.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-Œm4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grady CL, Mcintosh AR, Beig S, Keightly ML, Burian H, Black SE. Evidence from Functional Neuroimaging of a Compensatory Prefrontal Network in Alzheimer’s Disease. Vol. 23. Washington, DC: Society for Neuroscience; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solé-Padullés C, Bartrés-Faz D, Junqué C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2009;30(7):1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 104.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petrella JR, Wang L, Krishnan S, et al. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245(1):224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- 106.Duara R, Grady C, Haxby J, et al. Positron emission tomography in Alzheimer’s disease. Neurology. 1986;36(7):879–887. doi: 10.1212/wnl.36.7.879. [DOI] [PubMed] [Google Scholar]
- 107.Foster NL, Chase TN, Fedio P, Patronas NJ, Brooks RA, Chiro GD. Alzheimer’s disease. Neurology. 1983;33(8):961–965. doi: 10.1212/wnl.33.8.961. [DOI] [PubMed] [Google Scholar]
- 108.Grossman M, Payer F, Onishi K, et al. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1997;63(2):152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(11):2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 110.Scherder E, Oosterman J, Swaab D, et al. Recent developments in pain in dementia. BMJ. 2005;330(7489):461–464. doi: 10.1136/bmj.330.7489.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delacourte A, David J, Seargeant N, et al. The biochemical pathway of neurofibillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52(6):1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 112.Scherder E, Bouma A. Visual analogue scales for pain assessment in Alzheimer’s disease. Gerontology. 2000;46(1):47–53. doi: 10.1159/000022133. [DOI] [PubMed] [Google Scholar]
- 113.Vogt LJK, Hyman BT, Van Hoesen GW, Damasio AR. Pathological alterations in the amygdala in Alzheimer’s disease. Neuroscience. 1990;37(2):377–385. doi: 10.1016/0306-4522(90)90408-v. [DOI] [PubMed] [Google Scholar]
- 114.Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49(3):355–361. [PubMed] [Google Scholar]
- 115.Parvizi J, Van Hoesen GW, Damasio A. Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Ann Neurol. 2000;48(3):344–353. [PubMed] [Google Scholar]
- 116.Selden N, Mesulam MM, Geula C. Human striatum: the distribution of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1994;648(2):327–331. doi: 10.1016/0006-8993(94)91136-3. [DOI] [PubMed] [Google Scholar]
- 117.Lehericy S, Hirsch E, Cervera P, et al. Selective loss of cholinergic neurons in the ventral striatum of patients with Alzheimer’s disease. Proc Natl Acad Sci U S A. 1989;86(21):8580–8584. doi: 10.1073/pnas.86.21.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cole L, Farrell M, Gibson S, Egan G. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol Aging. 2010;31(3):494–503. doi: 10.1016/j.neurobiolaging.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 119.Hadjistavropolous T, LaChapelle D, MacLeod F, Snider B, Craig K. Measuring movement-exacerbated pain in cognitively impaired frail elders. Clin J Pain. 2000;16(1):54–63. doi: 10.1097/00002508-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Fisher-Morris M, Gellafly A. The experience and expression of pain in Alzheimer patients. Age Ageing. 1997;26(6):497–500. doi: 10.1093/ageing/26.6.497. [DOI] [PubMed] [Google Scholar]
- 121.Craft L. From fecal impaction to colon perforation. Am J Nurs. 2011;111(8):38–43. doi: 10.1097/01.NAJ.0000403360.82176.76. [DOI] [PubMed] [Google Scholar]
- 122.Montaldi D, Brooks DN, McColl JH, et al. Measurements of regional cerebral blood flow and cognitive performance in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1990;53(1):33–38. doi: 10.1136/jnnp.53.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scherer YK, Bruce SA, Montgomery CA, Ball LS. A challenge in academia: meeting the healthcare needs of the growing number of older adults. J Am Acad Nurse Pract. 2008;20(9):471–476. doi: 10.1111/j.1745-7599.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 125.Dresser R. Dementia research: ethics and policy for the twenty-first century. Georgia Law Rev. 2001;35(2):661–690. [PubMed] [Google Scholar]
- 126.Alzheimer Europe. Ethics of Dementia Research. [Accessed July 30, 2012];Alzheimer Europe. http://www.alzheimer-europe.org/Ethics/Ethical-issues-in-practice/Ethics-of-dementia-research/The-dementia-ethics-research-project - fragment-1.
- 127.Nuffield Council on Bioethics. [Accessed July 30, 2012];Dementia: ethical issues. www.nuffieldbioethics.org/dementia.
- 128.Mayo AM, Wallhagen MI. Considerations of informed consent and decision-making competence in older adults with cognitive impairment. Res Gerontol Nurs. 2009;2(2):103–111. doi: 10.3928/19404921-20090401-08. [DOI] [PubMed] [Google Scholar]
- 129.Jansen LA, Lebovits A, Brushwood DB. Of self-determination, care by proxy, and new techniques. Pain Med. 2004;5(1):94–97. doi: 10.1111/j.1526-4637.2004.04013.x. [DOI] [PubMed] [Google Scholar]
- 130.Snow AL, O’Malley KJ, Cody M, et al. A conceptual model of pain assessment for noncommunicative persons with dementia. Gerontologist. 2004;44(6):807–817. doi: 10.1093/geront/44.6.807. [DOI] [PubMed] [Google Scholar]
- 131.Laureys S, Faymonville M, Peigneux P, et al. Cortical processing of noxious somatosenwory stimuli in the persistent vegetative state. Neuroimage. 2002;17(2):732–741. [PubMed] [Google Scholar]
- 132.Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trend Cogn Sci. 2005;9(12):556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 133.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 134.Scherder E, Bouma A, Slaets J, Ooms M, Ribbe M, Blok A, Sergeant J. Repeated pain assessment in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12(6):400–407. doi: 10.1159/000051287. [DOI] [PubMed] [Google Scholar]
- 135.Kim Sh, Karlawish J, Kim H, Wall I, Bozoki A, Appelbaum P. Preservation of the capacity to appoint a proxy decision maker: implications for dementia research. Arch Gen Psychiatry. 2011;68(2):214–219. doi: 10.1001/archgenpsychiatry.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]