Abstract
Objectives
Cranial nerve injury (CNI) is the most common neurologic complication of carotid endarterectomy (CEA) and can cause significant chronic disability. Data from prior randomized trials are limited and provide no Health-Related Quality of Life (HRQOL) outcomes specific to CNI. Incidence of CNI and their outcomes for patients in CREST were examined to identify factors predictive of CNI and their impact on HRQOL.
Methods
Incidence of CNI, baseline and procedural characteristics, outcomes and HRQOL scores were evaluated in the 1151 patients randomized to CEA and undergoing surgery within 30 days. Patients with CNI were identified and classified using case report forms, adverse event data and clinical notes. Baseline and procedural characteristics were compared using descriptive statistics. Clinical outcomes at 1 and 12 months were analyzed. All data were adjudicated by two neurologists and a vascular surgeon. HRQOL was evaluated using the Medical Outcomes Short Form (SF-36) to assess general health and Likert Scales for disease specific outcomes at 2 weeks, 4 weeks and 12 months after CEA. The effect of CNI on SF-36 subscales was evaluated using random effects growth curve models and Likert Scale data were compared by ordinal logistic regression.
Results
CNI was identified in 53 (4.6%) patients. Cranial nerves injured were VII (30.2%), XII (24.5%), IX/X (41.5%) and 3.8% had Horner’s syndrome. CNI occurred in 52/1040 (5.0%) of patients receiving general anesthesia and 1/111 (0.9%) of patients operated under local anesthesia (p=0.05). No other predictive baseline or procedural factors were identified. Deficits resolved in 18 (34%) patients at 1 month and in 42 (80.8%) of 52 patients by 1 year. One patient died prior to the one year follow-up visit. HRQOL evaluation showed no statistical difference between groups with and without CNI at any interval. By Likert scale analysis, the group with CNI showed a significant difference in the difficulty eating/swallowing parameter at 2 and 4 weeks (p<0.001) but not at 1 year.
Conclusions
In CREST, CNI occurred in 4.6% of patients undergoing CEA with 34% resolution at 30 days and 80.8% at 1 year. The incidence of CNI was significantly higher in patients undergoing general anesthesia. CNI had a small and transient effect on HRQOL, negatively impacting only difficulty eating/swallowing at 2 and 4 weeks but not at 1 year. On the basis of these findings, we conclude that CNI is not a trivial consequence of CEA but rarely results in significant long-term disability.
Introduction
Injury to cranial nerves is the most common neurologic complication of carotid endarterectomy (CEA) and when unresolved may result in significant long term disability. These injuries have been a well-known complication of the procedure since its inception and have been the topic of numerous publications.1–9 Generally, it has been found that most of the injuries resolve and while there is potential for significant long-term disability, it is relatively rare.
Multiple surgical series have reported the incidence of cranial nerve injury (CNI) but rates are highly variable, ranging from 3% to 30 %.1–9 This variability is one of measurement error, largely a consequence of the intensity of evaluation and diagnostic modalities employed. In clinical trials that included a CEA arm, CNI has been reported as occurring in 5.1% to 8.6 % of cases.10–13 In studies where patients underwent detailed otolaryngological examination pre- and post-operatively to evaluate cranial nerve function, injury was found to occur following 11.5% to39% of operations.2,3,9,14,15 In contrast, two recent large series using the usual clinical criteria alone found an incidence of 5.5 and 5.6%.16, 17 The majority of these injuries resolve within a few weeks but the neurologic deficit can be shown to be persistent in as high as 7 – 12% of patients depending on the depth of scrutiny.14,17
Cranial nerves can be injured during CEA by the surgical dissection, traction, electrocautery, clamp injury or compression by a post-operative hematoma. The most commonly injured nerves are the recurrent or superior laryngeal branches of the vagus nerve (CN X), the hypoglossal nerve (CN XII), the marginal mandibular branch of the facial nerve (CN VII), and the glossopharyngeal nerve (CN IX). Depending on the nerve that is injured, deficits vary from being a minor nuisance to a severe disability that may require a feeding tube and/or tracheostomy.
The availability of carotid artery stenting (CAS) as an alternative therapy to endarterectomy for carotid artery stenosis has generated renewed interest in the topic of CNI because the former procedure does not put patients at risk for this complication. Some proponents of CAS have argued that the morbidity of CNI may be equivalent to that of a minor stroke and mitigates some of the benefit of the reduction in neurologic complications seen in the CEA arm in most clinical trials comparing the two procedures.13
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) compared CEA to CAS in 2,502 symptomatic and asymptomatic patients randomly assigned to undergo one of the two procedures. The primary results of the trial showed no difference in the composite endpoint of stroke, myocardial infarction (MI) and death between the two therapeutic options.18 The individual endpoints of peri-procedural MI and stroke were found to be more common in the CEA and CAS arms of the study respectively. In addition to the primary endpoint evaluations, patients in CREST underwent a Health-Related Quality of Life (HRQOL) assessment as part of the trial. The purpose of this study was to carefully examine the incidence, potential predictive factors and HRQOL outcomes in the patients experiencing CNI in the CREST study.
Methods
CREST is a prospective, randomized, multi-center trial with blinded endpoint adjudication that compared the safety of CEA versus CAS in patients with either symptomatic or asymptomatic high grade extra-cranial carotid stenosis. Details of the trial design have been previously reported.18,19 Participants were enrolled from December 2000 through July 2008 at 117 clinical centers in the United States and Canada. The protocol was approved by the institutional review boards/ethics committees at participating sites, and all participants provided signed informed consent.
Assessment of cranial nerve injury (CNI) at 1 and 6 months post-procedure was a pre-planned secondary analysis and these results have been previously reported.18 Although some studies have included injury to cervical sensory nerves in their reports, in this report it was decided to not include those injuries as they are common, do not cause significant disability and are largely unavoidable. For this analysis the assessment of CNI outcomes was extended to 12 months post-procedure. The study cohort included the 1151 patients who were assigned to the CEA arm of the study and were treated with CEA within 30 days of randomization. There were 5 additional patients with CNI excluded from this analysis because they did not receive CEA within the 30 day window or were crossovers from the CAS arm of the study. Their outcomes are described below. Patients with CNI were identified and classified using case report forms, adverse event data and clinical notes. Injuries were classified as resolved if stated as such in case report forms or clnical notes or if a deficit was no longer noted in clinical notes or on the NIH Stroke Scale evaluations. Sites were contacted regarding individual cases if the available data was unclear. Criteria used for diagnosis of CNI are contained in Table I. Adjudication of the CNIs was performed by two neurologists and a vascular surgeon. For the purpose of this study, injuries to the vagus and glossopharyngeal nerves were grouped together because the available data did not always allow a precise differentiation of which nerve had been injured.
Table I.
Cranial nerve injury definitions.
| Cranial Nerve | Symptoms |
|---|---|
| XII – Hypoglossal | Ipsilateral tongue deviation |
| VII – Facial | Ipsilateral facial droop Inability to depress ipsilateral corner of lip |
| X/IX – Vagus/Glossopharyngeal | Dysphagia, hoarseness Ipsilateral vocal cord paralysis on laryngoscopy* |
| Horner’s syndrome | Ipsilateral ptosis, miosis |
not systematically performed.
HRQOL was evaluated utilizing a standardized self-administered questionnaire at baseline (prior to procedure), 1 and 12 months post-procedure; and by telephone interview 2 weeks following the procedure. The Medical Outcomes Study Short-Form (SF-36) measures eight dimensions of health (physical functioning, physical role limitations, bodily pain index, vitality, general health, social functioning, emotional role limitations, and mental health) and has been validated in patients with cardiovascular disease and stroke.20,21,22 Six disease-specific Likert scales designed specifically for comparison of CAS versus CEA were used to evaluate aspects of functional status and symptoms that may be impacted by one or both of the treatments. The Likert scales included in this analysis were difficulty eating/swallowing, headaches, neck pain, difficulty walking, difficulty driving, and leg pain. These two measures of HRQOL (the SF-36 and Likert scales) were used to compare outcomes between patients who underwent CEA and were diagnosed with CNI versus those who did not have CNI.
Statistical Analysis
Baseline demographic characteristics and operative procedural characteristics were compared between the groups with and without CNI using chi-square for categorical and t-tests for continuous variables.
Random effects growth curve models were used to examine the effect of peri-procedural CNI on each of the SF-36 subscales over time (relative to no CNI). These models readily accommodate HRQOL score changes (linear or non-linear) over time, as well as missing data patterns commonly seen in longitudinal studies. Under the assumption of missing at random, subjects with missing data at one or more time points can be retained in the analysis, such that this approach can use all available data collected in the study. The outcome variable was each SF-36 subscale at 2 weeks, 1 month and 1 year follow-up. In addition to peri-procedural CNI, variables included in the models were baseline SF-36 scores, age, sex, symptomatic status, follow-up time, and the interaction between peri-procedural CNI and follow-up time, where the time variable was set as both random and fixed effects. The random effects growth curve model was implemented in SAS using PROC MIXED procedure in SAS for windows version 9.3 (SAS Institute, Inc., Cary, NC).
To assess the impact of peri-procedural CNI on each disease specific Likert scale, we treated each Likert scale as an ordinal outcome and used repeated ordinal logistic regression to analyze the data. Similar to the growth curve models, this method uses all available data under the assumption that any missing data are missing at random. In addition to peri-procedural CNI, variables included in the models were baseline value for each Likert scale, age, sex, symptomatic status, follow-up time, and the interaction between peri-procedural CNI and follow-up time. The time variable was set as both random and fixed effects. The results of this analysis are described in terms of odds ratios (with confidence intervals) that represented the adjusted odds of a 1-level increase in severity for the respective scales. The analysis was carried out using PROC GLIMMIX procedure in SAS for windows version 9.3.
Results
In CREST, CNI occurred in 53 (4.6%) of 1151 patients randomized to CEA who underwent the procedure within 30 days of randomization. The distribution of injuries is shown in Table II. Injuries that affected the ability to swallow or vocalize were most common, followed by injury to the marginal mandibular branch of the facial nerve and the hypoglossal nerve. A single patient had both tongue deviation and hoarseness/dysphagia but was classified as CN XII injury to simplify the analysis.
Table II.
Resolution of cranial nerve injuries over time.
| Type of injury | Present Immediately Post-op n (%) |
Present at 1-month n (%) |
Present at 12-months n (%) |
|---|---|---|---|
| Hypoglossal (XII) | 13 (24.5) | 6 (11.3) | 0 (0)a,b |
| Facial (VII) | 16 (30.2) | 10 (18.9) | 3 (5.8) |
| Dysphagia/hoarseness (IX, X) | 22 (41.5) | 18 (33.9) | 6 (11.5)c |
| Horner Syndrome | 2 (3.8) | 1 (1.9) | 1 (1.9) |
| Any CNI | 53 (100) | 35 (66) | 10 (19.2)a |
1 subject was diagnosed with lung cancer 1 month post-op and died 6 months post-op.
1 subject with tongue deviation and hoarseness/dysphagia was classified as having CN XII injury.
1 subject unknown status at 12 months, however when queried later on the subject did not recall experiencing any hoarseness, classified as resolved.
Status of the patient’s injuries was also assessed at the 1-month and 12-month follow-up visits and these results are also shown in Table II. Thirty-four percent (18/53) of the injuries had resolved within 1 month of operation while at 12 months, 80.8% (42/52) were no longer present in the surviving patients. One patient had died prior to the one year follow-up visit. There were no permanent hypoglossal nerve injuries but over 10% of injuries diagnosed as vagus or glossopharyngeal nerves were permanent.
Five patients were excluded from the analysis due to undergoing surgery beyond the 30 day post-randomization window (two) or were crossovers from the CAS group (three). Three injuries were to CN XII and two were to CN X. Four were still present at the 30 day follow-up but all 5 had resolved by 12 months following their operation.
Baseline demographic characteristics in patients who experienced CNI were compared with those without CNI to identify factors that might predict a higher likelihood of CNI. No significant differences were noted between the two groups with respect to age, sex, presence of symptoms, smoking status and whether the patients had a history of diabetes mellitus, hypertension, dyslipidemia or coronary disease (Table III).
Table III.
Baseline demographics and clinical characteristics of patients who experience cranial nerve injury compared to those who did not.
| Variable | CNI Patients (n=53) |
Non-CNI Patients (n=1098) |
p-value |
|---|---|---|---|
| Age, mean (SD) | 67.0 ± 9.2 | 69.2 ± 8.7 | 0.08 |
| Symptomatic,% | 57 | 53 | 0.63 |
| Female, % | 38 | 33 | 0.45 |
| Risk Factors | |||
| Diabetes | 32 | 31 | 0.84 |
| Prior CVD or CABG | 37 | 46 | 0.17 |
| Dyslipidemia | 89 | 86 | 0.52 |
| Hypertension | 83 | 86 | 0.51 |
| Current smoker | 33 | 26 | 0.27 |
CNI, cranial nerve injury; SD, standard deviation; CVD, cardiovascular disease; CABG, coronary artery bypass graft.
Details of operative variables including the side of operation, anesthetic type, operative time, reoperation for hematoma, and use of a patch or shunt were also compared between the groups with and without CNI (Table IV). CNI was found to be significantly higher in patients operated under general anesthesia, (5.0% versus 0.9%, P=0.05) when compared to those operated under local anesthesia. Shunts were used significantly more frequently in the general anesthesia group (59.8%) as compared to the local anesthesia group (31.2%, P < 0.0001). No other differences were detected that increased the probability of CNI.
Table IV.
Procedural characteristics of patients who experience cranial nerve injury compared to those who did not.
| Variable | CNI Patients (n=53) | Non-CNI Patients (n=1098) | p-value |
|---|---|---|---|
| Left side (%) | 55 | 52 | 0.74 |
| Local anesthesia (%)* | 1.9 | 10.0 | 0.05 |
| Operative time, minutes, mean (SD)** | 184.4 ± 51 | 170.7 ± 60 | 0.12 |
| Patch (%) | 70 | 70 | 0.99 |
| Shunts (%) | 58 | 57 | 0.82 |
| Re-exploration for hematoma (%) | 0 | 1.6 | 1.0 |
CNI, cranial nerve injury; SD, standard deviation.
CNI % in patients under local 1/111(0.9%), under general 52/1040 (5.0%)
procedure time is only available on those who received general anesthesia
The results of the comparison between the groups with and without CNI on the SF-36 surveys are shown in Figure 1. No significant differences were noted in any of the SF-36 subscales between the CNI and no CNI groups at any time point. Figure 2 shows the results of the comparison of responses to the Likert scales at the three time intervals following the surgical procedures. The group with CNI demonstrated greater difficulty with eating/swallowing at 2 weeks and 1 month following the surgical procedure when compared to the group without CNI, (p <0.001). At one year, there were no significant differences on any of the Likert scales between the CNI and no CNI group (although there was still a trend toward greater difficulty with swallowing/eating in the CNI group).
Figure 1.
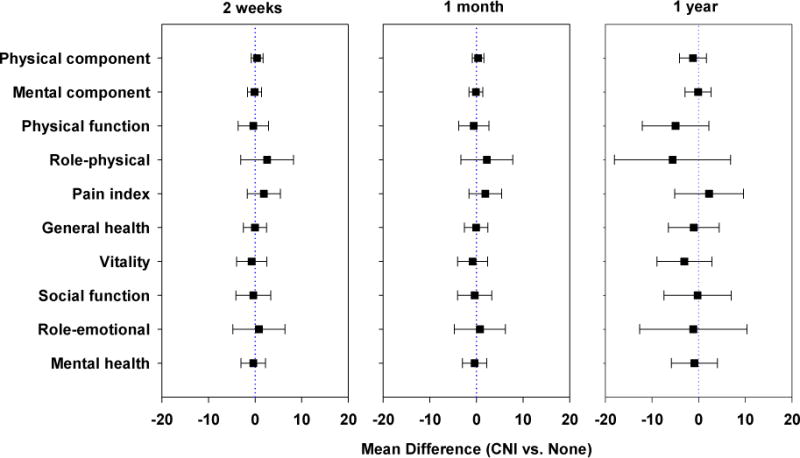
Comparison of results for the SF-36 at 2-weeks, 1-month and 1-year. No significant differences noted at any interval between the groups with and without CNI.
Figure 2.
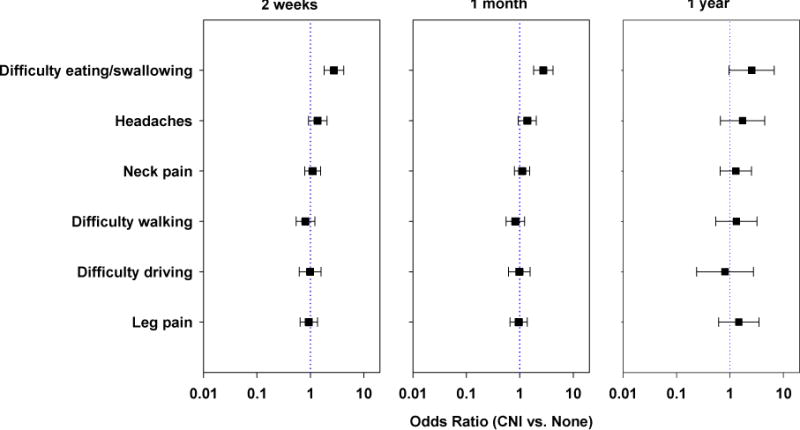
Comparison of Likert scale results at 2-weeks, 1-month and 1-year. A significantly worse outcome in Difficulty eating/swallowing was noted for the group with CNI versus without CNI at 2-weeks and 1-month, (p<0.001). At 1-year follow-up there was a non-significant trend toward a worse outcome for the same parameter for the group with CNI, (p=0.0586).
Discussion
We found a 4.6% rate of CNI among subjects who underwent CEA according to protocol in CREST. In previous reports from clinical trials, the incidence of CNI was 5.1 – 8.6%10–13, 23, consistent with these findings. Post-operative evaluation using laryngoscopy or by otolaryngologists were not systematically performed in this or any of the reference trials so the diagnosis of CNIs was made on examination by neurologists or by the surgeons who performed the operations. While this undoubtedly underestimates the true incidence of CNI, it is likely that the missed injuries had more minor manifestations, were trivial or transient.
The majority of CNI are due to trauma from dissection, traction, or retraction during the operation. Actual transection of nerves or injury due to cautery is rare so recovery is very likely. Few reports have included data regarding the timing of resolution of the deficits. For example, in the report from the International Carotid Stenting Study (ICSS)13,23, the incidence of CNI was 5.5% and little detail was provided regarding resolution of the deficits but two patients required percutaneous feeding tubes, at least temporarily. In CREST, we found that 34% of the injuries were resolved at the 1-month follow-up visit and 80.8% had resolved by the 12-month follow-up visit. The number of persistent deficits in CREST was similar to but higher than the findings in the analysis of the data from the ECST11 where a third of the injuries were resolved at discharge and over 90% resolved at the 4-month follow-up visit. That study also included patients with sensory nerve injuries and if those patients are excluded, the incidence of persistent injury at 4 months and 2-year follow-up is only 7%.
In a smaller single institution study, Zannetti14 reported 25% (13/51) of their CNI were still present at one year follow-up. Their experience was unique because a dedicated otolaryngologist performed all of the pre-procedural and post-procedural examinations. Finally, a recent large series using the Vascular Study Group of New England (VSGNE) database found that 12.3 % (47/382) of the CNI identified were still present at median follow-up of 10 months.17 Therefore, the number of persistent or permanent CNI identified in CREST is twice that reported from the ECST and VSGNE studies but similar to the findings of the Zanetti study. Overall, the surgical outcomes in CREST were superb and better than any previous report from a clinical trial with respect to the primary study endpoints. It is interesting that there was a higher percentage of persistent CNI in the CREST surgical cohort at one year than in the two studies cited above that also used primarily clinical criteria for diagnosis. It is possible that the follow-up evaluations in CREST were more thorough than in those studies. Since most of the persistent injuries in CREST were classified as cranial nerve IX or X injury on the basis of swallowing or speech abnormalities without a confirmatory otolaryngological evaluation, it also may be that those patients did not have actually have a CNI.
We were also interested in using the CREST data to identify any factors that might be predictive of CNI. Several previous studies have gone into great detail about the anatomic considerations that are important in preventing CNI2,9,15 and those are generally well-known to surgeons. The study that analyzed the CNI in the surgical cohort of the ECST trial found that operative duration was the only factor that was predictive of nerve injury.11 Operative duration is a surrogate for a technically difficult operation which intuitively might be associated with a greater likelihood of CNI. The recent VSGNE report also looked at predictors of CNI and found that urgent operations and re-exploration for bleeding or stroke either at the time of initial operation or in the immediate post-operative period were also associated with a higher likelihood of CNI.17 The number of re-explorations for bleeding was low in CREST and none of those patients suffered a CNI.
The recently published follow-up report from the ICSS investigators found additional operative factors associated with CNI.23 In their cohort of 821 patients undergoing CEA, CNI was more frequently seen in patients who suffered post-operative neck hematoma, were of female sex, or had cardiac failure. The degree of contralateral carotid stenosis and time from randomization to treatment also increased risk of CNI while use of a shunt decreased the risk. Risk of hematoma was associated with female sex, history of atrial fibrillation and pre-operative anticoagulation. Their findings contrast with those of CREST in which gender was not significant and patients undergoing re-exploration for hematoma, presumably the worst hematomas, suffered no CNI.
In CREST, it was interesting to note a significantly lower incidence of CNI in patients who underwent CEA under local anesthesia which has not been found in previous reports. In the data reported by VSGNE17 the incidence under general and loco-regional anesthesia was 5.6% and 5.1% respectively, while in ECST11 there was a non-significant trend (6.4% versus 1.7%) favoring local anesthesia. Why local anesthesia might result in a lower incidence of CNI does not have a clear explanation but may reflect a tendency toward less extensive dissection or gentler technique when patients are not under general anesthesia. While the frequency of shunt use was nearly identical in the groups with and without CNI in CREST, shunts were used significantly less often in the group operated under local anesthesia. Though interesting, the significance of this finding with respect to causation of CNI is questionable given the post-hoc analysis of a non-randomized variable. Finally, given that the diagnosis of injury to CN IX and X was based on findings of dysphagia/hoarseness, it is possible that the incidence of injury to those nerves was over-estimated. We were unable to identify any other pre-procedure or procedural characteristics that were associated with a higher or lower incidence of CNI.
If operative factors increasing the risk of CNI found in the studies cited above are summarized, most are indicative of a more technically difficult operation, While results vary between studies, it is reasonable to expect that CNI might be more likely to occur in technically difficult or complicated procedures.
The study design of CREST included HRQOL assessment that allowed direct comparison of patients experiencing CNI with those who did not. The overall HRQOL comparison between CAS and CEA in CREST has been previously reported.24 Others have also examined quality of life after CEA alone using a variety of measurement tools.25–31 Results were variable but most found no significant difference in HRQOL following CEA in general, however, none of those reports specifically examined outcomes in the sub-group of patients experiencing CNI. In CREST, the SF-36 generic health status instrument did not show any significant differences at any of the measured time points (2-weeks, 1-month and 1-year) between the two groups. While the SF-36 may not be sensitive enough to detect the degree of disability related to CNI, it was highly sensitive to detecting disability due to stroke in CREST.24,3 The Likert scales which assess more disease specific outcomes did show a negative impact on the difficulty eating/swallowing measurement at both the 2-week and 1-month assessment. At 1-year, this difference was no longer significant. Of the variables measured by the Likert scales, difficulty eating/swallowing is the most likely to be affected by a CNI so it is not surprising that this would differ between the two groups. The result at one year is likely a reflection of the resolution of the deficit in approximately 80% of the patients as well as accommodation to the deficit in the small group of patients with a persistent CNI.
The present study has several limitations. The diagnosis of CNI was made clinically, without systematic evaluation by an otolaryngologist or by laryngoscopy. Therefore it is likely that the true incidence of CNI was underestimated. On the other hand, all patients were examined by a neurologist and experienced surgeon so the majority of CNI should have been detected and reported. The case report forms used in CREST did contain a notation to document CNI at each of the follow-up visits, however they did not allow specific identification of the nerve involved unless the evaluator added an additional comment or it was identifiable in the NIH stroke scale assessment (e.g. the presence and degree of dysarthria and facial paresis for the right or left, were evaluated as part of each follow-up visit). Finally the minimal impact on HRQOL, especially over the long term, may reflect that the tools used in this study were not sufficiently sensitive to detect the more subtle impact of CNI on quality of life for these patients.
In summary, the outcomes for patients experiencing CNI due to carotid endarterectomy in CREST are consistent with prior reports documenting an incidence of about 5% and resolution of the neurologic deficit in the majority of patients within 1 year. We were unable to identify any pre-operative characteristics or intraoperative variables except operation under general anesthesia that predicted the occurrence of CNI in CREST. HRQOL was affected in patients with CNI only with respect to eating and swallowing at the 2-week and 1-month assessment but this finding was no longer present at 1 year. On the basis of these findings, we conclude that CNI is not a trivial consequence of CEA but rarely results in significant long-term disability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Twenty-ninth Annual Meeting of the Western Vascular Society, Coronado, CA September 20–23, 2014.
References
- 1.DeBord JR, Marshall WH, Wyffels PL, Marshall JS, Humphrey P. Carotid endarterectomy in a community hospital surgical practice. Am J Surg. 1991;57:627–33. [PubMed] [Google Scholar]
- 2.Hertzer NR, Feldman BJ, Beven EG, Tucker HM. A prospective study of the incidence of injury to the cranial nerves during carotid endarterectomy. Surg Gynecol Obstet. 1980;151:781–4. [PubMed] [Google Scholar]
- 3.Evans WE, Mendelowitz DS, Liapis CD, Wolfe V, Florence CL. Motor speech deficit following carotid endarterectomy. Ann Surg. 1982;196:461–4. doi: 10.1097/00000658-198210000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehn TC, Taylor GW. Cranial and cervical nerve damage associated with carotid endarterectomy. Br J Surg. 1983;70:365–8. doi: 10.1002/bjs.1800700619. [DOI] [PubMed] [Google Scholar]
- 5.Theodotu B, Mahaley MS., Jr Injury of the peripheral cranial nerves during carotid endarterectomy. Stroke. 1985;16:894–5. doi: 10.1161/01.str.16.5.894. [DOI] [PubMed] [Google Scholar]
- 6.Knight FW, Yeager RM, Morris DM. Cranial nerve injuries during carotid endarterectomy. Am J Surg. 1987;154:529–32. doi: 10.1016/0002-9610(87)90271-6. [DOI] [PubMed] [Google Scholar]
- 7.Aldoori MI, Baird RN. Local neurologic complication during carotid endarterectomy. J Cardiovasc Surg. 1988;29:432–6. [PubMed] [Google Scholar]
- 8.Forssell C, Kitzing P, Bergqvist D. Cranial nerve injuries after carotid artery surgery: A prospective study of 663 operations. Eur J Vasc Endovasc Surg. 1995;10:445–9. doi: 10.1016/s1078-5884(05)80167-4. [DOI] [PubMed] [Google Scholar]
- 9.Schauber MD, Fontenelle LJ, Solomon JW, Hanson TL. Cranial/cervical nerve dysfunction after carotid endarterectomy. J Vasc Surg. 1997;25:481–7. doi: 10.1016/s0741-5214(97)70258-1. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson GG, Eliasziw M, Barr HWK, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical results in 1415 patients. Stroke. 1999;30:1751–8. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham EJ, Bond R, Mayberg MR, Warlow CP, Rothwell PM. Risk of persistent cranial nerve injury after carotid endarterectomy. J Neurosurg. 2004;101:445–8. doi: 10.3171/jns.2004.101.3.0445. [DOI] [PubMed] [Google Scholar]
- 12.Mas J-L, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin J-P, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 13.International Carotid Stenting Study investigators. Carotid artery stenting compared with endarterectomy in patient with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomized controlled trial. Lancet. 2010;375:985–97. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zannetti S, Oarente B, De Rango P, Giordano G, Serafini G, Rossetti M, et al. Role of surgical techniques and operative findings in cranial and cervical nerve injuries during carotid endarterectomy. Eur J Vasc Endovasc Surg. 1998;15:528–31. doi: 10.1016/s1078-5884(98)80114-7. [DOI] [PubMed] [Google Scholar]
- 15.Ballotta E, Giuseppe DG, Renon L, Narne S, Saladini M, Abbruzzese E, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125:85–91. [PubMed] [Google Scholar]
- 16.Greenstein AJ, Chassin MR, Wang J, Rockman CB, Riles TS, Tuhrim S, et al. Association between minor and major surgical complications after carotid endarterectomy: results of the New York carotid artery surgery study. J Vasc Surg. 2007;46:1138–46. doi: 10.1016/j.jvs.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Fokkema M, de Borst GJ, Nolan BW, Indes J, Buck DB, Lo RC, et al. Clinical relevance of cranial nerve injury following carotid endarterectomy. Eur J Vasc Surg. 2014;47:2–7. doi: 10.1016/j.ejvs.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, et al. CREST Investigators Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) Int J Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 21.Failde I, Ramos I. Validity and reliability of the SF-36 Health Survey Questionnaire in patients with coronary artery disease. J Clin Epidemiol. 2000;53:359–65. doi: 10.1016/s0895-4356(99)00175-4. [DOI] [PubMed] [Google Scholar]
- 22.Kiebzak GM, Pierson LM, Campbell M, Cook JW. Use of the SF36 general health status survey to document health-related quality of life in patients with coronary artery disease: effect of disease and response to coronary artery bypass graft surgery. Heart Lung. 2002;31:207–13. doi: 10.1067/mhl.2002.124299. [DOI] [PubMed] [Google Scholar]
- 23.Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Brown MM, et al. Incidence, impact, and predictors of cranial nerve palsy and haematoma following carotid endarterectomy in the International Carotid Stenting Study. Eur J Endovasc Surg. 2014;48:498–504. doi: 10.1016/j.ejvs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen DJ, Stolker JM, Wang K, Magnuson EA, Clark WM, Demaerschalk BM, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy. JACC. 2011;58:1557–65. doi: 10.1016/j.jacc.2011.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd AJ, Hayes PD, London NJ, Bell PR, Naylor AR. Does carotid endarterectomy lead to a decline in cognitive function or health related quality of life? J Clin Exp Neuropsychol. 2004;36:817–25. doi: 10.1080/13803390490509420. [DOI] [PubMed] [Google Scholar]
- 26.Parker JC, Smarr KL, Granberg BW, Nichols WK, Hewett JE. Neuropsychological parameters of carotid endarterectomy: a two-year prospective analysis. J Consult Clin Psychol. 1986;54:676–81. doi: 10.1037//0022-006x.54.5.676. [DOI] [PubMed] [Google Scholar]
- 27.Vriens EM, Post MW, Jacobs HM, van Huffen AC, Eikelboom BC. Changes in health-related quality of life after carotid endarterectomy. Eur J Vasc Surg. 1998;16:395–400. doi: 10.1016/s1078-5884(98)80006-3. [DOI] [PubMed] [Google Scholar]
- 28.Sirkka A, Salenius JP, Portin R, Nummenmaa T. Quality of life and cognitive performance after carotid endarterectomy during long-term follow-up. Act Neurol Scand. 1992;85:58–62. doi: 10.1111/j.1600-0404.1992.tb03996.x. [DOI] [PubMed] [Google Scholar]
- 29.Dardik A, Minor J, Watson C, Hands IJ. Improved quality of life among patients with symptomatic carotid artery disease undergoing carotid endarterectomy. J Vasc Surg. 2001;33:329–33. doi: 10.1067/mva.2001.111735. [DOI] [PubMed] [Google Scholar]
- 30.De Leo D, Serraiotto L, Pellegrini C, Magni G, Francheschi L, Deriu GP. Outcome from carotid endarterectomy. Neuropsychological performances, depressive symptoms and quality of life: 8-month follow-up. Int J Psychiatry Med. 1987;17:317–25. doi: 10.2190/1grb-rkbh-nb2a-ppwr. [DOI] [PubMed] [Google Scholar]
- 31.Trudel L, Fabia J, Bouchard JP. Quality of life of 50 carotid endarterectomy survivors: a long-term follow-up study. Arch Phys Med Rehabil. 1984;65:310–12. [PubMed] [Google Scholar]


