Abstract
Brain imaging of cerebral blood flow and glucose metabolism has been playing key roles in describing pathophysiology of Parkinson’s disease (PD) and Huntington’s disease (HD), respectively. Many biomarkers have been developed in recent years to investigate the abnormality in molecular substrate, track the time course of disease progression, and evaluate the efficacy of novel experimental therapeutics. A growing body of literature has emerged on neurobiology of these two movement disorders in resting states and in response to brain activation tasks. In this paper, we review the latest applications of these approaches in patients and normal volunteers at rest conditions. The discussions focus on brain mapping studies with univariate and multivariate statistical analyses on a voxel basis. In particular, we present data to validate the reproducibility and reliability of unique spatial covariance patterns related with PD and HD.
Keywords: Neurodegenerative disorders, Parkinson’s disease, Huntington’s disease, Cerebral blood flow, Metabolism, Brain mapping, Spatial covariance analysis
Introduction
Pathogenesis of neurodegenerative disorders has been investigated extensively with functional brain imaging of radioligands that bind to relevant neurotransmitters and neuroreceptors as well as general radiotracers for the measurement of regional cerebral blood flow (rCBF) and cerebral metabolic rate of glucose (rCMRglc). Brain imaging of rCBF and rCMRglc can provide signature biomarkers to describe pathophysiological mechanisms and clinical correlates over the whole brain by using either region of interest (ROI) analysis or voxel-based brain mapping. Both univariate and multivariate statistical approaches have been developed for brain mapping analysis after transforming individual images into a standard anatomical space [1–3]. Brian images are also spatially smoothed or realigned between conditions as part of the preprocessing steps. Statistical parametric mapping (SPM) is a powerful univariate statistical method for detecting localized changes in brain function. By contrast, multivariate methods are more sensitive for assessing systematic changes that reflect potential interactions among a set of functional brain regions of interest.
Network analysis based on principal component analysis (PCA) is one of the versatile multivariate statistical brain mapping methods in neuropsychiatric disorders and normal aging [4–6]. This is a data reduction technique that accounts for variance in the images in decreasing degree of importance; it does so by projecting original images into a series of principal components after removing regional or subject mean from the data. This allows identification of unique 3-D topographic patterns to describe spatial covariation among a set of functionally dependent brain areas. Expressions of each pattern can not only discriminate between different patient groups but also predict behavior performance at rest and activation conditions. By applying this method to [18F]FDG and [15O]H2O positron emission tomography (PET) images of patients and control subjects, we and others have previously generated specific spatial covariance patterns for a series of neurodegenerative disorders such as Parkinson’s disease (PD), Huntington’s disease (HD), torsion dystonia, and Alzheimer’s disease (AD) [7–10]. A subject score can be computed prospectively on a single case basis and has been shown to correlate with independent measures of disease severity and response to treatment. The applications of this class of approaches have recently been reviewed in detail (see [11]).
Imaging methods based on these approaches have been used extensively in the study of neurodegenerative disorders. For instance, both rCBF and rCMRglc data from PET or single photon emission computed tomography (SPECT) can discriminate among controls, AD, and different forms of dementia as well as identify neural bases of corresponding cognitive dysfunction [12–16]. Imaging variables can serve as objective predictors of AD in individuals with mild cognitive impairment based on unique cerebral lesions related with clinical manifestation and genotype. This type of imaging work has also been pursued in mapping phenotype- or genotype-specific abnormal metabolism in primary torsion dystonia [17, 18] and investigating impaired motor activation response at baseline or after treatment with deep brain stimulations [19–21].
In this review, we discuss brain imaging studies at rest in PD and HD as examples of hypokinetic and hyperkinetic movement disorders, respectively. Both conditions are associated with localized deficits in basal ganglia that cause dysfunction at a systems level across the entire brain by disrupting signal transmission within different components of the cortico-striatopallidal-thalamocortical (CSPTC) circuitry. Because of the limited scope, this article is not meant to provide a systematic review of all the resting state investigations in this area. Instead, we focus on the work over the last decade in developing and validating biomarkers using quantitative measures of regional blood flow and glucose metabolism. Special emphasis will be given to novel applications of multivariate statistical analysis from our laboratory.
Parkinson’s Disease
PD results primarily from progressive losses of dopamine neurons in the nigrostriatal system leading to widespread motor symptoms and cognitive impairments. Imaging with PET and SPECT has been widely used to reveal regional abnormality in presynaptic and postsynaptic markers as well as hemodynamic and metabolic brain function in this disorder (e.g., [22]). Previous binding studies based on many radioligands have consistently shown dopamine deficiency and terminal degeneration in caudate and putamen with increased level of dopamine D2 receptors. Here we describe the use of CBF and FDG uptake in establishing clinical correlation in PD and assessing anti-parkinsonian therapies.
Imaging of Pathophysiology
Significant alternations in striatal and cortical function have been demonstrated in primate models of PD induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [23]. rCBF is increased in the globus pallidus but decreased in the caudate, putamen, thalamus, and primary motor cortex. Early investigations in humans with PET have shown that rCBF in striatal areas remains unchanged in PD as compared with the normal population [24], whereas a SPECT perfusion study has revealed that medication-induced hallucinations in PD may be related with significantly lower rCBF in left temporal regions [25]. Measurement of rCBF has limited use in resting states because of its relatively lower signal-to-noise ratio, but is more valuable in studying brain activation response and therapeutic interventions (see below).
PET imaging with 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) provides a more direct measure of brain function by quantifying regional glucose metabolism. The previous animal study on PD has revealed pallidal hypermetabolism with hypometabolism in striatal, thalamic, and motor regions in agreement with rCBF data [23]. In a recent study in primate models of PD, we have observed relative metabolic increases in the ipsilateral globus pallidus and the contralateral cerebellar hemisphere after unilateral MPTP injection [26]. As the most common imaging technique available, this method has been used mainly to distinguish PD from atypical parkinsonism such as multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) [27–29]. In a systematic analysis of FDG-PET scans from a large cohort of parkinsonian patients, we have used SPM to establish several characteristic patterns of abnormal metabolism for each of these movement disorders relative to age-matched normal controls [30]. A set of disease-specific brain templates and criteria were created for more accurate diagnosis of each entity. Imaging-assisted examination based on single-subject SPM comparison with controls improved the accuracy of differential diagnosis in each individual patient from 75% to 90%. This was achieved by blind comparison of the imaging result with clinical assessment made at two-year follow-up. Additionally, we found that glucose metabolism in PD increased in the dorsal lateral putamen, thalamic, cerebellar, and cortical motor areas with hypometabolism in the parietal cortex.
Brain network analysis
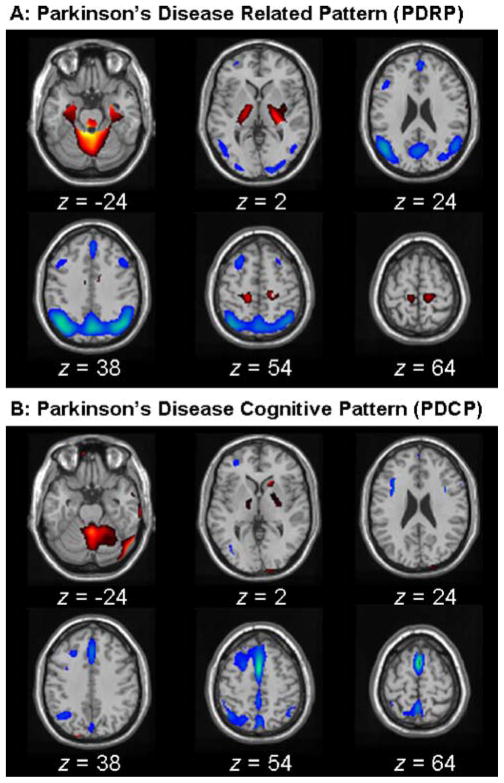
PCA-based network analysis has been successfully applied to identify PD-related covariance patterns associated with motor and cognitive functioning. The PD-related pattern (PDRP; [7, 31]) was identified on a voxel basis by applying PCA to FDG images acquired at rest from 33 PD patients (age 57±8 years; Hoehn & Yahr stage 2.6) and 33 age-matched normal controls (age 55±13 years). In this analysis, the PDRP represented the first principal component whose expression in individual subjects significantly (p<0.0001) discriminated PD patients from normal controls. Accounting for 21% of subject-voxel variance, this pattern (Fig. 1A) was marked by pallidal, thalamic, pontine, and cerebellar hypermetabolism, covarying with hypometabolism in the lateral premotor cortex, supplementary motor area, and posterior association cortices. This brain network is highly correlated with that reported by us previously [18, 32] and has been validated in the metabolic data from independent imaging centers [33, 34]. It has previously been shown that PDRP scores correlate with pallidal neuronal activity and clinical UPDRS motor ratings in PD patients (e.g., [35–37]).
Fig. 1.
(A) Parkinson’s disease-related pattern (PDRP) identified by network analysis of FDG-PET scans from 33 PD patients and 33 age-matched normal volunteers. This covariance pattern was characterized by pallidal, thalamic, pontine, and cerebellar hypermetabolism associated with metabolic decrements in the lateral premotor and posterior parietal areas. PDRP expression (subject scores) for this pattern was increased (p<0.00001) in the PD patients relative to normal subjects. (B) Parkinson’s disease cognitive pattern (PDCP) identified by network analysis of FDG-PET scans from 13 nondemented PD patients with mild-moderate motor symptoms. This network was marked by relative hypometabolism of prefrontal, preSMA, and superior parietal regions, associated with cerebellar and thalamic metabolic increases. Subject scores for this pattern correlated significantly with psychometric indices of verbal learning and visuospatial performance. (The display represents voxels that contribute significantly to the network at p=0.001. Voxels with positive region weights [metabolic increases] are color coded from red to yellow; those with negative region weights [metabolic decreases] are color coded from blue to purple. Both topographic patterns are overlaid on a standard single subject MRI brain template.)
The PD-related cognitive pattern (PDCP) was identified in the resting-state FDG images of 13 nondemented PD patients (age 59±10 years; Hoehn & Yahr stage 3.2; MMSE=28.4±2) [38]. The PDCP topography represented the second principal component whose expression in individual subjects was negatively correlated (R2=0.40; p<0.02) with performance on the California Verbal Learning Test and other neuropsychological tests of memory and executive function. Accounting for 19% of subject-voxel variance, this pattern (Fig. 1B) showed hypometabolism in the prefrontal, preSMA, and superior parietal cortices and hypermetabolism in the right caudate and bilateral thalamus and cerebellum. This abnormal brain network is in good agreement with that obtained previously with another multivariate network approach based on partial least squares [39].
Both PDRP and PDCP described here are very similar to topographic patterns generated from an early study, which conducted a PCA on ROI data of rCMRglc in a different cohort of PD patients and control subjects [33]. Two orthogonal principal components were detected in this cohort corresponding to PDRP and PDCP, respectively. The PDRP was found to separate the groups and correlate with UPDRS motor ratings in patients, whereas the PDCP correlated only with cognitive performance measures, but not with UPDRS motor scores. These results suggest that topographically distinct brain networks may subserve motor and cognitive dysfunction in PD involving different components within the CSPTC loop.
We have investigated the test–retest reproducibility of PD-related brain networks in the context of clinical trial settings involving distinct patient populations [7]. We first chose control subjects and PD patients who were scanned with both tracers to examine the compatibility of rCBF and rCMRglc data in quantifying network activity of these two abnormal metabolic patterns. We then used within-session H2O-PET scan pairs in normal controls and several groups of PD patients selected to participate in a longitudinal study or receive levodopa infusion and deep brain stimulations (DBS) at the internal globus pallidus (GPi) and subthalamus (STN), respectively. We also used between-session FDG-PET scan pairs acquired both off and on dopaminergic medications separated by up to two months. To test the within-subject reliability, subject scores of both PDRP and PDCP were computed prospectively from all of these PET scans using an automated algorithm (see [40] for details).
CBF-FDG Correlation
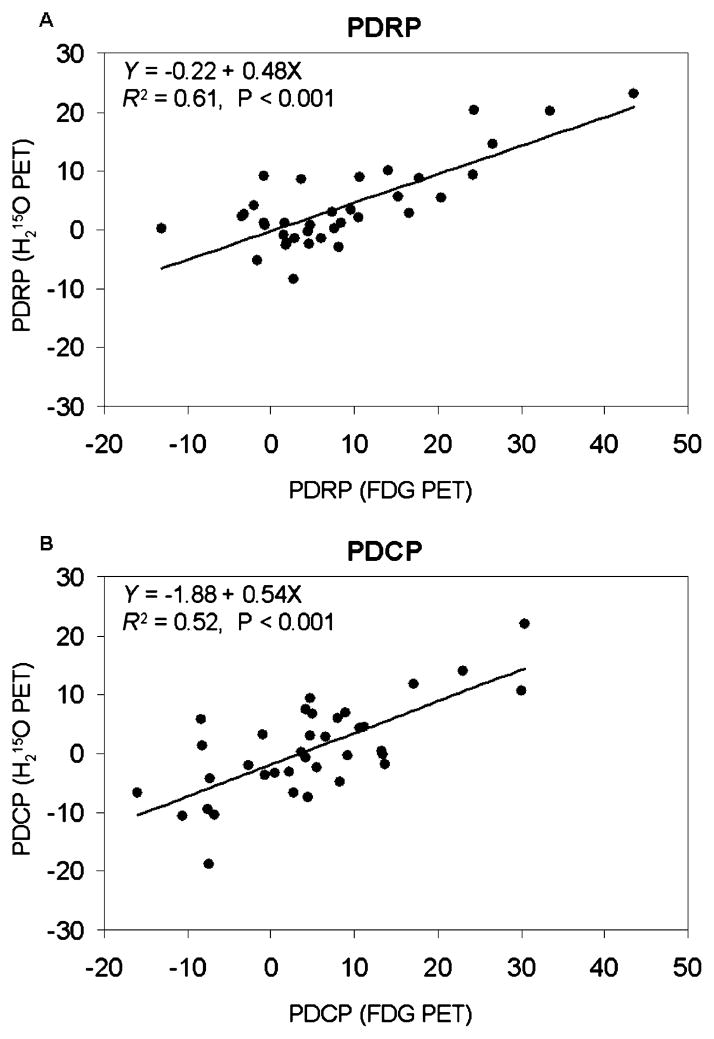
PDRP scores from rCBF and rCMRGlc scans were highly correlated with each other within PD patients (Fig. 2A; R2=0.61; p<0.001). The corresponding PDCP scores were also correlated to a high degree in the same PD group (Fig. 2B; R2=0.52; p<0.001). In addition, correlations were significant in the combined group of PD patients and healthy volunteers for both PDRP (R2=0.62; p<0.001) and PDCP (R2=0.48; p<0.001). Normal volunteers had less variability in PDRP or PDCP subject scores with a mean of zero.
Fig. 2.
Regression analysis revealing significant linear relationships between network scores obtained from concurrent CBF and FDG-PET scans in patients with PD. A: PDRP [F(1, 34)=52.4, p<0.001 and R2=0.61]. B: PDCP [F(1, 34)=35.8, p<0.001 and R2=0.52]. The same relationships remained after including the normal controls.
We have observed a strong correlation between PDRP scores calculated from CBF and FDG images measured in the same individuals. Likewise, the correlation between CBF and FDG subject scores for PDCP is also highly significant. These results indicate that CBF and FDG uptake over the whole brain are coupled to a high degree and network activity can be reliably quantified with CBF data as has been shown in PD with [99 mTc]-ECD SPECT for imaging cerebral perfusion [41, 42]. This creates the basis for identifying disease-related brain networks in CBF scans obtained using noninvasive arterial spin label imaging methods based on magnetic resonance imaging (MRI) [43–46]. This technique is highly reproducible in normal volunteers and agrees well with measurements from H2O PET.
Although CBF images can be used to evaluate functional abnormality in PD on a system level, we found that only 50–60% of variability in network activity was shared across the CBF and FDG data (Fig. 2). This suggested that CBF and glucose metabolism may to some degree be uncoupled in PD on a regional level. Indeed, the relatively weaker correlation in the PDCP scores suggests an increase in this uncoupling in regions associated with cognitive dysfunction in PD [38]. This potential dissociation is an ongoing topic of investigation.
Prospective Validation
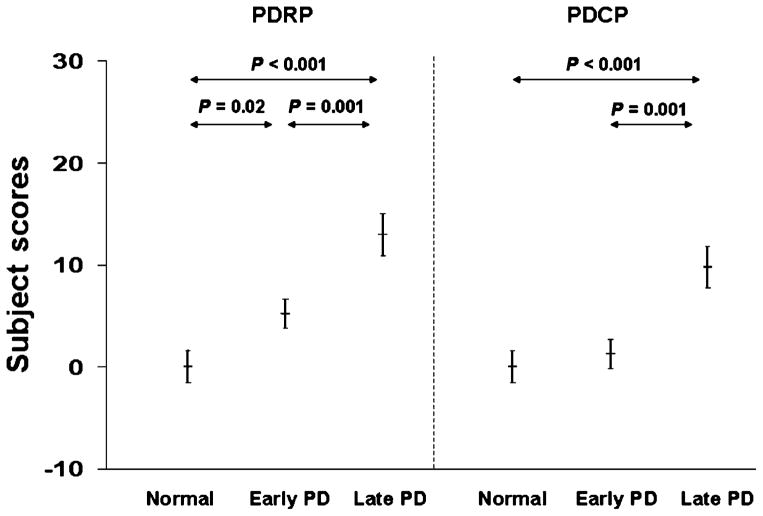
We observed that subject scores prospectively computed from CBF data discriminated PD and normal groups with a slight difference (Fig. 3). PDRP activity separated both early and late PD patients from controls, whereas PDCP activity discriminated only late PD patients from controls. Network activity was significantly higher in late PD patients than in early PD patients for both PDRP and PDCP. This behavior compared favorably with the results obtained from FDG images in several independent cohorts of patients and controls [7, 31]. However, PDCP activity did not discriminate between early PD and normal groups, reflecting preserved cognitive function in early PD. This is in contrary to PDRP expression, which is more related to motor disability. Indeed, this cross-sectional difference between the PDRP and PDCP networks in relation to disease severity has recently been observed in a longitudinal FDG-PET study of disease progression [47].
Fig. 3.
Disease discrimination by PDRP and PDCP expressions measured by resting-state CBF PET images. Both scores significantly discriminated late PD and early PD or control groups with comparable accuracy (p<0.001). PDRP scores were abnormally elevated while PDCP scores remained unchanged in early PD relative to controls. Subject scores came from a prospective individual case analysis conducted using an automated routine that was blind to diagnostic category. Error bars represent standard errors of the mean.
Using the same CBF data in Fig. 3, we found a very strong correlation (p<0.0005) between PDRP and PDCP network values in the controls (R2=0.71) and all PD patients (R2=0.68). This indicated that both patterns are highly related as a pair of key features of PD pathology although we had shown elsewhere that only PDRP activity correlates moderately with clinical ratings of disease severity. To compare their potential diagnostic value, we performed the ROC analysis on this dataset. As expected, we observed better discrimination between the controls and all PD patients by PDRP [area under the curve (AUC)=0.84] than PDCP (AUC=0.67). We also found that the group separation improved slightly (AUC=0.89) compared with those of the individual networks by using a linear regression with both network expressions. This shows that a combination biomarker may be more useful in clinical diagnosis and functional assessment of PD in single patients.
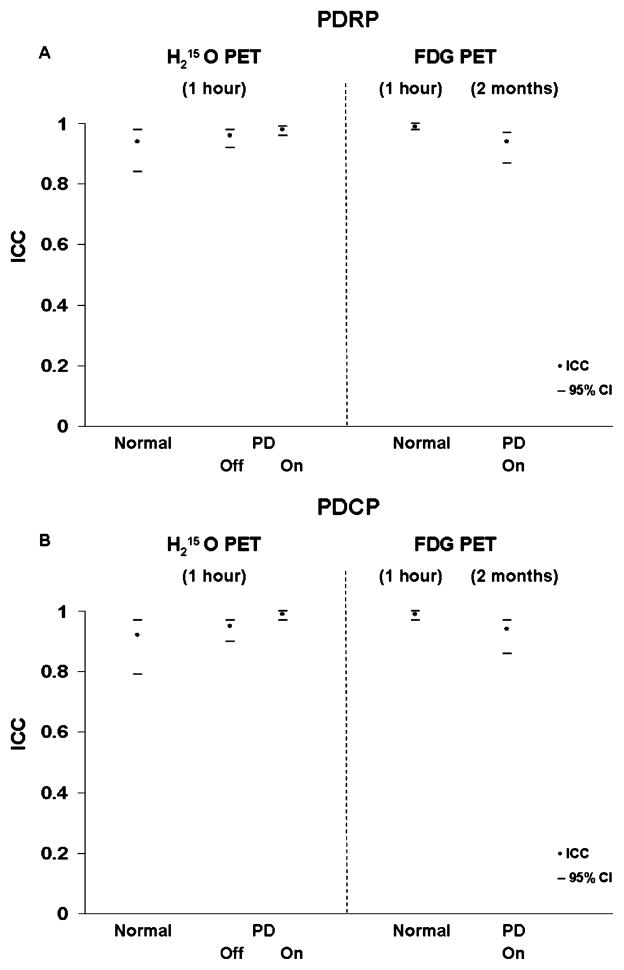
We found excellent within-subject reproducibility for PDRP and PDCP subject scores in resting state H2O PET scans conducted within one hour (Fig. 4). Both subject scores exhibited a very high reliability (intraclass correlation coefficient [ICC] of 0.95 and 95% confidence of interval [CI] of 0.90 to 0.98) in early and advanced PD patients in the absence of any acute treatment, as well as in each subset of patients after levodopa infusion and DBS procedures, respectively. These scores were also highly reproducible (ICC of 0.94 and 95% CI of 0.87 to 0.97) in PD patients scanned over two months by FDG-PET while on dopaminergic medication. This is comparable with high reproducibility shown by within-session CBF measurements made on levodopa, but also agrees with that given by FDG data taken one day apart and off medication from five early-stage PD patients. For comparison, both PDRP and PDCP scores show excellent test–retest reliability in normal controls as well using CBF and FDG scan pairs measured within session.
Fig. 4.
Summary of test–retest reliability showing intraclass correlation coefficient (filled symbols) and 95% confidence of interval (horizontal bars) in normal subjects and PD patients. Data came from CBF scans collected twice within one hour in normals and PD patients at baseline and following medical and surgical therapies (conducted 12 hours off medication for patients) and FDG scans repeated within one hour in normals and over eight weeks in PD patients (on medications).
In addition to PD-related brain networks, we have recently generated specific metabolic covariance patterns for MSA, PSP, and dopa-responsive dystonia to further help differential diagnosis of clinical parkinsonism [18, 40]. This allows us to test the probability of having any one of these conditions by computing the expression of respective brain networks in an individual patient on an automated, high throughput basis. The accuracy of diagnosis at early clinical stages is expected to improve in conjunction with single-subject SPM analysis based on disease-specific brain templates [30]. This approach is currently being validated for diagnostic use.
Therapeutic Interventions
PET imaging of CBF and FDG uptake has been particularly valuable in revealing functional mechanisms of medical and surgical interventions in PD. The specific action of therapy is delivered by either enhancing endogenous dopamine level or modulating different nodes within the CSPTC circuitry. rCBF measurement is proving most useful in charting treatment-mediated brain activation response in PD under task conditions. For example, it has been shown by SPM analysis that STN stimulation decreased motor cortex activity at rest [48] and enhanced movement-related activity of motor-association cortex [49], but suppressed activation of the right orbitofrontal cortex and verbal fluency-associated activation within a left-sided frontotemporal network [50]. These observations help to explain the improved motor function and worsening verbal-fluency performance after STN DBS. Neural bases of impaired sequence learning in PD during disease progression and effective therapies have been explored with both SPM and PCA-based network methods (see review [51]).
We have previously revealed different effects on metabolic brain function after levolopa infusion, DBS at GPi, and ablative lesioning at STN [8, 32, 52, 53]. These interventions consistently restore abnormal glucose metabolism on a regional basis and suppress the PDRP, with the degree of regional change or network modulation correlating with the clinical treatment response. We have recently compared metabolic responses between STN lesioning and DBS [54]. SPM analysis revealed that metabolism was reduced in the GPi and caudal midbrain but elevated in the posterior parietal cortex by both STN procedures (p<0.01). Although reductions in GPi were more evident with lesion, elevations in posterior parietal metabolism were more pronounced with stimulation. Furthermore, we noted that PDRP activity declined significantly (p<0.02) with both STN lesioning and stimulation.
In another study, we found that both STN DBS and levodopa therapy [37] were associated with significant (p<0.001) metabolic reductions in the putamen/globus pallidus, sensorimotor cortex, and cerebellar vermis, as well as increases in the precuneus (BA 7). The metabolic effects of the two interventions differed in the STN and medial prefrontal cortex, with relative increases with stimulation in the former structure and decreases in the latter. Network quantification disclosed reductions in PDRP activity with both interventions, which correlated with clinical improvement (p<0.05). Metabolic increase in the parietal areas and decreases in the cerebellum we observed above agree with an earlier study on STN DBS [55]. We conclude that suppression of pathological brain networks is a critical feature of the treatment response in parkinsonism. Nevertheless, glucose metabolism at a regional level may differ among these interventions.
To demonstrate the independence of the cognitive pattern in PD, we have also examined therapeutic responses of the PDCP network to the treatment modalities described above, which target mainly motor symptoms. Interestingly, we observed that PDCP subject scores did not change significantly before and after these interventions. It is expected that PDCP expression is more sensitive to therapies targeting cognitive symptoms in PD patients. Nonetheless, in a recent study of oral donepezil for PD cognitive symptoms, we found no significant change in PDCP expression with treatment. Further studies using different treatment strategies will be of interest.
Cellular-based transplantation neurosurgery represents a new class of neurorestorative therapies for advanced PD. PET with dopaminergic markers has mainly been used in clinical trials of dopamine cell transplantation and infusion of nerve growth factors. Functional recovery is also been examined by using brain activation paradigms with CBF PET. These aspects have recently been reviewed (e.g., [22]). However, there is some evidence that PET imaging with FDG can also be useful for evaluating the viability of neural grafts. For instance, human differentiated chromaffin cells can be grafted into the caudate nucleus of PD patients [56]. This procedure was capable of generating substantial clinical improvement that was correlated with increased regional glucose metabolism while allowing a 70% decrease in levodopa medications.
Gene therapy is a novel technology capable of restoring functional integrity in PD at advanced stages. One of the promising methods aims to inhibit hyperactivity in STN output by transfection with adeno-associated virus (AAV) containing the gene for glutamic acid decarboxylase (GAD). In a recent animal study, 13 macaques were rendered hemiparkinsonian by right intracarotid MPTP injection [26]. Seven animals were injected with AAV-GAD into the right STN, whereas six received an AAV gene for green fluorescent protein (GFP). Motor ratings were taken over approximately one year followed by FDG-PET and histological examination at the end. The GAD animals exhibited an increase in glucose utilization in the ipsilateral motor cortex relative to GFP controls (p<0.001). Metabolism in this region correlated with clinical ratings at endpoint (p<0.01). Histology confirmed GAD expression in treated animals. These findings suggest that STN AAV-GAD is well tolerated and potentially effective in a primate model of PD.
In the first open-label, safety-tolerability dose-escalation trial of this novel intervention we have used FDG-PET to assess the effects of AAV vector delivery of GAD into the STN in PD [57]. Twelve advanced PD patients received unilateral treatment at the right STN and were evaluated at baseline and six months post-surgery. SPM analysis revealed decreases in brain metabolism in the internal globus pallidus and ventrolateral thalamus ipsilateral to AAV-GAD, without any significant changes on the contra-lateral side. Improvements in UPDRS motor ratings correlated with increases in brain metabolism in the primary (R2=0.69; p<0.001) and supplementary (R2=0.73; p<0.001) motor areas on the side ipsilateral to the AAV-GAD. The results from both animal models and human trials are consistent with imaging studies in other subthalamic surgical therapies for PD, suggesting that STN AAV-GAD therapy is potentially effective in the treatment of advanced PD.
In summary, brain imaging of rCBF and rCMRglc allows identification of signature biomarkers for differential diagnosis of clinical parkinsonism. Multivariate network analysis can generate specific topographic patterns related to motor and cognitive abnormalities, respectively. Recent work has demonstrated that these covariance patterns are highly reliable descriptors of abnormal cerebral blood flow and metabolism underlying PD in a resting state. Both provide objective imaging markers for assessing the time course of disease progression and therapeutic interventions.
Huntington’s Disease
HD is a hereditary disorder in which degeneration of neurons in the striatum leads to motor, cognitive, and behavioral dysfunction. The genetic mutation is an unstable expanded DNA trinucleotide [cytosine–adenosine–guanosine (CAG)] repeat whose length predominantly determines the expected age of onset of motor symptoms in affected patients. Prior investigations with MRI have revealed progressively widespread brain atrophy in striatal and selective cortical areas in both presymptomatic and symptomatic HD subjects [58–60]. PET studies have used radiotracers that measure dopamine D1/D2 receptor binding, cerebral blood flow, and glucose metabolism. It has been reported that radiotracer binding to dopamine D1/D2 receptors may be normal or lower in HD mutation carriers in the preclinical period, but is significantly reduced in HD patients [61–63]. Dopamine receptor losses occur mainly in the striatum [64–66] as well as in the cortex [67]. These postsynaptic abnormalities have been shown to correlate with clinical or cognitive symptoms and become more pronounced with progression of HD. In the following sections, we summarize brain imaging studies using both rCBF and rCMRglc measurements.
Evaluation of Pathophysiology
MicroPET technology is increasingly becoming available in exploring cerebral dysfunction in transgenic animal models of HD. One study showed that energy utilization ipsilateral to the lesion in rats was reduced severely one week after intrastriatal excitotoxin injections [68]. The decrements in energy metabolism were even more prominent five to seven weeks later. This type of imaging techniques has paved the way for evaluating behavior correlates and response to therapies in living animals.
FDG-PET has been employed to investigate abnormal metabolic substrate in both clinically unaffected subjects at risk for HD and symptomatic patients. Striatal glucose metabolism is reported to be normal or reduced in presymptomatic HD (p-HD) individuals [61, 63, 69]. By contrast, striatal hypometabolism is consistently observed in symptomatic HD patients and correlated with different aspects of impaired functional status [70–73]. Thalamic hypermetabolism as well as cortical hypometabolism and hypoperfusion have also been seen in early stage and symptomatic HD patients with PET or SPECT imaging [74–76]. A recent study has shown significantly decreased glucose metabolism in the striatum and temporal and frontal cortical lobes in both preclinical and affected HD subjects [77]. Progressively decreased glucose uptake is seen in the striatum and cortex when assessed longitudinally.
As part of a multimodality longitudinal study, we have acquired FDG-PET and MRI data at baseline and 1.5 years in 12 p-HD gene carriers (CAG repeat length=42±2; age=47±12 years). The time to clinical onset was estimated to be 10±9 years at baseline using a lookup table [78]. We first used SPM analysis to detect local changes in glucose metabolism in the p-HD gene carriers relative to 11 age-matched controls (41±15 years) and between two time points. In addition to confirming the regional abnormalities summarized above, we also revealed hypometabolism in the cingulate cortex as well as hypermetabolism in the cerebellum and occipital cortex (Fig. 5). Altered glucose metabolism was more spread and progressed differently in these anatomical areas of interest over the course of 1.5 years.
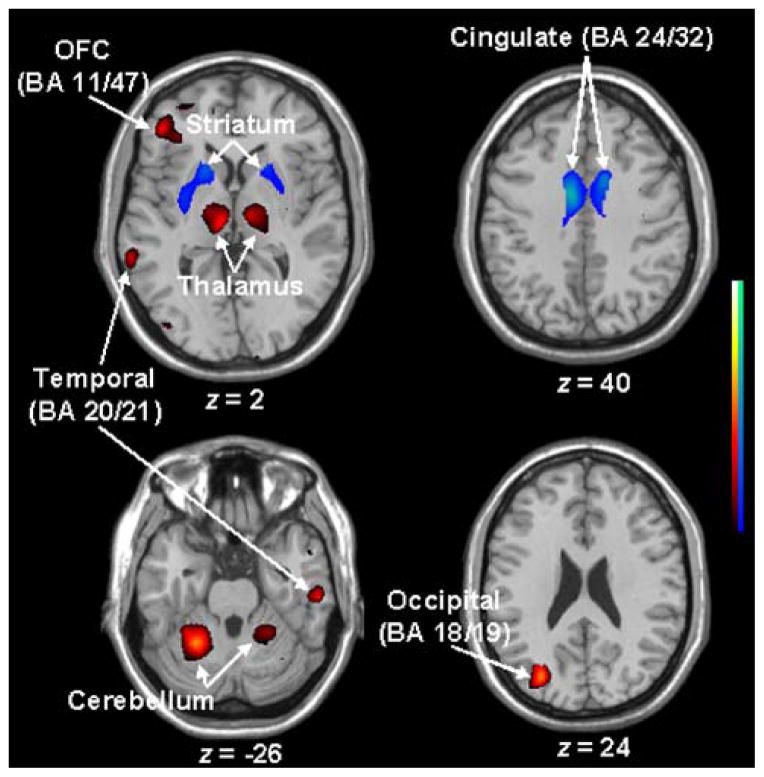
Fig. 5.
Altered glucose metabolism detected by SPM analysis (p<0.01; T>2.5) of FDG-PET scans in 11 presymptomatic HD gene carriers relative to 12 gene-negative normal controls. This abnormality included bilateral hypometabolism in the striatum and cingulate, and hypermetabolism in the bilateral thalamus and cerebella as well as in temporal and frontal cortices. Regions of abnormal metabolism were overlaid on a standard single subject MRI brain template.
We then performed PCA using the combined group of p-HD gene carriers at baseline and normal controls to identify a specific abnormal metabolic topography [79]. We found a significant HD-related covariance pattern (HDRP) as the first principal component accounting for 18% of subject-voxel variance. This pattern was characterized by relative bilateral increases in thalamic, occipital, and cerebellar glucose metabolism associated with bilateral covariate decreases in striatal metabolism (Fig. 6A). Subject scores in the p-HD gene carriers at baseline were elevated (p<0.05) as compared with normal controls (Fig. 6B). Region weights of this pattern were highly correlated (R2=0.76; p<0.0001) with those reported in the previous HDRP pattern from a different p-HD cohort [80].
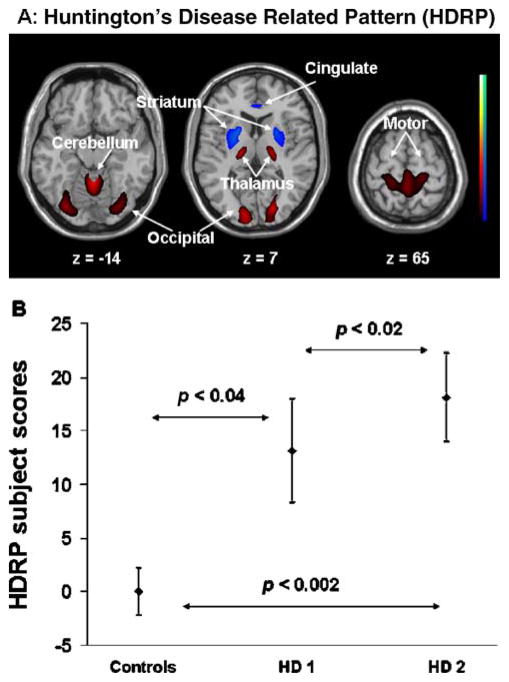
Fig. 6.
HD-related spatial covariance pattern (HDRP) identified by network analysis of FDG-PET scans in 11 pre-symptomatic HD gene carriers and 12 gene-negative controls. (A) HDRP overlaid on a standard single subject MRI brain template showing bilateral hypometabolism in the striatum and cingulate, associated with bilateral hypermetabolism in the thalamus and cerebella as well as in occipital and motor cortices. (B) Subject scores in the preclinical HD gene carriers increased at baseline as compared with normal controls and elevated further at 1.5 years. This graph plots the mean and standard error of the mean.
We subsequently found that mean subject scores in the p-HD cohort increased further by prospectively calculating the expression of HDRP in each of the asymptomatic gene carriers at follow-up. In addition, HDRP scores at each time point correlated with the estimated years to onset (R2>0.66; p<0.01) and caudate D2 receptor binding (R2>0.62; p<0.01) measured concurrently in the same cohort. It is of interest to note that on a regional level we observed a strong positive correlation (R2>0.7; p<0.005) between dopamine D2 receptor binding and glucose metabolism in caudate and putamen at both time points. This finding supports the notion that abnormal metabolic brain network in HD is related to dopamine D2 receptor loss. However, the brain network can capture aspects of functional abnormality not reflected by impaired neuroreceptor integrity.
In a preliminary study, we have investigated the effect of brain atrophy on metabolic change in preclinical HD. FDG-PET images from the p-HD subjects and controls were corrected for brain atrophy using the coregistered MRI data [81]. Striatal hypometabolism at baseline observed by SPM analysis disappeared to a large extent after atrophy correction. Striatal hypometabolism was present in an enlarging area at the later time point, but appeared mainly in the posterior putamen. These data indicate that striatal hypometabolism reflects mainly the presence of atrophy but begins to decline more quickly as the p-HD subjects move closer to symptom onset.
The HDRP topography generated from FDG images with atrophy correction was similar to that described above but with less hypometabolism in the caudate and putamen, consistent with the SPM analysis. The mean network activity in the p-HD gene carriers was elevated at baseline but did not progress over time. This suggests the existence of a different covariance network after atrophy correction. It remains to be seen whether a unique and new topographic pattern will emerge when these p-HD subjects become symptomatic.
PET imaging of CBF has been primarily utilized to examine abnormal brain activation responses during cognitive tasks in HD. To study the mechanism of this cognitive abnormality in p-HD, we have used H2O PET to record rCBF in these subjects while they perform a set of kinematically controlled motor sequence learning and execution tasks [82]. We found that sequence learning was impaired in p-HD subjects despite normal motor performance. Activation responses during learning were abnormally increased in the left mediodorsal thalamus and orbitofrontal cortex. Impaired learning performance in these subjects was associated with increased activation in the precuneus. These data suggest that enhanced activation of thalamocortical pathways during motor learning can compensate for striatal degeneration in p-HD. Nonetheless, this mechanism may not be sufficient to sustain a normal level of task performance, even during the presymptomatic stage of the disease.
Assessment of Experimental Therapeutics
Although effective symptomatic treatments are not yet available in HD, there are growing efforts in developing promising new therapies not only to delay the clinical onset but also to slow down the disease progression [41, 83]. This has been focusing on improving energy metabolism with agents such as coenzyme Q and neural grafting with embryonic striatum. FDG-PET can be very useful for studying the efficacy of neuroprotective and therapeutic agents. In R6/2 transgenic HD mice, for example, exponentially decreasing glucose utilization was observed in the striatum, cortex and cerebellum as a function of age [84]. A dose-dependent neuroprotective effect was evident in the striatum by comparing changes in glucose metabolism between cystamine-treated and control animals.
There is evidence that fetal striatal allografts can reverse functional deficits in phenotypic models of HD developed in primates. FDG-PET has been proved valuable in testing the transplantation strategy of striatal fetal tissue in a nonhuman primate model of HD [85]. A recent open-label pilot study has shown some clinical improvement in three out of five HD patients who received bilateral striatal grafts of fetal neurons [86]. Clinical changes in these three patients were associated with a reduction of striatal and cortical hypometabolism, indicating that grafts were able to restore the function of striato-cortical loops. Conversely, in the two patients not improved by the grafts, striatal and cortical hypometabolism progressed over the two-year follow-up. Further analysis revealed considerable heterogeneity in the anatomic and metabolic profiles of grafted tissue, both within and between HD patients.
By contrast, there has been no benefit on PET indices in a second human transplant trial in which HD patients underwent intrastriatal fetal cell grafting [87]. These patients showed widespread reductions in striatal glucose uptake with no significant change over two years. Whereas striatal D1 receptor binding did not change significantly after transplantation, there was a significant loss of D2 receptor binding. These findings may reflect loss of graft viability and/or disease progression. In addition, there was no significant relationship between changes in PET measures and clinical function. These results demonstrate the usefulness of PET imaging of brain glucose metabolism in understanding the effects of fetal grafts in patients with HD.
DBS may also be a promising alternative for the treatment of motor symptoms in HD based on its effectiveness in the neurosurgical management of PD. A pilot study with bilateral GPi DBS was performed in a HD patient with severe chorea [88]. Stimulation at 40 and 130 Hz improved chorea, but with slight worsening in bradykinesia at 130 Hz. A H2O PET activation experiment under task conditions showed increased rCBF in motor decision making and execution areas that were more evident at 40 Hz. This suggests that adjustment of stimulation parameters in GPi DBS may have the potential to optimize the motor response in HD and improve chorea without aggravating bradykinesia.
In summary, brain imaging of rCBF and rCMRglc can detect widespread functional abnormality in HD and predict the onset time of motor symptoms even during presymptomatic stages. Progressively abnormal metabolism in presymptomatic HD may be related to neuropsychological dysfunction that is known to precede the onset of motor symptoms. Combining these data with advanced analytic tools will help elucidate the functional basis for the transition from presymptomatic to symptomatic phases of the disease, and for novel treatment approaches.
Conclusion
Measurements of cerebral blood flow and glucose metabolism are valuable in assessing neuronal function and are often associated with clinical and/or behavioral changes in neurodegenerative disorders. Statistical parametric mapping and spatial covariance analysis are complementary tools for quantifying brain function in these disorders on the local and system-wide levels, respectively. In the cases of PD and HD, we have demonstrated the existence of pathological brain networks, which have proven to be reproducible across populations and within individual subjects. Biomarkers based upon these and other pathological brain networks have become simpler to quantify in patients, especially with the greater availability of clinical PET and improved noninvasive MRI-based perfusion methods. Network approaches using images of cerebral blood flow and metabolism are likely to impact on the development of novel therapies for the neurodegenerative disorders.
Acknowledgments
This work was supported by NIH RO1 NS 35069 and 37564.
References
- 1.Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the response of PET and fMRI data using multivariate linear models. NeuroImage. 1997;6:305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- 2.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuro-Image. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 3.Lin FH, McIntosh AR, Agnew JA, Eden GF, Zeffiro TA, Belliveau JW. Multivariate analysis of neuronal interactions in the generalized partial least squares framework: simulations and empirical studies. NeuroImage. 2003;20:625–642. doi: 10.1016/S1053-8119(03)00333-1. [DOI] [PubMed] [Google Scholar]
- 4.Alexander G, Moeller J. Application of the scaled subprofile model to functional imaging in neuropsychiatric disorders: a principal component approach to modeling brain function in disease. Hum Brain Mapp. 1994;2:1–16. [Google Scholar]
- 5.Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 6.Zuendorf G, Kerrouche N, Herholz K, Baron JC. Efficient principal component analysis for multivariate 3D voxel-based mapping of brain functional imaging data sets as applied to FDG-PET and normal aging. Hum Brain Mapp. 2003;18:13–21. doi: 10.1002/hbm.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: test–retest reproducibility. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600358. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, et al. Metabolic correlates of levodopa response in Parkinson’s disease. Neurology. 2001;57:2083–2088. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- 9.Trost M, Carbon M, Edwards C, Ma Y, Raymond D, Mentis MJ, et al. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann Neurol. 2002;52:853–856. doi: 10.1002/ana.10418. [DOI] [PubMed] [Google Scholar]
- 10.Scarmeas N, Habeck CG, Zarahn E, Anderson KE, Park A, Hilton J, et al. Covariance PET patterns in early Alzheimer’s disease and subjects with cognitive impairment but no dementia: utility in group discrimination and correlations with functional performance. Neuro-Image. 2004;23:35–45. doi: 10.1016/j.neuroimage.2004.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert T, Eidelberg D. Neuroimaging and therapeutics in movement disorders. NeuroRx. 2005;2:361–371. doi: 10.1602/neurorx.2.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Eidelberg D, Habeck C, Moeller J, Svensson L, Tarabula T, et al. Imaging markers of mild cognitive impairment: multivariate analysis of CBF SPECT. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.05.017. (in press) [DOI] [PubMed] [Google Scholar]
- 14.Salmon E, Kerrouche N, Herholz K, Perani D, Holthoff V, Beuthien-Baumann B, et al. Decomposition of metabolic brain clusters in the frontal variant of frontotemporal dementia. NeuroImage. 2006;30:871–878. doi: 10.1016/j.neuroimage.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Kerrouche N, Herholz K, Mielke R, Holthoff V, Baron JC. 18FDG-PET in vascular dementia: differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab. 2006;26:1213–1221. doi: 10.1038/sj.jcbfm.9600296. [DOI] [PubMed] [Google Scholar]
- 16.Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron JC, et al. ‘In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain. 2004;127:1549–1560. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- 17.Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 2004;62:1384–1390. doi: 10.1212/01.wnl.0000120541.97467.fe. [DOI] [PubMed] [Google Scholar]
- 18.Asanuma K, Ma Y, Huang C, Carbon M, Edwards C, Raymond D, et al. The metabolic pathology of dopa-responsive dystonia. Ann Neurol. 2005;57:596–600. doi: 10.1002/ana.20442. [DOI] [PubMed] [Google Scholar]
- 19.Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- 20.Detante O, Vercueil L, Thobois S, Broussolle E, Costes N, Lavenne F, et al. Globus pallidus internus stimulation in primary generalized dystonia: a H215O PET study. Brain. 2004;127:1899–1908. doi: 10.1093/brain/awh213. [DOI] [PubMed] [Google Scholar]
- 21.Yianni J, Bradley K, Soper N, O’Sullivan V, Nandi D, Gregory R, et al. Effect of GPi DBS on functional imaging of the brain in dystonia. J Clin Neurosci. 2005;12:137–141. doi: 10.1016/j.jocn.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Dhawan V, Freed C, Fahn S, Eidelberg D. PET and embryonic dopamine cell transplantation in Parkinson’s disease. In: Broderick PA, Rabni DN, Kolodny EH, editors. Bioimaging in neuro-degeneration. New Jersey: Humana Press; 2005. pp. 45–58. [Google Scholar]
- 23.Brownell AL, Canales K, Chen YI, Jenkins BG, Owen C, Livni E, et al. Mapping of brain function after MPTP-induced neurotoxicity in a primate Parkinson’s disease model. NeuroImage. 2003;20:1064–1075. doi: 10.1016/S1053-8119(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka M, Ichiya Y, Hosokawa S, Kuwabara Y, Tahara T, Fukumura T, et al. Striatal blood flow, glucose metabolism and 18F-dopa uptake: difference in Parkinson’s disease and atypical parkinsonism. J Neurol Neurosurg Psychiatry. 1991;54:898–904. doi: 10.1136/jnnp.54.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada K, Suyama N, Oguro H, Yamaguchi S, Kobayashi S. Medication-induced hallucination and cerebral blood flow in Parkinson’s disease. J Neurol. 1999;246:365–368. doi: 10.1007/s004150050364. [DOI] [PubMed] [Google Scholar]
- 26.Emborg ME, Carbon M, Holden JE, During MJ, Ma Y, Tang C, et al. Subthalamic glutamic acid decarboxylase gene therapy: changes in motor function and cortical metabolism. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600364. (in press) [DOI] [PubMed] [Google Scholar]
- 27.Otsuka M, Ichiya Y, Kuwabara Y, Hosokawa S, Sasaki M, Yoshida T, et al. Glucose metabolism in the cortical and subcortical brain structures in multiple system atrophy and Parkinson’s disease: a positron emission tomographic study. J Neurol Sci. 1996;144:77–83. doi: 10.1016/s0022-510x(96)00172-4. [DOI] [PubMed] [Google Scholar]
- 28.Antonini A, Kazumata K, Feigin A, Mandel F, Dhawan V, Margouleff C, et al. Differential diagnosis of parkinsonism with [18F]fluorodeoxyglucose and PET. Mov Disord. 1998;13:268–274. doi: 10.1002/mds.870130212. [DOI] [PubMed] [Google Scholar]
- 29.Klein RC, de Jong BM, de Vries JJ, Leenders KL. Direct comparison between regional cerebral metabolism in progressive supranuclear palsy and Parkinson’s disease. Mov Disord. 2005;20:1021–1030. doi: 10.1002/mds.20493. [DOI] [PubMed] [Google Scholar]
- 30.Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, et al. FDG-PET in the differential diagnosis of parkinsonian disorders. NeuroImage. 2005;26:912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, et al. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med. 1999;40:1264–1269. [PubMed] [Google Scholar]
- 32.Trost M, Su PC, Barnes A, Su SL, Yen RF, Tseng HM, et al. Evolving metabolic changes during the first postoperative year after subthalamotomy. J Neurosurg. 2003;99:872–878. doi: 10.3171/jns.2003.99.5.0872. [DOI] [PubMed] [Google Scholar]
- 33.Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson’s disease: FDG-PET and network analysis. Hum Brain Mapp. 2004;22:236–245. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaasinen V, Maguire RP, Hundemer HP, Leenders KL. Corticostriatal covariance patterns of 6-[18F]fluoro-L-dopa and [18F]fluorodeoxyglucose PET in Parkinson’s disease. J Neurol. 2006;253:340–348. doi: 10.1007/s00415-005-0993-7. [DOI] [PubMed] [Google Scholar]
- 35.Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, et al. Assessment of disease severity in parkinsonism with fluorine-18-fluorodeoxyglucose and PET. J Nucl Med. 1995;36:378–383. [PubMed] [Google Scholar]
- 36.Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, et al. Metabolic correlates of pallidal neuronal activity in Parkinson’s disease. Brain. 1997;120:1315–1324. doi: 10.1093/brain/120.8.1315. [DOI] [PubMed] [Google Scholar]
- 37.Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, et al. Network modulation in the treatment of Parkinson’s disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. NeuroImage. 2006;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, et al. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. Am J Psychiatry. 2002;159:746–754. doi: 10.1176/appi.ajp.159.5.746. [DOI] [PubMed] [Google Scholar]
- 40.Spetsieris P, Ma Y, Dhawan V, Moeller JR, Eidelberg D. Highly automated computer-aided diagnosis of neurological disorders using functional brain imaging. Proc SPIE Int Soc Opt Eng-Medical Imaging. 2006;6144(5M):1–12. [Google Scholar]
- 41.Feigin A, Antonini A, Fukuda M, De Notaris R, Benti R, Pezzoli G, et al. Tc-99m ethylene cysteinate dimer SPECT in the differential diagnosis of parkinsonism. Mov Disord. 2002;17:1265–1270. doi: 10.1002/mds.10270. [DOI] [PubMed] [Google Scholar]
- 42.Eckert T, Van Laere KV, Tang C, Lewis DE, Santens P, Eidelberg D. Quantification of PD-related network expression with ECD SPECT. Eur J Nucl Med Mol Imaging. 2006 doi: 10.1007/s00259-006-0261-9. (in press) [DOI] [PubMed] [Google Scholar]
- 43.Carroll TJ, Teneggi V, Jobin M, Squassante L, Treyer V, Hany TF, et al. Absolute quantification of cerebral blood flow with magnetic resonance, reproducibility of the method, and comparison with H2(15)O positron emission tomography. J Cereb Blood Flow Metab. 2002;22:1149–1156. doi: 10.1097/00004647-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Yen YF, Field AS, Martin EM, Ari N, Burdette JH, Moody DM, et al. Test–retest reproducibility of quantitative CBF measurements using FAIR perfusion MRI and acetazolamide challenge. Magn Reson Med. 2002;47:921–928. doi: 10.1002/mrm.10140. [DOI] [PubMed] [Google Scholar]
- 45.Spilt A, Box FM, van der Geest RJ, Reiber JH, Kunz P, Kamper AM, et al. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. J Magn Reson Imaging. 2002;16:1–5. doi: 10.1002/jmri.10133. [DOI] [PubMed] [Google Scholar]
- 46.Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- 47.Huang C, Feigin A, Ma Y, Eidelberg D. Imaging measures of longitudinal change in Parkinson’s disease. Neurology. 2005;64:A235. [Google Scholar]
- 48.Sestini S, Scotto di Luzio A, Ammannati F, De Cristofaro MT, Passeri A, Martini S, et al. Changes in regional cerebral blood flow caused by deep-brain stimulation of the subthalamic nucleus in Parkinson’s disease. J Nucl Med. 2002;43:725–732. [PubMed] [Google Scholar]
- 49.Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, et al. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder U, Kuehler A, Lange KW, Haslinger B, Tronnier VM, Krause M, et al. Subthalamic nucleus stimulation affects a frontotemporal network: a PET study. Ann Neurol. 2003;54:445–450. doi: 10.1002/ana.10683. [DOI] [PubMed] [Google Scholar]
- 51.Carbon M, Eidelberg D. Functional imaging of sequence learning in Parkinson’s disease. J Neurol Sci. 2006;248:72–77. doi: 10.1016/j.jns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Su PC, Ma Y, Fukuda M, Mentis MJ, Tseng HM, Yen RF, et al. Metabolic changes following subthalamotomy for advanced Parkinson’s disease. Ann Neurol. 2001;50:514–520. doi: 10.1002/ana.1232. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, et al. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease: a PET study of resting-state glucose metabolism. Brain. 2001;124:1601–1609. doi: 10.1093/brain/124.8.1601. [DOI] [PubMed] [Google Scholar]
- 54.Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. NeuroImage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- 56.Drucker-Colin R, Verdugo-Diaz L, Morgado-Valle C, Solis-Maldonado G, Ondarza R, Boll C, et al. Transplant of cultured neuron-like differentiated chromaffin cells in a Parkinson’s disease patient. A preliminary report. Arch Med Res. 1999;30:33–39. doi: 10.1016/s0188-0128(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 57.Feigin A, Tang C, Ma Y, Dhawan V, During MJ, Kaplitt MG, et al. Gene therapy for Parkinson’s disease with AAV-GAD: interim FDG-PET results. Neurology. 2006;66(5, Suppl 2) [Google Scholar]
- 58.Aylward EH, Codori AM, Rosenblatt A, Sherr M, Brandt J, Stine OC, et al. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington’s disease. Mov Disord. 2000;15:552–560. doi: 10.1002/1531-8257(200005)15:3<552::AID-MDS1020>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 59.Rosas HD, Goodman J, Chen YI, Jenkins BG, Kennedy DN, Makris N, et al. Striatal volume loss in HD as measured by MRI and the influence of CAG repeat. Neurology. 2001;57:1025–1028. doi: 10.1212/wnl.57.6.1025. [DOI] [PubMed] [Google Scholar]
- 60.Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, et al. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125:1815–1818. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- 61.Antonini A, Leenders KL, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington’s disease. Brain. 1996;119:2085–2095. doi: 10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 62.Weeks RA, Piccini P, Harding AE, Brooks DJ. Striatal D1 and D2 dopamine receptor loss in asymptomatic mutation carriers of Huntington’s disease. Ann Neurol. 1996;40:49–54. doi: 10.1002/ana.410400110. [DOI] [PubMed] [Google Scholar]
- 63.van Oostrom JC, Maguire RP, Verschuuren-Bemelmans CC, Veenmavan der Duin L, Pruim J, Roos RA, et al. Striatal dopamine D2 receptors, metabolism, and volume in preclinical Huntington disease. Neurology. 2005;65:941–943. doi: 10.1212/01.wnl.0000176071.08694.cc. [DOI] [PubMed] [Google Scholar]
- 64.Backman L, Robins-Wahlin TB, Lundin A, Ginovart N, Farde L. Cognitive deficits in Huntington’s disease are predicted by dopaminergic PET markers and brain volumes. Brain. 1997;120:2207–2217. doi: 10.1093/brain/120.12.2207. [DOI] [PubMed] [Google Scholar]
- 65.Antonini A, Leenders KL, Eidelberg D. [11C]raclopride-PET studies of the Huntington’s disease rate of progression: relevance of the trinucleotide repeat length. Ann Neurol. 1998;43:253–255. doi: 10.1002/ana.410430216. [DOI] [PubMed] [Google Scholar]
- 66.Andrews TC, Weeks RA, Turjanski N, Gunn RN, Watkins LH, Sahakian B, et al. Huntington’s disease progression. PET and clinical observations. Brain. 1999;122:2353–2363. doi: 10.1093/brain/122.12.2353. [DOI] [PubMed] [Google Scholar]
- 67.Pavese N, Andrews TC, Brooks DJ, Ho AK, Rosser AE, Barker RA, et al. Progressive striatal and cortical dopamine receptor dysfunction in Huntington’s disease: a PET study. Brain. 2003;126:1127–1135. doi: 10.1093/brain/awg119. [DOI] [PubMed] [Google Scholar]
- 68.Araujo DM, Cherry SR, Tatsukawa KJ, Toyokuni T, Kornblum HI. Deficits in striatal dopamine D(2) receptors and energy metabolism detected by in vivo microPET imaging in a rat model of Huntington’s disease. Exp Neurol. 2000;166:287–297. doi: 10.1006/exnr.2000.7514. [DOI] [PubMed] [Google Scholar]
- 69.Kuwert T, Noth J, Scholz D, Schwarz M, Lange HW, Topper R, et al. Comparison of somatosensory evoked potentials with striatal glucose consumption measured by positron emission tomography in the early diagnosis of Huntington’s disease. Mov Disord. 1993;8:98–106. doi: 10.1002/mds.870080118. [DOI] [PubMed] [Google Scholar]
- 70.Hayden MR, Martin WR, Stoessl AJ, Clark C, Hollenberg S, Adam MJ, et al. Positron emission tomography in the early diagnosis of Huntington’s disease. Neurology. 1986;36:888–894. doi: 10.1212/wnl.36.7.888. [DOI] [PubMed] [Google Scholar]
- 71.Young AB, Penney JB, Starosta-Rubinstein S, Markel DS, Berent S, Giordani B, et al. PET scan investigations of Huntington’s disease: cerebral metabolic correlates of neurological features and functional decline. Ann Neurol. 1986;20:296–303. doi: 10.1002/ana.410200305. [DOI] [PubMed] [Google Scholar]
- 72.Berent S, Giordani B, Lehtinen S, Markel D, Penney JB, Buchtel HA, et al. Positron emission tomographic scan investigations of Huntington’s disease: cerebral metabolic correlates of cognitive function. Ann Neurol. 1988;23:541–546. doi: 10.1002/ana.410230603. [DOI] [PubMed] [Google Scholar]
- 73.Kuwert T, Lange HW, Langen KJ, Herzog H, Aulich A, Feinendegen LE. Cortical and subcortical glucose consumption measured by PET in patients with Huntington’s disease. Brain. 1990;113:1405–1423. doi: 10.1093/brain/113.5.1405. [DOI] [PubMed] [Google Scholar]
- 74.Grafton ST, Mazziotta JC, Pahl JJ, St George-Hyslop P, Haines JL, Gusella J, et al. Serial changes of cerebral glucose metabolism and caudate size in persons at risk for Huntington’s disease. Arch Neurol. 1992;49:1161–1167. doi: 10.1001/archneur.1992.00530350075022. [DOI] [PubMed] [Google Scholar]
- 75.Martin WR, Clark C, Ammann W, Stoessl AJ, Shtybel W, Hayden MR. Cortical glucose metabolism in Huntington’s disease. Neurology. 1992;42:223–229. doi: 10.1212/wnl.42.1.223. [DOI] [PubMed] [Google Scholar]
- 76.Sax DS, Powsner R, Kim A, Tilak S, Bhatia R, Cupples LA, et al. Evidence of cortical metabolic dysfunction in early Huntington’s disease by single-photon-emission computed tomography. Mov Disord. 1996;11:671–677. doi: 10.1002/mds.870110612. [DOI] [PubMed] [Google Scholar]
- 77.Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47:215–222. [PubMed] [Google Scholar]
- 78.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, Feigin A, Rachakonda S, Dhawan V, Eidelberg D. Evolution of metabolic brain networks in presymptomatic Huntington’s disease: a longitudinal PET study. J Nucl Med. 2006;47:209p. [Google Scholar]
- 80.Feigin A, Leenders KL, Moeller JR, Missimer J, Kuenig G, Spetsieris P, et al. Metabolic network abnormalities in early Huntington’s disease: an [(18)F]FDG-PET study. J Nucl Med. 2001;42:1591–1595. [PubMed] [Google Scholar]
- 81.Ma Y, Feigin A, Okulski J, Dhawan V, Chaly T, Eidelberg D. Implementation of atrophy correction in metabolic mapping studies of presymptomatic Huntington’s disease. J Cereb Blood Flow Metab. 2003:S629. [Google Scholar]
- 82.Feigin A, Ghilardi MF, Huang C, Ma Y, Carbon M, Guttman M, et al. Preclinical Huntington’s disease: compensatory brain responses during learning. Ann Neurol. 2006;59:53–59. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hersch SM. Huntington’s disease: prospects for neuroprotective therapy 10 years after the discovery of the causative genetic mutation. Curr Opin Neurol. 2003;16:501–506. doi: 10.1097/01.wco.0000084229.82329.03. [DOI] [PubMed] [Google Scholar]
- 84.Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, et al. Cerebral PET imaging and histological evidence of trans-glutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntington’s disease. J Neurol Sci. 2005;231:57–66. doi: 10.1016/j.jns.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Schumacher JM, Hantraye P, Brownell AL, Riche D, Madras BK, Davenport PD, et al. A primate model of Huntington’s disease: functional neural transplantation and CT-guided stereotactic procedures. Cell Transplant. 1992;1:313–322. doi: 10.1177/096368979200100409. [DOI] [PubMed] [Google Scholar]
- 86.Gaura V, Bachoud-Levi AC, Ribeiro MJ, Nguyen JP, Frouin V, Baudic S, et al. Striatal neural grafting improves cortical metabolism in Huntington’s disease patients. Brain. 2004;127:65–72. doi: 10.1093/brain/awh003. [DOI] [PubMed] [Google Scholar]
- 87.Furtado S, Sossi V, Hauser RA, Samii A, Schulzer M, Murphy CB, et al. Positron emission tomography after fetal transplantation in Huntington’s disease. Ann Neurol. 2005;58:331–337. doi: 10.1002/ana.20564. [DOI] [PubMed] [Google Scholar]
- 88.Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, et al. Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol. 2004;56:290–294. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]