Abstract
The striatonigral projection is a striatal output pathway critical to motor control, cognition, and emotion regulation. Its axon terminals in the substantia nigra pars reticulata (SNr) express a high level of serotonin (5-HT) type 1B receptors (5-HT1BRs), whereas the SNr also receives an intense 5-HT innervation that expresses 5-HT transporters, providing an anatomic substrate for 5-HT and selective 5-HT reuptake inhibitor (SSRI)-based antidepressant treatment to regulate the striatonigral output. In this article we show that 5-HT, by activating presynaptic 5-HT1BRs on the striatonigral axon terminals, potently inhibited the striatonigral GABA output, as reflected in the reduction of the striatonigral inhibitory postsynaptic currents in SNr GABA neurons. Functionally, 5-HT1BR agonism reduced the striatonigral GABA output-induced pause of the spontaneous high-frequency firing in SNr GABA neurons. Equally important, chronic SSRI treatment with fluoxetine enhanced this presynaptic 5-HT1BR-mediated pause reduction in SNr GABA neurons. Taken together, these results indicate that activation of the 5-HT1BRs on the striatonigral axon terminals can limit the motor-promoting GABA output. Furthermore, in contrast to the desensitization of 5-HT1 autoreceptors, chronic SSRI-based antidepressant treatment sensitizes this presynaptic 5-HT1BR-mediated effect in the SNr, a novel cellular mechanism that alters the striatonigral information transfer, potentially contributing to the behavioral effects of chronic SSRI treatment.
Keywords: basal ganglia, medium spiny neuron, selective serotonin reuptake inhibitor, 5-HT1B receptor sensitization, substantia nigra pars reticulata
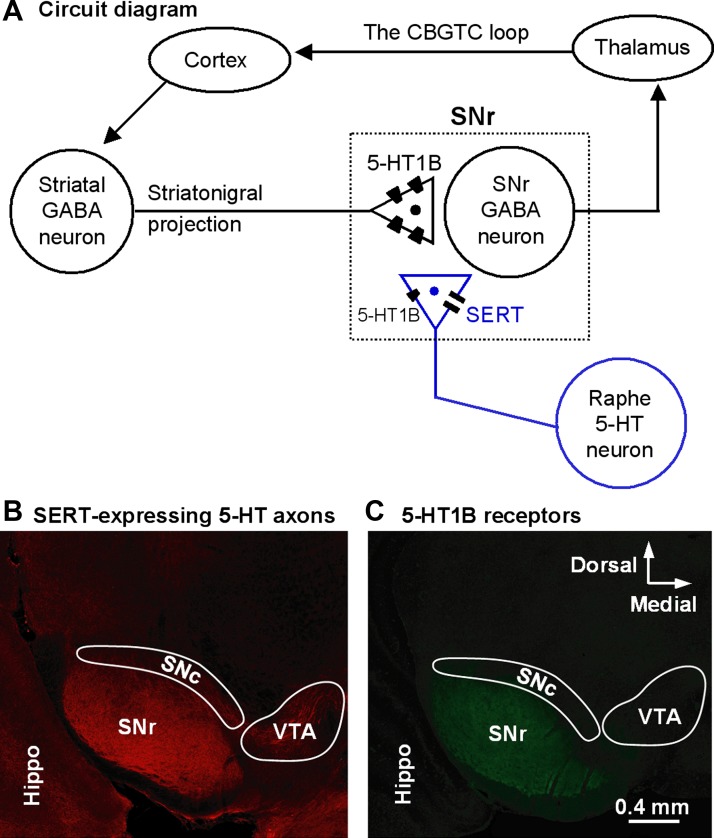
the substantia nigra pars reticulata (SNr) is a critical node in the vast cortico-basal ganglia-thalamo-cortical (CBGTC) loop (Fig. 1A) (Haber 2003; Hikosaka 2007) that is important to motor control (Friend and Kravitz 2014) and also to cognition (Simpson et al. 2010), emotion (Marchand et al. 2012; Révy et al. 2014), and learning and habit formation (Graybiel and Smith 2014; Sesack and Grace 2010). A main characteristic of the SNr is that its GABA projection neurons fire autonomous high-frequency spikes that are paused by the massive GABA input from the striatonigral projection (Hikosaka et al. 2000, 2014; Sano et al. 2013; Schultz 1986; Zhou and Lee 2011). Another prominent anatomic feature of the SNr is its dense serotonin (5-HT) innervation, originated in the dorsal raphe (Fig. 1B) (Moukhles et al. 1997; Parent et al. 2011). 5-HT neurons fire tonic spikes at 1–2 Hz, likely leading to spontaneous tonic 5-HT release and an ambient 5-HT level in the SNr (Jacobs and Azmitia 1992). Matching the heavy 5-HT innervation, the SNr has an expression of 5-HT type 1B receptors (5-HT1BRs) on the striatonigral axon terminals that is the highest in the brain (Fig. 1C) (Boschert et al. 1994; Ding and Zhou 2014; Maroteaux et al. 1992; Riad et al. 2000; Sari, 2004; Voigt et al. 1991). 5-HT1BRs are commonly coupled to the Gi/o protein and are inhibitory (Hannon and Hoyer 2008). Thus there is an anatomic basis for 5-HT to inhibit striatonigral GABA output.
Fig. 1.
Serotonin (5-HT) innervation and 5-HT type 1B receptor (5-HT1BR) expression in the substantia nigra pars reticulata (SNr). A: circuit diagram showing the position of the striatonigral projection in the cortico-basal ganglia-thalamo-cortical (CBGTC) loop. B: strikingly dense 5-HT innervation in the SNr revealed by serotonin transporter (SERT) immunostaining in coronal brain sections. C: relatively dense 5-HT1BR expression in the SNr revealed by 5-HT1BR immunostaining in coronal brain sections. As described in materials and methods, B and C show results obtained with different staining methods in different tissue sections from two 50-day-old male mice. These images illustrate the point that the SNr had an exceptionally dense 5-HT innervation and a relatively heavy expression of 5-HT1BRs in our experimental mice. Hippo, hippocampus; SNc, substantia nigra pars compacta; VTA, ventral tegmental area.
Another key feature of the SNr is the densely expressed 5-HT transporters (SERTs) on the 5-HT axons (Fig. 1B). SERTs are the target of selective 5-HT reuptake inhibitor (SSRI)-based antidepressant treatment (Wong et al. 2005). SSRIs block SERT-mediated 5-HT reuptake and increase the extracellular 5-HT level (Wong et al. 2005). Chronic increase in the extracellular 5-HT level can desensitize the 5-HT1A and 5-HT1B autoreceptors in 5-HT neurons and their axon terminals via diminished receptor-G protein coupling (Castro et al. 2003; Cornelisse et al. 2007; Hensler 2002) or receptor internalization (Descarries and Riad 2012), thus contributing to their antidepressant effect (Blier and El Mansari 2013), although the precise neuronal mechanisms are not established for either depression or antidepressant treatment.
To our knowledge, although studies have examined the molecular and behavioral aspects of 5-HT1BRs in the brain (Zhuang et al. 1999; Woehrle et al. 2013), neurophysiological studies on chronic SSRI effects on presynaptic 5-HT1 heteroreceptors on non-5-HT axon terminals are lacking, and currently there are no data on the potential chronic SSRI treatment-induced alteration of the 5-HT1BRs on the striatonigral axon terminals. Given the importance of the nigral node in the emotion-regulating cortico-basal ganglia-thalamo-cortical (CBGTC) loop (Marchand et al. 2012), it is critical to understand how chronic SSRI treatment affects these 5-HT1BRs and the functional consequences. Based on the prevailing idea that 5-HT1 autoreceptors are desensitized following chronic SSRI-type antidepressant (Blier and El Mansari 2013; Descarries and Riad 2012; Newman et al. 2004), we initially reasoned that chronic SSRI treatment may desensitize 5-HT1BRs on the striatonigral axon terminals; our data, however, indicate a substantial sensitization, a novel finding that is the opposite of the changes for 5-HT1 autoreceptors reported in the literature.
MATERIALS AND METHODS
Electrophysiology
Preparation of brain slices.
Wild-type 25- to 35-day-old male and female C57BL mice were used. Equal numbers of male and female mice were used; no difference in 5-HT effects was noticed and data were pooled. Young mice were used because brain slices from young animals survive tissue sectioning better and thus facilitate recording. We have determined that the baseline 5-HT1B effect was stable during this period. Additionally, it has been reported that in rodents, the 5-HT system develops rapidly, reaching adult levels at postnatal day 21 (Murrin et al. 2007), and SSRIs produced antidepressant-like effects in 21-day old juvenile rats (Reed et al. 2008). Furthermore, depression is a common psychiatric disorder in children and adolescents, and SSRIs are the common pharmacological treatment (Birmaher 2014; Kennard et al. 2014; Kessler et al. 2001; March et al. 2004). Thus data from juvenile and adolescent animals are important.
All procedures were carried out in accordance with Institutional Animal Care and Use Committee of The University of Tennessee Health Science Center (UTHSC) and followed the guidelines of the National Institutes of Health. The procedure to prepare 15° angular sagittal slices containing the SNr, the external globus pallidus (GPe), and the striatum (Fig. 2A) has been described in detail (Connelly et al. 2010; Ding et al. 2013, 2015). Briefly, mice were killed by decapitation under deep anesthesia, and brains were quickly dissected out and immediately immersed in an oxygenated ice-cold cutting solution containing (in mM) 220 glycerol, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 20 d-glucose. Parasagittal slices 300 μm thick were cut on a Leica Zero Z VT1200S vibratome (Leica Microsystems, Wetzlar, Germany) at a 15° angle to the sagittal plane using a precision metal wedge. The brain slices were transferred to a holding chamber filled with a standard artificial cerebrospinal fluid (ACSF; in mM: 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2, and 10 d-glucose) that was kept at 34°C and continuously bubbled with 95% O2 and 5% CO2. After an initial 30-min incubation at 34°C, the brain slices were kept at room temperature (25°C). Ascorbic acid (0.4 mM) was added to all brain slice-bathing solutions, including the cutting solution to protect the tissue (Rice 1999).
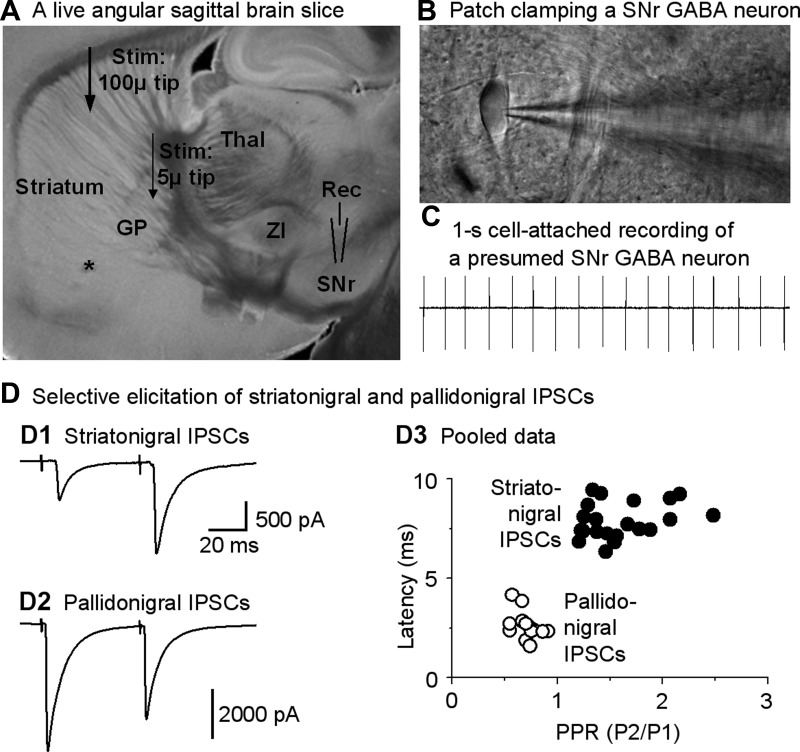
Fig. 2.
Focal stimulation in the striatum or globus pallidus (GP) evokes relatively pure striatonigral and pallidonigral inhibitory postsynaptic currents (IPSCs) in SNr GABA neurons. A: image of a live, 15° angular sagittal brain slice taken with a ×1 objective. The SNr, GP, and striatum are clearly identifiable. Other structures such as the thalamus (Thal) and zona incerta (ZI) are also clearly visible, as marked. B: image taken under a ×60 objective shows a typical SNr GABA neuron being patch-clamped in cell-attached mode. C: typical spontaneous spikes (action potentials, ∼17 Hz) in a presumed SNr GABA neuron recorded in cell-attached mode. D: independent elicitation of striatonigral IPSCs and pallidonigral IPSCs. D1 and D2: example traces of striatum-evoked typical striatonigral facilitating IPSCs and GP-evoked depressing pallidonigral IPSCs, respectively. Timescale applies to both striatonigral IPSCs and pallidonigral IPSCs. D3: pooled data for paired-pulse ratios (PPR; P1 and P2, pulse 1 and pulse 2) and latencies of the first evoked IPSCs induced by a paired-pulse protocol with an interval of 50 ms for striatonigral IPSCs and pallidonigral IPSCs. For striatonigral IPSC data, n = 26 cells from 26 brain slices of 22 mice; for pallidonigral IPSC data, n = 11 cells from 11 brain slices of 11 mice.
Fluoxetine treatment.
Fluoxetine hydrochloride was dissolved in distilled and autoclaved water and then administered subcutaneously with a daily dose of 20 mg/kg. Briefly, starting on postnatal day 15, male and female C57BL mice received 2 subcutaneous doses of 10 mg/kg fluoxetine at 8:00 AM and 5:00 PM. Injection of 0.9% NaCl saline served as control. Mice were killed at 10:00 AM on the appropriate treatment days. For 1-day treatment to test the acute effect of fluoxetine, mice received the first fluoxetine injection at 5:00 PM the day before and the second fluoxetine injection at 8:00 AM on the day of experiment and were killed 2 h later. To test the effect of chronic fluoxetine treatment, mice received fluoxetine treatment for up to 20 days; the effects during 10–20 days were similar, and the data from these treatment days were pooled. The daily 20 mg/kg fluoxetine dose was chosen because literature reports indicate that 5–30 mg·kg−1·day−1 fluoxetine produces antidepressant-like effects (Cryan et al. 2005) and also biochemical changes in 5-HT1AR and 5-HT1BR signaling pathways (Hensler 2002; Le Poul et al. 2000; Pejchal et al. 2002; Shen et al. 2002). This dose did not affect the body weight of the mice. A protocol of two subcutaneous injections per day was chosen because the blood half-life of fluoxetine in rodents is only about 6 h, whereas it is 12 h in nonhuman primates and 2 days in humans (Ansorge et al. 2008; Czachura and Rasmussen 2000; Hirano et al. 2005; Sawyer and Howell 2011).
Whole cell patch-clamp recording.
Slices were placed in a recording chamber mounted on the microscope stage and continuously perfused at 2 ml/min with the standard ACSF saturated with 95% O2 and 5% CO2. Recordings were made at 30°C (TC 324B temperature controller; Warner Instruments) under visual guidance of a video microscope (an Olympus BX51WI and a Zeiss Axiocam MRm digital camera) equipped with Nomarski optics and a ×60 water-immersion lens (Fig. 2B). Patch pipettes were pulled from borosilicate glass capillary tubing (KG-33, 1.1-mm inner diameter, 1.65-mm outer diameter; King Precision Glass, Claremont, CA) using a PC-10 puller (Narishige, Tokyo, Japan) and had resistances of 1.5–2.5 MΩ when filled with one of the following intracellular solutions. A KCl-based intracellular solution (in mM: 135 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine, pH 7.25, 280–290 mosM) was used to record inhibitory postsynaptic currents (IPSCs) in voltage-clamp recording mode. To record hyperpolarizing inhibitory postsynaptic potentials (IPSPs) in current-clamp mode, we used a KMeSO4-based intracellular solution (in mM: 130 KMeSO4, 5 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine, pH 7.25, 280–290 mosM). When evoked IPSCs were recorded, the Na+ channel blocker lidocaine N-ethyl bromide (5 mM) was added to the intracellular solution to block Na+ spikes. All recordings were made in the presence of 10 μM 6,7-dinitroquinoxaline-2,3-dione and 20 μM 2-amino-5-phosphonovaleric acid to block ionotropic glutamate receptors.
Electrical stimulation to evoke synaptic responses.
To evoke striatonigral IPSCs, a bipolar tungsten electrode (Microprobes, Gaithersburg, Maryland) was placed in the striatum. A paired pulse with an interpulse interval of 50 ms was generated by a Master-8 pulse generator (A.M.P.I., Jerusalem, Israel) and delivered at 0.05 Hz. The stimulation intensity was from 50 to 300 μA at a constant duration of 0.2 ms. To evoke pallidonigral IPSCs, a minimal stimulation method was used to minimize the activation of the passing striatonigral axons. Briefly, a theta capillary pipette with a tip diameter of 5 μm was placed in the GPe. Paired pulses with an inter pulse interval of 50 ms were delivered at 0.05 Hz. The stimulation intensities ranging from 10 to 50 μA were first adjusted to elicit all-or-none IPSCs with 20–50% failure rate. The stimulation intensities were then increased a few microamperes to decrease the failure rate to <10%.
Data acquisition and analysis.
A Multiclamp 700B amplifier, pClamp 9.2 software, and Digidata 1322A interface (Molecular Devices, Sunnyvale, CA) were used to acquire data. For voltage-clamp recording, cells were held at −70 mV, leading to inward IPSCs with our KCl-based intracellular solution. Access resistance was monitored by a 10-mV, 50-ms pulse before every evoked IPSC. Cells in which the access resistance increased by >15% were discarded. Liquid junction potentials were not corrected because our experimental questions did not have any voltage dependence.
The peak IPSC amplitudes were measured using Clampfit 9.2 software. Averages of 10 consecutive IPSCs before or during drug administration were used to evaluate baseline and drug response, respectively. Paired-pulse ratio (PPR) was calculated by dividing the peak amplitude of the second IPSCs by the peak amplitude of the first IPSCs. To analyze the effect of a train of IPSPs on spontaneous firing of SNr GABA neuron, the spike number during the 300 ms immediately after the first stimulation artifact was measured.
To obtain the IC50, we fitted the averaged data points in the dose-response plot to the Hill equation: Y = A·Xn[1/(Kn + Xn)], where A is the maximal inhibition, X is the 5-HT concentration, and K is the IC50.
Drugs.
All drugs used in electrophysiology experiments were made into stock solutions in ddH2O or dimethyl sulfoxide. Stock solutions of drugs were diluted at least 1:1,000 to the desired concentration in ACSF immediately before their application. dl-2-Amino-5-phosphonovalerate, 6-cyano-7-nitroquinoxaline-2,3-dione, lidocaine N-ethyl bromide (QX-314), 5-HT, picrotoxin, CP93129, and NAS-181 were purchased from either Tocris or Sigma-Aldrich. Drugs were bath-applied. Fluoxetine hydrochloride was also obtained from the National Institute of Mental Health Chemical Synthesis and Drug Supply Program.
Immunohistochemistry
Serotonin transporter immunostain.
Under overdose urethane anesthesia, 40- to 50-day-old mice (n = 3) were intracardially perfused with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde dissolved in PBS. The brains were further postfixed in the same 4% paraformaldehyde at 4°C overnight. Coronal brain sections (50 μm thick) were cut on a Leica VT1200S vibratome. Free-floating sections were incubated with 2% fat-free milk, 1% bovine serum albumin (BSA), and 0.4% Triton X-100 in PBS for 1 h at room temperature (RT) to block nonspecific binding and permeabilize the cell membrane, respectively. After thorough rinsing, the free-floating sections were incubated for 48 h at 4°C with the primary antibody (see below) and then rinsed in PBS three times for 5 min each, followed by incubation with the secondary antibody (see below) for 3 h at RT in the dark. Both the primary and secondary antigen-antibody reactions occurred in PBS containing 3% normal donkey serum, 1% BSA, and 0.1% Triton X-100. The primary antibody was a polyclonal SERT antibody raised in goat (Santa Cruz Biotechnology; diluted at 1:800). The secondary antibody was a donkey anti-goat IgG antibody conjugated with red Alexa Fluor 568 (diluted at 1:200). At the age of the mice used here, SERT immunostain reliably labels SERT-expressing 5-HT axon terminals (Nielsen et al. 2006).
5-HT1BR immunostain.
Under overdose urethane anesthesia, mice were intracardially perfused with PBS. For this particular antigen and antibody pair, we used the following procedure to enhance the detection of 5-HT1BR protein. The brains were quickly dissected out and immediately frozen and stored at −80°C. Brains were sectioned on a Leica cryostat at 20-μm thickness. The sections were collected onto Superfrost Plus microscope slides (Fisher Scientific) and stored at −80°C until use. To process the tissue sections for 5-HT1BR immunostain, the slides were removed from −80°C, quickly thawed, air-dried, and lightly fixed in 4% paraformaldehyde for 6 min, then in Bouin's solution for 5 min, and finally in 80% ethanol for 5 min. These sections were then incubated in 2% fat-free milk, 1% BSA, and 0.4% Triton X-100 in PBS for 1 h at RT to block nonspecific binding and permeabilize the cell membrane, respectively. After thorough rinsing, the sections were incubated for 48 h at 4°C with the primary antibody (see below) and then rinsed in PBS three times for 5 min each, followed by incubation with the secondary antibody (see below) for 3 h at RT in the dark. Both the primary and secondary antigen-antibody reactions occurred in PBS containing 3% normal donkey serum, 1% BSA, and 0.2% Triton X-100. The primary antibody was a polyclonal 5-HT1BR antibody raised in rabbit (Santa Cruz Biotechnology; diluted at 1:1,000). The secondary antibody was donkey anti-rabbit IgG antibody conjugated with green Alexa Fluor 488 (Invitrogen; diluted at 1:200).
Fluorescence images were acquired on a Zeiss 710 laser scanning confocal microscope at the UTHSC Neuroscience Imaging Center (Memphis, TN).
Statistics
Data are means ± SE. The paired t-test was used to make comparisons of evoked events before and during drug administration. Unpaired t-test, one-way ANOVA, and two-way ANOVA were also used when appropriate. When two-way ANOVA was used, drug treatment and dose of 5-HT were the independent variables. A P value <0.05 was considered statistically significant.
RESULTS
Independent Elicitation of Striatonigral and Pallidonigral IPSCs
The main neuron type in the SNr is the GABA projection neurons that fire spontaneously around 10 Hz under in vitro conditions (Atherton and Bevan 2005; Ding et al. 2011a, 2011b; Zhou and Lee 2011). The SNr also contains sparse dopamine neurons that fire spontaneously around 1.5 Hz under similar in vitro conditions (Connelly et al. 2010; Ding et al. 2011a, 2011b; Zhou et al. 2006). Therefore, we first briefly recorded action potentials of neurons in SNr in a cell-attached mode in our angular sagittal brain slices (Fig. 2, A–C). Neurons in the SNr with a spontaneous firing rate ≥5 Hz were presumed to be GABA neurons (Connelly et al. 2010; Ding et al. 2011a, 2011b; Zhou et al. 2006). After cell identification, we proceeded to whole cell recording mode and started striatal or GPe stimulation to activate striatonigral or pallidonigral GABAergic transmission, respectively. As shown in Fig. 2D, focal stimulation in the striatum consistently evoked IPSCs in SNr GABA neurons. These evoked IPSCs and also the spontaneous IPSCs were blocked by bath-applied picrotoxin (100 μM), indicating that these IPSCs were mediated by GABAA receptors, as expected. Focal GPe minimal stimulation also consistently evoked IPSCs in SNr GABA neurons that were blocked by bath-applied 100 μM picrotoxin, too.
We also used low-intensity stimuli and a paired-pulse protocol to ensure that our focal stimulation in the dorsal striatum and in the GPe evoked isolated striatonigral IPSCs and pallidonigral IPSCs, respectively. We found that in our sampled cells, as shown in Fig. 2D, the latency was 7.8 ± 0.2 ms (n = 23 cells) for the striatum-evoked IPSCs and 2.5 ± 0.2 ms (n = 11) for GPe-evoked IPSCs (unpaired t-test, P < 0.000 for the 2 latency values). The PPR was 1.6 ± 0.1 (n = 23 cells) for the striatum-evoked IPSCs and 0.7 ± 0.1 (n = 11 cells) for GPe-evoked IPSCs (unpaired t-test, P < 0.000 for the 2 PPR values). These are consistent with data showing that the striatonigral IPSC has a long latency (∼7 ms, due to the long-distance pathway) and a facilitating PPR (∼2), whereas pallidonigral IPSC had a short latency (∼2 ms) and a depressing PPR (∼0.7) (Connelly et al. 2010; Ding et al. 2015). Thus, under our experimental conditions, the striatum-evoked IPSCs in SNr GABA neurons were predominantly striatonigral IPSCs, whereas the GPe-evoked IPSCs in SNr GABA neurons were predominantly pallidonigral IPSCs. This allowed us to study 5-HT effects on these relatively pure striatonigral IPSCs and pallidonigral IPSCs.
5-HT Inhibits Striatonigral IPSCs via a Presynaptic Mechanism
After obtaining a stable baseline recording of striatonigral IPSCs, we started bath application of 5-HT. We initially used 10 μM 5-HT and found that it reduced the striatonigral IPSC amplitude by a profound 94% on the average (Fig. 3A). By using 5-HT concentrations ranging 0.5 to 20 μM, we determined that 5-HT inhibited striatonigral IPSCs in a dose-response manner with an IC50 of 1.2 μM and a Hill coefficient of 1.3, based on fitting the data points to the Hill equation (Fig. 3, A and C). In contrast, bath application of 0.5 to 20 μM 5-HT did not significantly affect pallidonigral IPSCs (Fig. 3, B and C). These results indicate that 5-HT can potently inhibit the striatonigral GABAergic transmissions while having no effect on the pallidonigral GABAergic transmissions.
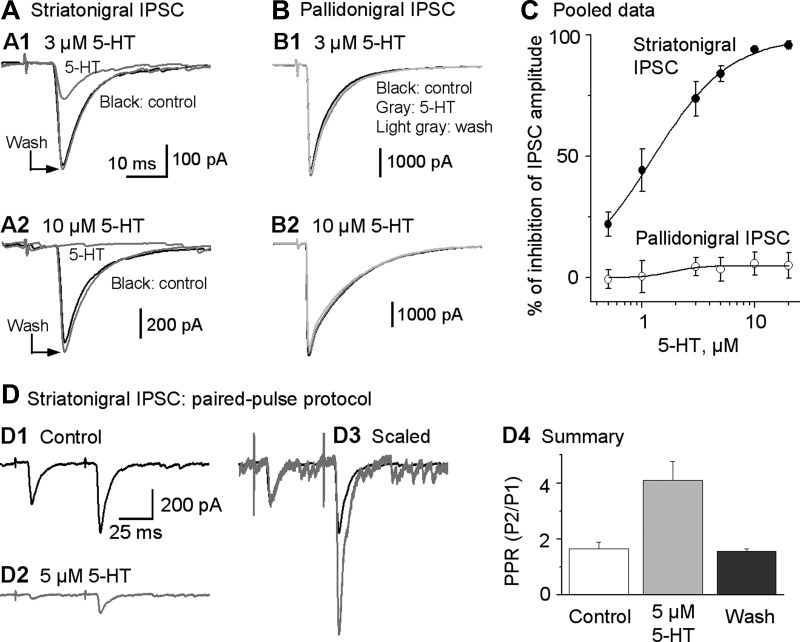
Fig. 3.
5-HT strongly inhibits striatonigral IPSCs but does not affect pallidonigral IPSCs. A1 and A2: example traces of striatonigral IPSCs affected by bath application of 3 and 10 μM 5-HT, respectively. B1 and B2: example traces of pallidonigral IPSCs not affected by bath application of 3 and 10 μM 5-HT, respectively. C: dose-response relationship of the 5-HT inhibition of striatonigral IPSCs and pallidonigral IPSCs. The continuous line is the Hill equation fit. Each data point represents 6–8 experiments. For striatonigral IPSC data, n = 27 neurons from 27 brain slices of 25 mice; for pallidonigral IPSC data, n = 15 neurons from 15 brain slices of 14 mice. D: 5-HT increases PPR of striatonigral IPSCs. D1 and D2: example traces of striatonigral paired IPSCs (evoked by 2 stimuli 50 ms apart) before (control) and during bath application of 5 μM 5-HT, respectively. D3: the same traces from D1 and D2 scaled to the peak of their 1st IPSC to show the relative increase of the 2nd IPSC peak. D4: summary showing a clear 5-HT-induced increase in PPR of striatonigral paired IPSCs.
Next, we determined if 5-HT was reducing the striatonigral IPSC amplitude by a presynaptic mechanism. To answer this question, we used a paired-pulse protocol with a pair of stimulating pulses separated by 50 ms (20 Hz). This frequency was chosen because 20 Hz is a common firing frequency when the striatonigral neurons are phasically active in freely moving mice (Miller et al. 2008; unpublished data, Sagot B and Zhou F.-M.). Using this paired-pulse protocol, we found that under control conditions, striatonigral IPSCs exhibited a paired-pulse facilitation with a PPR value of 1.66 ± 0.22 (Fig. 3, D1 and D4). During bath application of 5 μM 5-HT, the peak amplitude of the first of the paired striatonigral IPSCs was reduced to a larger extent than that of the second IPSC, thus increasing the PPR from 1.66 ± 0.22 under control conditions to 4.10 ± 0.67 during 5-HT application (P < 0.05, paired t-test, n = 6; Fig. 3, D2–D4). Because an increased PPR is an indication of inhibition of presynaptic vesicular release (Fioravante and Regehr 2011), these results suggest that 5-HT inhibits striatonigral IPSCs by acting presynaptically to inhibit transmitter release. To further support our conclusion of presynaptic 5-HT inhibition of GABA release of the striatonigral axon terminals, we calculated the coefficient of variation (CV) of the striatonigral IPSCs by dividing the standard deviation by the mean of the 10 or more individual IPSCs, as described in Michaeli and Yaka (2010). Under control conditions, the baseline CV was 0.43 ± 0.08 (n = 6 cells); during 5 μM 5-HT application, the CV was increased to 0.78 ± 0.05. The increase in CV was significant with P < 0.05 (paired t-test), indicating a decreased vesicular release probability during application of 5 μM 5-HT application.
5-HT1B Agonism Mimics and 5-HT1B Antagonism Blocks 5-HT Effects on Striatonigral IPSCs
Next, we asked this question: Are presynaptic 5-HT1BRs mediating the inhibitory effect of 5-HT on striatonigral IPSCs? To answer this question, we evoked striatonigral IPSCs with the same paired-pulse protocol described above and bath-applied 1 μM CP93129, a 5-HT1BR agonist. As shown in Fig. 4A, after bath application of 1 μM CP93129, the peak amplitude of the first of the paired striatonigral IPSCs was reduced to 11.0 ± 3.0% of control, a 89% reduction (P < 0.000, paired t-test, n = 6). Similar to the effect of 5-HT, 1 μM CP93129 significantly increased the PPR from 1.67 ± 0.21 to 4.08 ± 0.72 (P < 0.05, paired t-test, n = 6), indicating a presynaptic 5-HT1BR-mediated inhibition of vesicular GABA release. This is consistent with the literature reporting that 5-HT1BR activation decreases vesicular transmitter release (Mizutani et al. 2006).
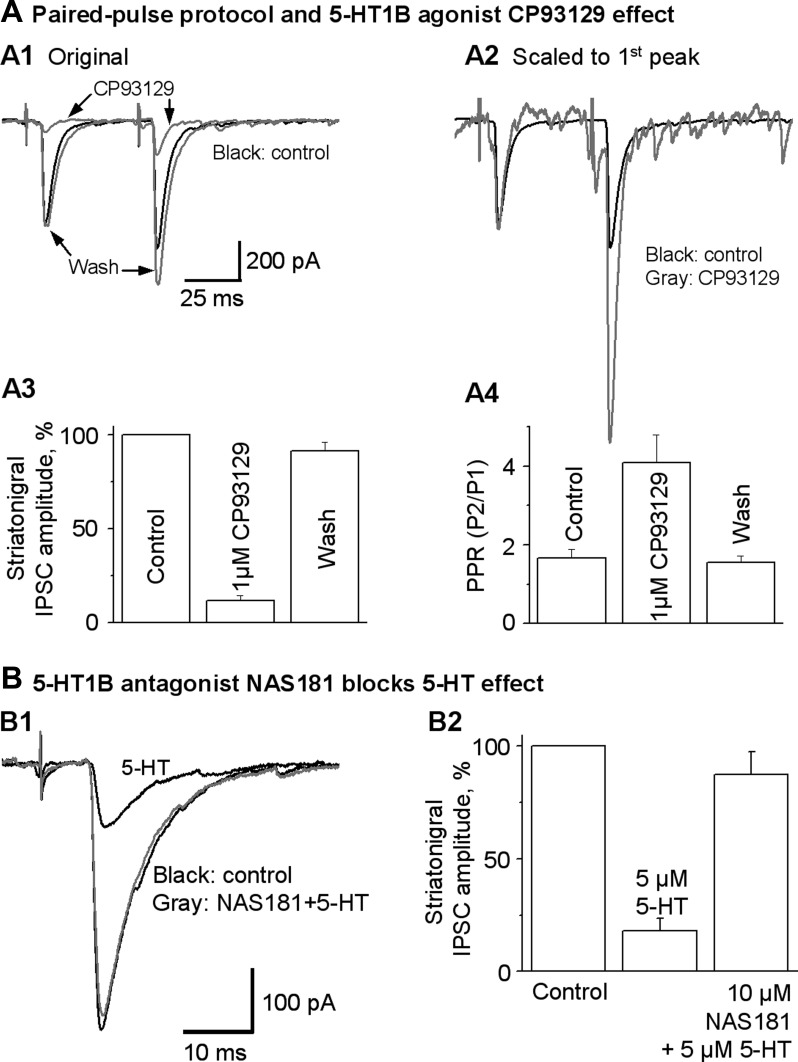
Fig. 4.
5-HT1BR agonist CP93129 mimics and 5-HT1BR antagonist NAS-181 inhibits the 5-HT effects on striatonigral IPSCs. A1: superimposed averaged striatonigral IPSCs evoked by the paired-pulse protocol before (control), during (CP93129), and after (wash) 1 μM CP93129 application. A2: current traces in A1 normalized to the peak of the 1st IPSC in each pair to show clearly that the 2nd IPSC is increased during 1 μM CP93129 application, leading to increased PPR. A3: pooled data showing the inhibitory effect of 1 μM CP93129 on the peak amplitude of the 1st of the paired IPSCs in 6 SNr GABA neurons. A4: pooled data showing the increased PPR during 1 μM CP93129 in the 6 SNr GABA neurons. B: 5-HT1BR antagonist NAS-181 blocked the effect of 5-HT on striatonigral IPSCs. B1: superimposed averaged IPSCs evoked by striatum stimulation before (control) and during application of 5 μM 5-HT or 10 μM NAS-181 + 5 μM 5-HT (NAS181+5-HT). B2: pooled data showing the blockade effect of NAS-181 on the effect of 5-HT on striatonigral IPSCs (n = 6 neurons from 6 brain slices of 5 mice).
Furthermore, we tested if NAS-181, a 5-HT1BR antagonist, can block the effect of 5-HT on striatonigral IPSCs. As shown in Fig. 4, E and F, bath application of 5 μM 5-HT reduced the striatonigral IPSCs to 18.0 ± 3.0% of control, a 82% reduction (P < 0.0001, paired t-test, n = 6). Bath application of 10 μM NAS-181 almost completely blocked the inhibitory effect of 5 μM 5-HT, restoring the striatonigral IPSC amplitude to 87.5 ± 10.1% of control (P < 0.005, paired t-test, n = 6; Fig. 4, E and F). Taken together, these results indicate that 5-HT potently inhibits the striatonigral IPSCs through the presynaptic 5-HT1BRs on striatonigral axon terminals.
5-HT and 5-HT1B Receptor Agonist CP93129 Reduces the Striatonigral Inhibition of SNr GABA Neuron Firing
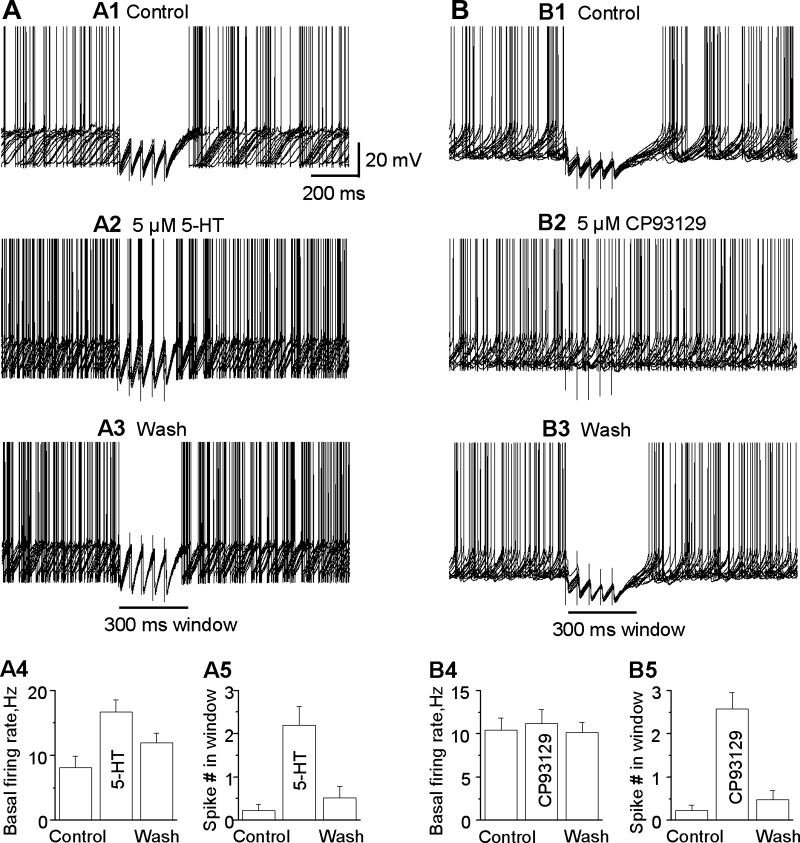
Next, we examined the functional consequences of the robust presynaptic 5-HT1BR inhibition of the GABA release from striatonigral axon terminals, a critical issue that had never been studied. We predicted that 5-HT may reduce the inhibitory effect of striatonigral IPSPs on the spontaneous firing of SNr GABA neurons. To test this idea, we evoked a train of 5 striatonigral IPSPs by a train of 5 stimuli at 20 Hz, mimicking a common firing pattern of the striatonigral neurons in freely moving mice (Miller et al. 2008; unpublished data, Sagot B and Zhou F.-M.). As shown in Fig. 5, A and B, under the control conditions, the striatonigral IPSP train strongly inhibited or paused the spontaneous firing of SNr GABA neurons. After bath application of 5 μM 5-HT, the amplitude of the striatonigral IPSPs, and hence the effect of the striatonigral IPSPs on the spontaneous firing of SNr GABA neurons, was reduced substantially. To quantify the effect of 5-HT on the striatonigral IPSP-induced pause in SNr GABA neurons, we calculated the spike number in the 300-ms window that starts at the first stimulus, as illustrated in Fig. 5. The spike number in the 300-ms window was only 0.23 ± 0.14 (spike firing was almost completely inhibited or paused) under control conditions, but it was increased to 2.20 ± 0.43 under 5 μM 5-HT (the pause was substantially reduced) (P < 0.005, paired t-test, n = 6). This effect is likely mediated by presynaptic 5-HT1BRs that inhibit the striatonigral IPSPs. At the same time, bath application of 5 μM 5-HT significantly increased the baseline spontaneous firing of SNr GABA neurons from 8.4 ± 1.7 to 16.7 ± 1.8 Hz (P < 0.001, paired t-test, n = 6), probably through activation of postsynaptic 5-HT2C receptors known to express in SNr GABA neurons (Clemett et al. 2000).
Fig. 5.
5-HT reduces the pausing effect of striatonigral inhibitory postsynaptic potentials (IPSPs) on the spontaneous firing of SNr GABA neurons. A1–A3: a train of 5 striatonigral IPSPs evoked by stimulating the striatum with a train of 5 pulses with an interval of 50 ms paused the spontaneous firing in SNr GABA neuron, which was strongly reduced by bath application of 5 μM 5-HT. A4: summary showing the effect of 5 μM 5-HT on baseline spontaneous firing frequency. A5: summary showing the effect of 5 μM 5-HT on the spike number in the 300-ms period immediately after the first stimulus artifact (n = 5 neurons from 5 brain slices of 5 mice). B1–B3: 5-HT1BR agonist CP93129 reduces the pausing effect of striatonigral IPSPs on spontaneous firing of SNr GABA neuron. A train of 5 striatonigral IPSPs evoked by stimulating striatum with a train of 5 pulses with an interval of 50 ms paused the spontaneous firing in SNr GABA neuron, which was strongly reduced by bath application of 5 μM CP93129. B4: summary showing the effect of 5 μM CP93129 on baseline spontaneous firing frequency. B5: summary showing the effect of 5 μM CP93129 on the spike number in the 300-ms period immediately after the first stimulus artifact (n = 9 neurons from 9 brain slices of 8 mice).
To further test our idea, we studied the effect of the 5-HT1B agonist CP93129 on striatonigral IPSP-induced pause in the spontaneous firing of SNr GABA neurons with the same stimulation protocol. As shown in Fig. 5, A4, A5, B4, and B5, the spike number in the 300-ms window was 0.23 ± 0.13 under control conditions and increased to 2.58 ± 0.38 during bath application of 5 μM CP93129 (P < 0.001, paired t-test, n = 8). The effect was recovered upon washing out 5 μM CP93129. Unlike 5-HT, 5 μM CP93129 had no significant effect on the baseline spontaneous firing of SNr GABA neurons (10.4 ± 1.4 Hz under control vs. 11.2 ± 1.6 Hz under CP93129, P > 0.05, paired t-test, n = 8), because SNr GABA neurons are not known to express postsynaptic 5-HT1BRs (Sari 2004). Together, these results suggest that 5-HT can reduce striatonigral IPSPs by activating the presynaptic 5-HT1BRs and soften the pausing effect of the striatonigral output on the spontaneous high-frequency firing in SNr GABA neurons.
Chronic Fluoxetine Treatment Sensitizes 5-HT1BRs on Striatonigral Axon Terminals
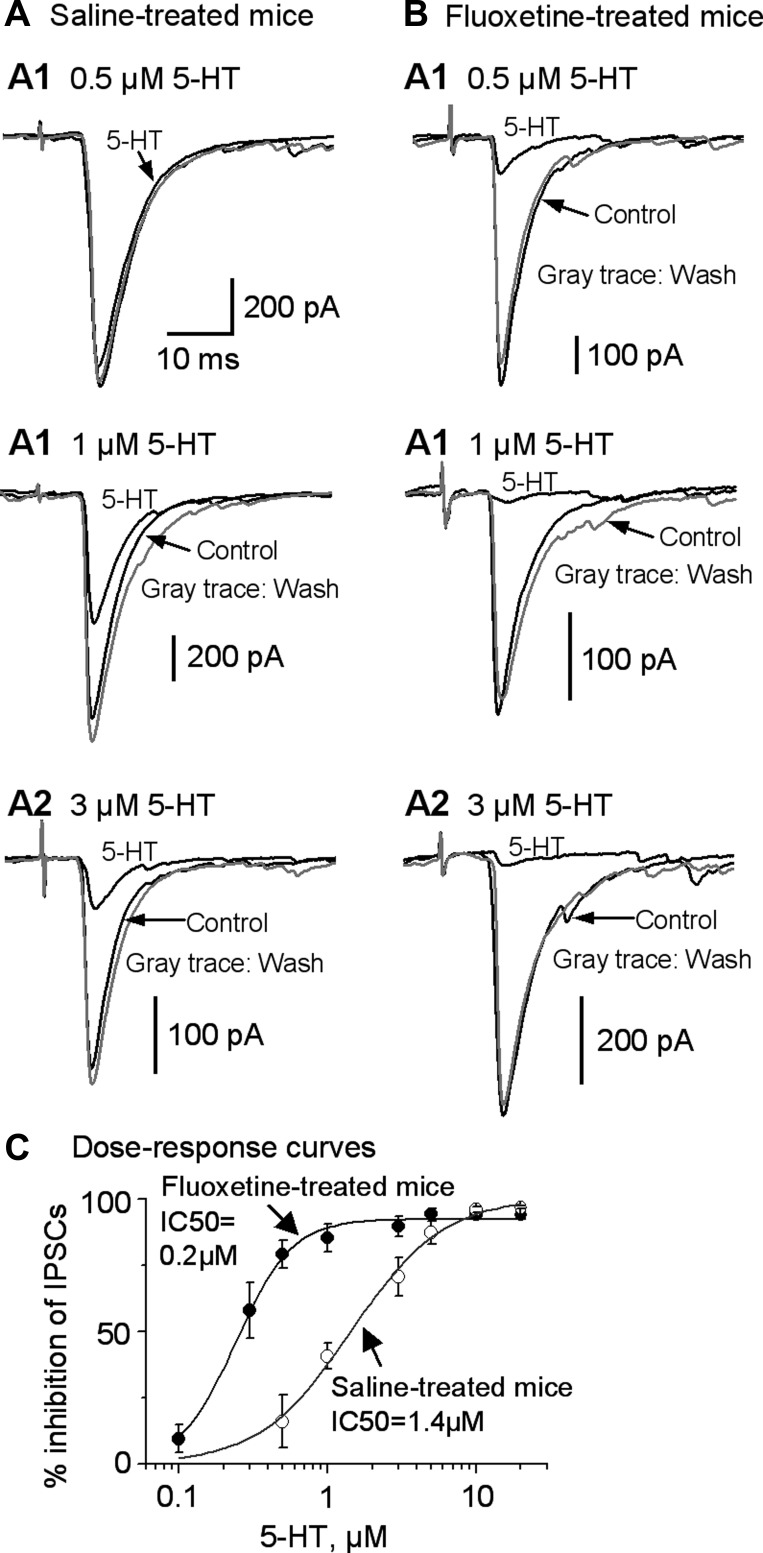
To determine the potential effects of chronic SSRI treatment on the functional status of the 5-HT1BRs on striatonigral axon terminals, we injected mice with either saline or fluoxetine using the protocol described in materials and methods. The effects during 10–20 days were similar, and thus data from these treatment days were pooled. As shown in Fig. 6, A–C, 5-HT inhibited the striatonigral IPSCs in a dose-dependent manner in both chronically fluoxetine-treated and saline-treated mice [2-way ANOVA, F(7) = 38.826, P = 0.000]. However, at 0.5 μM, 5-HT reduced the striatonigral IPSC by 79.3 ± 5.2% (n = 6) in chronically fluoxetine-treated mice and by only 16.1 ± 9.9% (n = 6) in saline-treated mice [2-way ANOVA, F(1,71) = 66.56, P = 0.000]. Similarly, at 1 μM, 5-HT inhibited the striatonigral IPSC to a larger extent in chronically fluoxetine-treated mice than in saline-treated mice [85.4 ± 5.3% for fluoxetine-treated mice (n = 6) and 40.9 ± 4.7% for saline-treated mice (n = 7); 2-way ANOVA, F(1,71) = 35.65, P = 0.000]. At 3 μM, 5-HT also had larger inhibitory effects on the striatonigral IPSCs in chronically fluoxetine-treated mice than in age-matched saline-treated mice [89.8 ± 3.9 and 66.3 ± 7.3% for fluoxetine-treated mice and saline-treated mice, respectively (n = 6 in each group); 2-way ANOVA, F(1,71) = 9.21, P = 0.0034]. However, at 5, 10, and 20 μM, the 5-HT inhibition of the striatonigral IPSCs was a similarly total or near total in both chronically fluoxetine-treated and saline-treated mice [2-way ANOVA, F(1,71) = 2.01, 0.07, and 0.11, P = 0.16, 0.80, and 0.74 for 5, 10, and 20 μM 5-HT, respectively]. When we fitted these data points to the Hill equation, we found that the IC50 was 0.24 ± 0.01 and 1.4 ± 0.2 μM for fluoxetine-treated mice and saline-treated mice, respectively (Fig. 6C). There was a clear leftward shift in the dose-response curve of 5-HT inhibition of the striatonigral IPSCs in chronic fluoxetine-treated mice compared with that of the age-matched saline-treated mice, indicating that 5-HT1BRs on the striatonigral axon terminals became more sensitive to 5-HT after chronic fluoxetine treatment.
Fig. 6.
Chronic fluoxetine treatment sensitizes 5-HT1BRs on the striatonigral axon terminals. A: example traces of striatonigral IPSCs in saline-treated mice before (A1), during (A2), and after (A3) bath application of 0.5, 1, and 3 μM 5-HT. B: example traces of striatonigral IPSCs in chronically fluoxetine-treated mice before (B1), during (B2), and after (B3) bath application of 0.5, 1, and 3 μM 5-HT, respectively. C: dose-response relationship of the 5-HT inhibition of striatonigral IPSCs in saline-treated mice and in chronically fluoxetine-treated mice. The continuous line is the Hill equation fit. Each data point represents 6–8 experiments (n = 28 neurons from 28 brain slices of 23 chronically fluoxetine-treated mice; n = 24 neurons from 24 brain slices of 17 saline-treated mice).
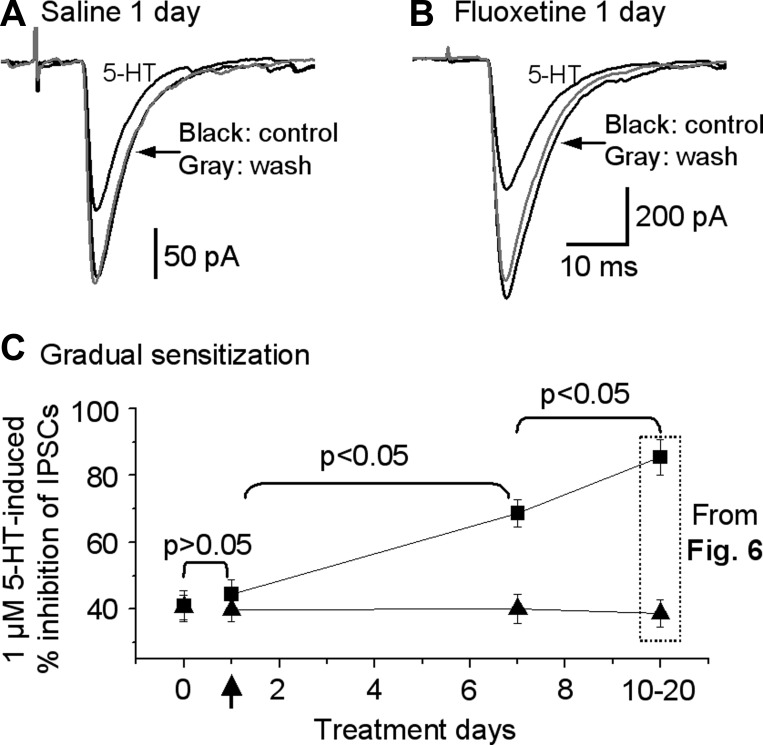
We have also tested the potential effects of 1-day and 7-day fluoxetine treatment (see materials and methods for dosing details). As shown in Fig. 7, after 1-day treatment with fluoxetine, 1 μM 5-HT inhibited striatonigral IPSC by 44.4 ± 4.5% (n = 6 cells) in mice and by 40.9 ± 4.7% (n = 7 cells) in saline-treated control mice (1-way ANOVA, P > 0.05). In mice treated with fluoxetine for 7 days, 1 μM 5-HT inhibited striatonigral IPSC by 68 ± 4.1% (n = 7), significantly more than in saline-treated control mice and also 1-day fluoxetine treatment mice (Fig. 7B; 1-way ANOVA, P < 0.05). However, compared with that in mice treated with fluoxetine for 10–20 days (chronic fluoxetine treatment), the inhibitory effect of 1 μM 5-HT on striatonigral IPSCs in the 7-day fluoxetine treatment group was significantly smaller (1-way ANOVA, P < 0.05). Starting on fluoxetine treatment day 10, the 1 μM 5-HT-induced inhibition of the striatonigral IPSCs was at 85%, near the maximal 94% inhibition induced by 10 μM 5-HT. Thus 10-day fluoxetine treatment-induced sensitization was near the saturating level, and pooling of data from 10–20 days of treatment was justified. These results indicated that fluoxetine treatment-induced sensitization of 5-HT1BRs on the striatonigral axon terminals is a gradual process and that the timeline is similar to that of 5-HT1A autoreceptor desensitization in 5-HT neurons in intact animals (Blier and De Montigny 1983).
Fig. 7.
Time course of fluoxetine treatment-induced sensitization of 5-HT1BRs on the striatonigral axon terminals. A: example traces showing effect of 1 μM 5-HT on striatonigral IPSCs in mice treated with saline or fluoxetine for 1 day. B: pooled data showing that the inhibitory effect of 1 μM 5-HT on striatonigral IPSCs in untreated mice, saline-treated control mice, and mice treated with fluoxetine for 1 day (n = 7 neurons from 7 brain slices of 6 acute fluoxetine-treated mice), 7 days (n = 7 neurons from 7 brain slices of 6 fluoxetine-treated mice for 1 wk), and 10–20 days. Arrow indicates treatment day 1.
Chronic Fluoxetine Treatment Upregulates Presynaptic 5-HT1BR-Mediated Reduction of the Pause in SNr GABA Neuron Firing
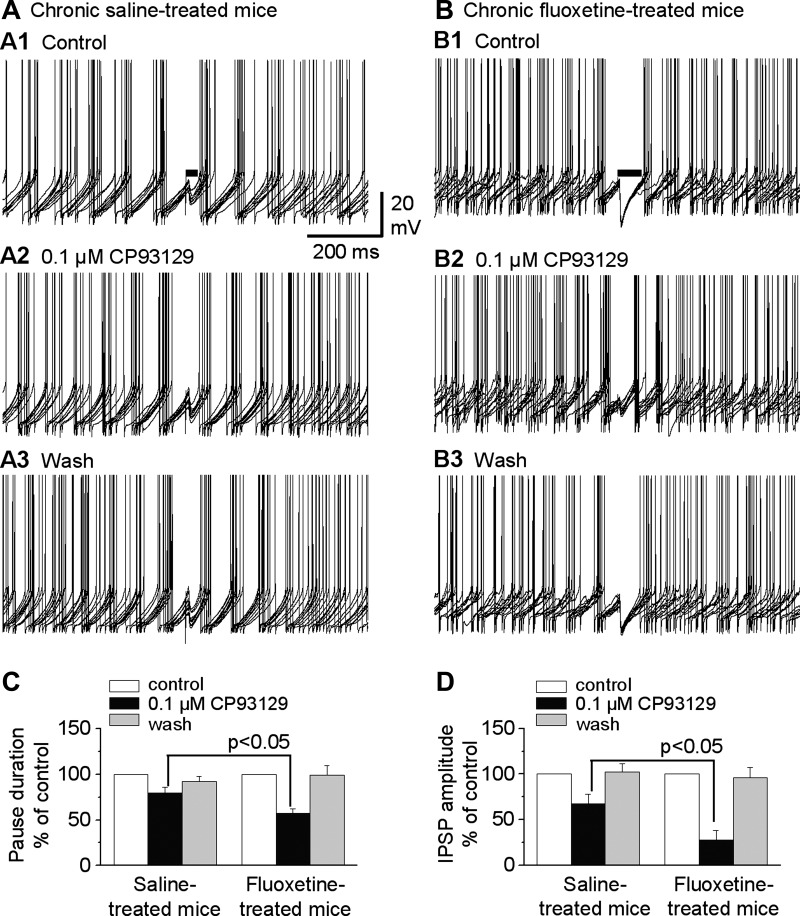
Because our data showed that chronic fluoxetine treatment sensitizes 5-HT1BRs on the striatonigral axon terminals (Fig. 6), we reasoned that the same treatment might upregulate 5-HT1BR-mediated reduction in the striatonigral IPSP-induced pause of SNr GABA neuron firing. To test our hypothesis, we treated mice with saline or fluoxetine using the same protocol described above. In brain slices from these treated mice, we tested the effects of the 5-HT1BR agonist CP93129 at a low concentration of 0.1 μM on the striatonigral IPSP-induced pause in SNr GABA neuron firing; 5-HT was not used in order to avoid 5-HT activation of somatic 5-HT2C receptors that would increase SNr GABA neuron firing and complicate data interpretation. To quantify the effect of CP93129 on the pause, we measured the pause duration induced by single striatonigral IPSPs. This pause duration was defined as the time window between the artifact of the stimulus that evoked the striatonigral IPSP and the first spike after the stimulus artifact (Fig. 8, A1 and B1). Because the pausing effect of the striatonigral IPSP on SNr GABA neuron firing was also dependent on where the IPSP was located on the membrane potential trajectory, in addition to its amplitude, we selected sweeps in which the IPSPs were evoked at the same position on the rising part of interspike membrane potential trajectory before, during, and after drug application; in these sweeps, we examined how the drug-induced changes in IPSP amplitudes affected the pausing of SNr GABA neuron firing.
Fig. 8.
Chronic fluoxetine treatment enhances presynaptic 5-HT1BR-mediated reduction of the pause of the spontaneous firing in SNr GABA neurons. A: example traces showing the pausing effect of striatonigral IPSPs in a SNr GABA neuron before (A1), during (A2), and after (A3) bath application of 0.1 μM CP93129 in a saline-treated control mouse. B: example traces showing the pausing effect of striatonigral IPSPs on the firing of a SNr GABA neuron before (B1), during (B2), and after (B3) bath application of 0.1 μM CP93129 in a chronic fluoxetine-treated mouse. Horizontal bars in A1 and B1 indicate the pause duration: the time window between the stimulus artifact and the first spike after the stimulus artifact in each sweep. C: pooled data showing that 0.1 μM CP93129 reduced the pause duration to a larger extent in chronic fluoxetine-treated mice than in saline-treated mice. D: pooled data showing that 0.1 μM CP93129 reduced the peak amplitude of the striatonigral IPSPs more strongly in chronic fluoxetine-treated mice than in saline-treated mice (n = 6 neurons from 6 slices of 5 fluoxetine-treated mice; n = 7 neurons from 7 slices of 6 saline-treated mice).
We found that bath application of 0.1 μM CP93129 reduced the pause duration to 79.6 ± 6.1% of control condition in saline-treated mice [from control pause duration of 79.3 ± 13.7 ms; F(1,5) = 43.678, P < 0.001, 1-way repeated-measures ANOVA; Fig. 8, A and C]. In chronic fluoxetine-treated mice, 0.1 μM CP93129 reduced the pause duration to 57.6 ± 4.6% of control condition [from control pause duration of 93.6 ± 13.7 ms; F(1,5) = 52.281, P < 0.001, 1-way repeated-measures ANOVA; Fig. 8, B and C]. The effect of 0.1 μM CP93129 on the pause was larger in chronic fluoxetine-treated mice than in saline-treated mice (n = 6 for each group, P < 0.05, unpaired t-test; Fig. 8C). Also, as expected, bath application of 0.1 μM CP93129 reduced the peak amplitude of striatonigral IPSPs to 67.5 ± 10.3% of control in saline-treated mice [from control IPSP amplitude of 8.8 ± 1.4 mV; F(1,5) = 32.497, P < 0.001, 1-way repeated-measures ANOVA] and to 27.7 ± 10.3% of control in chronic fluoxetine-treated mice [from control IPSP amplitude of 9.7 ± 2.4 mV; F(1,5) = 5.83, P < 0.05, 1-way repeated-measures ANOVA; Fig. 8D], and this effect was also larger in chronic fluoxetine-treated mice than in saline-treated mice (n = 6 for each group, P < 0.05, unpaired t-test). Additionally, saline or fluoxetine treatment did not alter the basal firing frequency of SNr GABA neurons recorded in our brain slices; CP93129 also did not affect the spontaneous firing in these neurons: in saline-treated mice, the spontaneous firing rate was 9.95 ± 1.1 Hz under control, 10.9 ± 1.6 Hz under 0.1 μM CP93129, and 10.5 ± 1.4 Hz after washout; in fluoxetine-treated mice, the spontaneous firing rate was 11.1 ± 1.5 Hz under control, 12.1 ± 2.0 Hz under 0.1 μM CP93129, and 12.1 ± 2.2 Hz after washout. Together, these data clearly indicate that 5-HT1BR-mediated reduction of SNr GABA neuron firing pause was enhanced after chronic fluoxetine treatment.
DISCUSSION
The main finding of this study is that 5-HT potently inhibits the striatonigral GABA output via the 5-HT1BRs on the striatonigral axon terminals. Consequently, 5-HT reduces the striatonigral IPSP-induced pause of the high-frequency spontaneous firing in SNr GABA neurons. Moreover, after chronic fluoxetine treatment, these 5-HT1BRs became more sensitive to 5-HT, in contrast to the common, chronic SSRI-induced desensitization of 5-HT1 autoreceptors. Thus our present study has revealed a novel aspect of chronic SSRI-based antidepressant treatment.
Presynaptic 5-HT1B Heteroreceptors Potently Reduce Striatonigral GABA Output
We found that 5-HT can completely inhibit the striatonigral IPSCs while not affecting the GPe-evoked pallidonigral IPSCs; 5-HT also increased the PPR. Additionally, the 5-HT effects were mimicked by the 5-HT1BR agonist CP93129 and blocked by the 5-HT1BR antagonist NAS-181, clearly indicating that presynaptic 5-HT1BRs on striatonigral axon terminals were mediating these effects. Our data are consistent with the anatomic fact that 5-HT1BRs are expressed at a high level on the striatonigral axon terminals, whereas 5-HT1BR gene expression is absent or very low in pallidal neurons (Boschert et al. 1994; Maroteaux et al. 1992; Riad et al. 2000; Sari 2004; Sari et al. 1999; Voigt et al. 1991). Our results also substantially expand the study of Stanford and Lacey (1996), who reported that 10 μM 5-HT induced a 60% inhibition of locally evoked IPSCs of unknown origin. In our present study, 10 μM 5-HT induced a virtually complete (94%) inhibition of the striatum-evoked, relatively pure striatonigral IPSCs while having no effect on the pallidonigral IPSCs. The smaller 5-HT effect of Stanford and Lacey (1996) (60% vs. our 94% inhibition with 10 μM 5-HT) is probably due to their activation of mixed striatonigral and pallidonigral IPSCs, because pallidonigral IPSCs are insensitive to the presynaptic 5-HT inhibition. We also provided more compelling pharmacological evidence for the mediation of 5-HT1BRs by using the specific 5-HT1BR agonist CP93129 and antagonist NAS-181.
Presynaptic 5-HT1B Heteroreceptors Reduce the Pause in High-Frequency Firing in Nigral GABA Neurons
Our present study explored the functional importance of the potent presynaptic 5-HT1BR inhibition of the GABA release from striatonigral axon terminals, a critical issue that had never been studied until now. We found that striatonigral IPSPs can inhibit or pause the endogenously generated high-frequency firing in SNr GABA neurons, consistent with previous studies (Connelly et al. 2010; Hikosaka et al. 2000, 2014; Kravitz at al. 2010). We further demonstrated that 5-HT and the 5-HT1BR agonist CP93129 reduced striatonigral IPSP-induced pause of the spontaneous high-frequency firing of SNr GABA neurons. This is important because the pause in the high-frequency firing of SNr GABA neurons releases the downstream targets such as the thalamus and brainstem motor nuclei (Basso and Sommer 2011; Hikosaka et al. 2000, 2014; Kaneda et al. 2008; Kravitz at al. 2010; Sano et al. 2013), and consequently, a reduction in the pause is likely to affect the activity of these downstream nuclei.
We need to note here that 5-HT has other effects on the SNr. For example, 5-HT increases the basal spontaneous firing in SNr GABA neurons by activating 5-HT2C receptors in these neurons (Stanford and Lacey 1996; Zhou and Lee 2011). Additionally, presynaptic 5-HT1BRs reduce subthalamic glutamatergic input-induced burst firing in SNr GABA neurons (Ding et al. 2013). Clearly, 5-HT exerts different effects on different targets in the SNr but with an apparently common goal: to keep SNr GABA neurons in their default autonomous high-frequency firing state. In other words, 5-HT exerts a homeostatic effect: the 5-HT2CRs help SNr GABA neurons maintain their basal high-frequency firing and prevent them from firing too slowly; the 5-HT1BRs on the subthalamonigral axon terminals prevent the glutamatergic input from triggering too much firing in SNr GABA neurons; and finally, the 5-HT1BRs on the striatonigral axon terminals prevent the striatal GABA input from inhibiting SNr GABA neuron firing too strongly.
Chronic Fluoxetine Treatment Sensitizes 5-HT1BRs on Striatonigral Axon Terminals
Our results showed that following chronic fluoxetine treatment, the sensitivity of the presynaptic 5-HT1BRs to 5-HT increased substantially, enabling a low dose of 5-HT to more strongly reduce the GABA release from the striatonigral axon terminals. This is a novel cellular mechanism for chronic SSRI treatment that has never been reported before, to our knowledge. The prevailing idea is that SSRIs block SERT-mediated 5-HT reuptake and increase the extracellular 5-HT level that desensitizes the 5-HT1A and 5-HT1B inhibitory autoreceptors in 5-HT neurons and their axon terminals via diminished receptor-G protein coupling (Castro et al. 2003; Cornelisse et al. 2007; Hensler 2002) or receptor internalization (Descarries and Riad 2012); this in turn leads to sustained increase in extracellular 5-HT and thus contributes to the antidepressant effect of SSRI treatment (Blier and El Mansari 2013; Wong et al. 2005). Reports indicate that postsynaptic 5-HT1ARs may also be desensitized via similar mechanisms (Hensler 2002; Li et al. 1997).
Thus we were initially surprised by our results indicating that chronic fluoxetine treatment sensitized the 5-HT1BRs on the striatonigral axon terminals. However, we quickly realized that although there is no published study on the potential effects of chronic SSRI treatment on presynaptic 5-HT1 heteroreceptors (presynaptic 5-HT1 receptors on non-5-HT axons), there are reports on the potential effects of chronic SSRI treatment on postsynaptic 5-HT1 heteroreceptors (5-HT1 receptors on the somata and dendrites of non-5-HT neurons). In particular, an electrophysiological study indicated that postsynaptic 5-HT1A receptors in the hippocampal neurons in rats were sensitized by chronic fluoxetine treatment (Beck et al. 1997). Biochemical studies indicate that chronic SSRI treatment may sensitize postsynaptic 5-HT1A receptors by increasing the receptor-G protein coupling (Castro et al. 2003; Shen et al. 2002; Zanoveli et al. 2007), providing a possible molecular basis for our unexpected observation.
Although the mechanisms underlying the sensitization of the presynaptic 5-HT1BRs on the striatonigral axon terminals was beyond the scope of our current study, the substantial leftward shift in the dose-response curve (IC50 changed from 1.4 to 0.2 μM; Fig. 6C) indicates an increased receptor binding affinity or more efficient downstream signaling mechanisms. Because the maximal effect was a total inhibition under both conditions, this parameter is less informative. An alternative and nonexclusive possibility is that the 5-HT1BR expression at the striatonigral axon terminals is upregulated, also leading to a leftward shift in the dose-response curve. This possibility is consistent with a previous study reporting a 127% increase over the baseline in the 5-HT1B mRNA level following chronic fluoxetine-treated in rats, although the host cell was not identified (Le Poul et al. 2000). Thus de novo increase in receptor expression may be another factor that allows lower concentrations of 5-HT to achieve the maximal and total inhibition of the striatonigral IPSC. A third possible mechanism for the increased 5-HT sensitivity or the leftward shift of the 5-HT dose-response curve (Fig. 6C) is an increased plasma membrane insertion on the striatonigral axon terminals after chronic fluoxetine treatment, a process that is opposite to the 5-HT1AR internalization underlying 5-HT1AR desensitization (Descarries and Riad 2012).
Functional Implications
We found that 5-HT can almost completely inhibit the GABA release from the striatonigral axon terminal with an IC50 at 1 μM. Although the endogenous 5-HT is unlikely to fully activate these 5-HT1BRs, this remarkable capacity, coupled with the intense 5-HT innervation in the SNr, still gives these receptors the power to regulate the striatonigral GABA output both in the normal brain and under pathological conditions and thus has important implications.
An obvious implication is in motor control. It is now established that striatonigral GABA output promotes motor activity by inhibiting the SNr GABA neurons (Friend and Kravtiz 2014; Hikosaka et al. 2000, 2014; Kravitz et al. 2010). Our data show that the presynaptic 5-HT1BRs serve to reduce this motor-promoting striatonigral GABA output. We speculate that because too much motor activity can be harmful to the animal, these presynaptic 5-HT1BRs are expressed there as a safety device to limit the striatonigral output. This may also be an evolutionary purpose for the exceptionally dense 5-HT innervation in the SNr. It will be interesting to determine the functional status of these presynaptic 5-HT1Rs in Parkinson's disease when there is a deficient or abnormal striatonigral output and in dyskinetic disorders when there is too much motor activity.
Another implication is in the regulation of emotion and antidepressant treatment. The precise anatomic location of emotional regulation is not known, but a proper function of the CBGTC loop is essential to emotional well-being, and dysfunction of the CBGTC loop is involved in depression pathogenesis (Hamilton et al. 2012; Hamon and Blier 2013; Marchand and Yurgelun-Todd 2010; Price and Drevets 2012). Aberrant functional connectivity in this loop has been indicated to be a primary pathology in depression (Marchand et al. 2012). Because the SNr is a narrow gate in the CBGTC loop, the information transfer in the SNr may critically affect the emotional regulation aspect of the loop. When the 5-HT1BRs on striatonigral axon terminals are sensitized during chronic SSRI treatment, these 5-HT1BRs can reduce GABA release and the striatonigral information transfer in a heightened manner, altering the information processing in the CBGTC loop and potentially contributing to the antidepressant effect of chronic fluoxetine treatment.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01NS058850 (to F.-M. Zhou). S. Ding was the recipient of a University of Tennessee Neuroscience Institute FY2014 fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.D. and F.-M.Z. conception and design of research; S.D., L.L., and F.-M.Z. performed experiments; S.D., L.L., and F.-M.Z. analyzed data; S.D. and F.-M.Z. interpreted results of experiments; S.D. and F.-M.Z. prepared figures; S.D. and F.-M.Z. drafted manuscript; S.D. and F.-M.Z. edited and revised manuscript; S.D., L.L., and F.-M.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michèle Darmon and Hongbing Wang for advice on 5-HT1B receptor immunostaining.
REFERENCES
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci 28: 199–207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci 25: 8272–8281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Sommer MA. Exploring the role of the substantia nigra pars reticulata in eye movements. Neuroscience 198: 205–212, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SG, Birnstiel S, Choi KC, Pouliot WA. Fluoxetine selectively alters 5-hydroxytryptamine1A and γ-aminobutyric acidB receptor-mediated hyperpolarization in area CA1, but not area CA3, hippocampal pyramidal cells. J Pharmacol Exp Ther 281: 115–122, 1997. [PubMed] [Google Scholar]
- Birmaher B. Improving remission and preventing relapse in youths with major depression. Am J Psychiatry 171: 1031–1033, 2014. [DOI] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci 3: 1270–1278, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci 368: 20120536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 58: 167–182, 1994. [DOI] [PubMed] [Google Scholar]
- Castro M, Diaz A, del Olmo E, Pazos A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology 44: 93–101, 2003. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39: 123–132, 2000. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Schulz JM, Lees G, Reynolds JN. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J Neurosci 30: 14854–14861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse LN, Van der Harst JE, Lodder JC, Baarendse PJ, Timmerman AJ, Mansvelder HD, Spruijt BM, Brussaard AB. Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol 98: 196–204, 2007. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29: 571–625, 2005. [DOI] [PubMed] [Google Scholar]
- Czachura JF, Rasmussen K. Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arch Pharmacol 362: 266–275, 2000. [DOI] [PubMed] [Google Scholar]
- Descarries L, Riad M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos Trans R Soc Lond B Biol Sci 367: 2416–2425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Nigral dopamine loss induces a global upregulation of presynaptic dopamine D1 receptor facilitation of the striatonigral GABAergic output. J Neurophysiol 113: 1697–1711, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Presynaptic serotonergic gating of the subthalamonigral glutamatergic projection. J Neurosci 33: 4875–4885, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Matta SG, Zhou FM. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol 105: 554–570, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wei W, Zhou FM. Molecular and functional differences in voltage-activated sodium currents between GABA projection neurons and dopamine neurons in the substantia nigra. J Neurophysiol 106: 3019–3034, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Zhou FM. Serotonin regulation of subthalamic neurons. Rev Neurosci 25: 605–619, 2014. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 21: 269–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Kravitz AV. Working together: basal ganglia pathways in action selection. Trends Neurosci 37: 301–303, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Smith KS. Good habits, bad habits. Sci Am 310: 38–43, 2014. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26: 317–330, 2003. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 169: 693–703, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry 45: 54–63, 2013. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res 195: 198–213, 2008. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology 26: 565–573, 2002. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res 160: 209–226, 2007. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value-guided behavior. Annu Rev Neurosci 37: 289–306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, Hashimoto H, Yamada S. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol 144: 695–702, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 72: 165–229, 1992. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci 28: 11071–11078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard BD, Emslie GJ, Mayes TL, Nakonezny PA, Jones JM, Foxwell AA, King J. Sequential treatment with fluoxetine and relapse-prevention CBT to improve outcomes in pediatric depression. Am J Psychiatry 171: 1083–1090, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry 49: 1002–1014, 2001. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, Hamon M, Lanfumey L. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology 39: 110–122, 2000. [DOI] [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther 282: 1581–1590, 1997. [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J; Treatment for Adolescents With Depression Study (TADS) Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 292: 807–820, 2004. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Suchy Y, Johnson S, Thatcher J, Gale P. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci Lett 514: 86–90, 2012. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Yurgelun-Todd D. Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disord 12: 764–785, 2010. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci USA 89: 3020–3024, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli A, Yaka R. Dopamine inhibits GABAA currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience 165: 1159–1169, 2010. [DOI] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington's disease. J Neurophysiol 100: 2205–2216, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H, Hori T, Takahashi T. 5-HT1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. Eur J Neurosci 24: 1946–1954, 2006. [DOI] [PubMed] [Google Scholar]
- Moukhles H, Bosler O, Bolam JP, Vallee A, Umbriaco D, Geffard M, Doucet G. Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 76: 1159–1171, 1997. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol 73: 1225–1236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME, Shalom G, Ran A, Gur E, Van de Kar LD. Chronic fluoxetine-induced desensitization of 5-HT1A and 5-HT1B autoreceptors: regional differences and effects of WAY-100635. Eur J Pharmacol 486: 25–30, 2004. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse 59: 270–276, 2006. [DOI] [PubMed] [Google Scholar]
- Parent M, Wallman MJ, Gagnon D, Parent A. Serotonin innervation of basal ganglia in monkeys and humans. J Chem Neuroanat 41: 256–265, 2011. [DOI] [PubMed] [Google Scholar]
- Pejchal T, Foley MA, Kosofsky BE, Waeber C. Chronic fluoxetine treatment selectively uncouples raphe 5-HT1A receptors as measured by [35S]-GTPγS autoradiography. Br J Pharmacol 135: 1115–1122, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 16: 61–71, 2012. [DOI] [PubMed] [Google Scholar]
- Reed AL, Happe HK, Petty F, Bylund DB. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology (Berl) 197: 433–441, 2008. [DOI] [PubMed] [Google Scholar]
- Révy D, Jaouen F, Salin P, Melon C, Chabbert D, Tafi E, Concetta L, Langa F, Amalric M, Kerkerian-Le Goff L, Marie H, Beurrier C. Cellular and behavioral outcomes of dorsal striatonigral neuron ablation: new insights into striatal functions. Neuropsychopharmacology 39: 2662–2672, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417: 181–194, 2000. [PubMed] [Google Scholar]
- Rice ME. Use of ascorbate in the preparation and maintenance of brain slices. Methods 18: 144–149, 1999. [DOI] [PubMed] [Google Scholar]
- Sano H, Chiken S, Hikida T, Kobayashi K, Nambu A. Signals through the striatopallidal indirect pathway stop movements by phasic excitation in the substantia nigra. J Neurosci 33: 7583–7594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y. Serotonin 1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28: 565–582, 2004. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience 88: 899–915, 1999. [DOI] [PubMed] [Google Scholar]
- Sawyer EK, Howell LL. Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology 88: 44–49, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Activity of pars reticulata neurons of monkey substantia nigra in relation to motor, sensory, and complex events. J Neurophysiol 55: 660–677, 1986. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35: 27–47, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Li H, Meller E. Repeated treatment with antidepressants differentially alters 5-HT1A agonist-stimulated [35S]GTPγS binding in rat brain regions. Neuropharmacology 42: 1031–1038, 2002. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65: 585–596, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci 16: 7566–7573, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt MM, Laurie DJ, Seeburg PH, Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J 10: 4017–4023, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehrle NS, Klenotich SJ, Jamnia N, Ho EV, Dulawa SC. Effects of chronic fluoxetine treatment on serotonin 1B receptor-induced deficits in delayed alternation. Psychopharmacology (Berl) 227: 545–551, 2013. [DOI] [PubMed] [Google Scholar]
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 4: 764–774, 2005. [DOI] [PubMed] [Google Scholar]
- Zanoveli JM, Nogueira RL, Zangrossi H Jr. Enhanced reactivity of 5-HT1A receptors in the rat dorsal periaqueductal gray matter after chronic treatment with fluoxetine and sertraline: evidence from the elevated T-maze. Neuropharmacology 52: 1188–1195, 2007. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Lee CR. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience 198: 69–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou FM. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol 96: 1581–1591, 2006. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 21, Suppl 2: 52S–60S, 1999. [DOI] [PubMed] [Google Scholar]