Abstract
Training people to suppress motor representations voluntarily could improve response control. We evaluated a novel training procedure of real-time feedback of motor evoked potentials (MEPs) generated by transcranial magnetic stimulation (TMS) over motor cortex. On each trial, a cue instructed participants to use a mental strategy to suppress a particular finger representation without overt movement. A single pulse of TMS was delivered over motor cortex, and an MEP-derived measure of hand motor excitability was delivered visually to the participant within 500 ms. In experiment 1, we showed that participants learned to reduce the excitability of a particular finger beneath baseline (selective motor suppression) within 30 min of practice. In experiment 2, we performed a double-blind study with 2 training groups (1 with veridical feedback and 1 with matched sham feedback) to show that selective motor suppression depends on the veridical feedback itself. Experiment 3 further demonstrated the importance of veridical feedback by showing that selective motor suppression did not arise from mere mental imagery, even when incentivized with reward. Thus participants can use real-time feedback of TMS-induced MEPs to discover an effective mental strategy for selective motor suppression. This high-temporal-resolution, trial-by-trial-feedback training method could be used to help people better control response tendencies and may serve as a potential therapy for motor disorders such as Tourette's and dystonia.
Keywords: inhibitory control, mental imagery, transcranial magnetic stimulation
inhibitory response control (hereafter, inhibitory control, IC) refers to the neural processes by which individuals suppress movement. IC is thought to be implemented by top-down (prefrontal) control over response channels in basal ganglia and/or M1 (reviewed by Aron et al. 2014; Bari and Robbins 2013; Coxon et al. 2009; Spierer et al. 2013). Different forms of IC might be employed depending on the context (Aron 2011; Spierer et al. 2013). For instance, one can rapidly stop an action when a sudden event occurs, which is a reactive form of IC modeled by stop-signal or go/no-go tasks (Verbruggen and Logan 2009). One can also employ IC proactively to dampen the motor system in preparation for having to stop. For example, in one kind of proactive suppression paradigm, participants are cued that they might have to stop a particular muscle later in a trial. By implementing motor suppression well in advance, they are better able to stop action in a targeted way when needed (Cai et al. 2011; Chikazoe et al. 2009; Jahfari et al. 2012; Majid et al. 2013; Smittenaar et al. 2013).
Much research has shown that impairments in IC partly underlie impulse control disorders such as attention deficit hyperactivity disorder and Tourette's syndrome (Chamberlain and Sahakian 2007; Chambers et al. 2009; Channon et al. 2009; Lijffijt et al. 2005; Shahana and Gilbert 2013). Consequently, it is important to discover behavioral regimens that improve IC (reviewed by Enriquez-Geppert et al. 2013; Spierer et al. 2013).
Training studies of IC have mostly focused on having participants repeatedly practice the reactive stopping of action; however, these have had mixed success (e.g., Berkman et al. 2014; Chevalier et al. 2014; Cohen and Poldrack 2008; Enge et al. 2014; Logan and Burkell 1986; Schapkin et al. 2007; and reviewed by Spierer et al. 2013). Interestingly, in one study that showed faster stopping speeds with training, IC improvements seemed to be related to greater emphasis on the brain circuitry for preparing to suppress (Berkman et al. 2014). This suggests that training proactive suppression of the motor system is a good avenue for improving IC. There are also other considerations in favor of trying to train proactive IC. First, it might be more amenable to training than the reactive system, which is very quick, dependent on the speed of detection and signaling via white matter pathways. Second, it is probable that fewer situations in real life require rapid reactive IC; instead, one often has to prepare to suppress a response in advance, and this requires translating goals into action influences, i.e., “implementation intentions” (Burkard et al. 2013), which are probably more amenable to training.
Here, we tested a novel method of training proactive IC. On each trial, we instructed the participant to try to suppress a right-hand muscle. Then, on the same trial, we delivered a single pulse of transcranial magnetic stimulation (TMS), recorded the motor evoked potential (MEP), and immediately returned the MEP to the participant as visual feedback. The participant's task was to try to discover an effective mental strategy to suppress the muscle. Experiment 1 investigated whether this feedback method allowed participants to “drive down” the excitability of a particular muscle beneath baseline. Experiment 2 examined whether veridical feedback is key by performing a rigorous double-blind study comparing real and sham feedback groups. Experiment 3 addressed an alternative possibility for the effects, that veridical feedback is not important and that selective motor suppression can instead be achieved through mere mental imagery.
EXPERIMENT 1
Methods
Participants.
Fourteen healthy, right-handed participants (4 men, 10 women, mean age: 20.9 ± 4.1 yr) provided written, informed consent in accordance with Institutional Review Board (IRB) guidelines at University of California, San Diego (UCSD), completed a TMS safety questionnaire (Rossi et al. 2009), and were paid $15/h. All 3 experiments reported in this study were approved by the UCSD IRB (no. 071912).
Behavioral task and mental procedures.
Participants sat in front of a 19-in. monitor with the right hand resting on a table. Electromyography (EMG) was recorded using a pair of 10-mm silver electrodes for two separate muscles: 1) the first dorsal interosseous (FDI) of the index finger; and 2) the abductor digiti minimi (ADM) of the pinky (Fig. 1). Ground electrodes were placed on the radial and ulnar wrist protuberances for the FDI and ADM, respectively. Single-pulse TMS was delivered over contralateral M1 to produce MEPs in both muscles simultaneously.
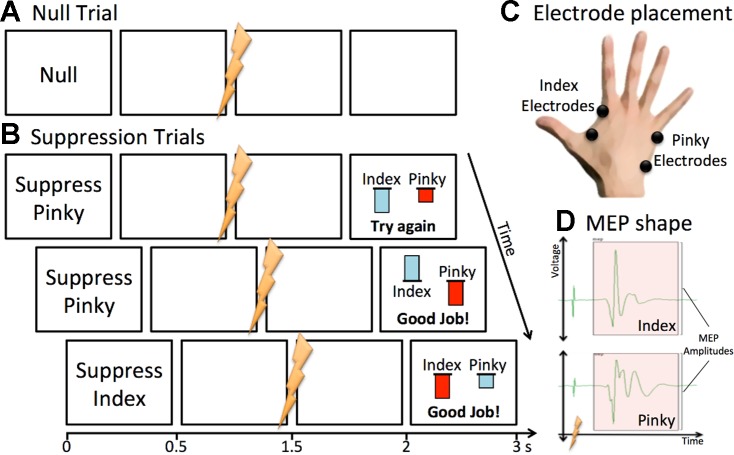
Fig. 1.
Feedback-driven task. A: Null trials at the start of each block. B: Suppression trials followed Null trials. Motor evoked potential (MEP) amplitudes compared with baseline were fed back visually on each trial. A red bar corresponded to the muscle cued for suppression. Participants saw “Good Job!” if 1) the cued MEP was reduced compared with baseline (i.e., the red bar was pointing downward), and 2) the cued MEP was reduced compared with the uncued MEP (i.e., the red bar was more negative than the blue bar). Otherwise, participants were told to “Try Again.” C: placement of the first dorsal interosseous (FDI, index) and abductor digiti minimi (ADM, pinky) electrodes. D: qualitative shape of MEP in both FDI (index) and ADM (pinky). Lightning bolt indicates time of stimulation with associated artifact. MEP amplitudes were measured in microvolts from peak to trough.
Each participant performed 10 blocks of 36 trials each. The 1st 12 trials of each block were baseline trials for the MEP normalization procedure (see below). For these 12 trials, participants saw the “Null” cue for 500 ms, indicating they should rest. A blank screen then followed for an additional 1 s before TMS was delivered and the resulting MEPs were recorded. The screen then remained blank for another 2.5 s before the start of the next trial. After these 12 Null trials, the mean MEP amplitude for each muscle was calculated to serve as a resting baseline.
The remaining 24 trials of each block began with a 500-ms cue that instructed participants either to “Suppress Index” or “Suppress Pinky” (12 each, randomly presented). Participants were instructed to experiment with different mental strategies to reduce the excitability of the muscle recorded from the cued finger, using the real-time feedback (see Results for a description of post hoc subjective reports). After the Suppress cue disappeared, the screen turned blank for 1 s before TMS was delivered and MEPs were obtained. The screen remained blank for another 500 ms before feedback was presented for 1 s. Participants saw a feedback bar for the index on the left and another for the pinky on the right. A red bar indicated that the finger was cued for suppression, and a blue bar indicated it was not. The bar height was calculated as log(trial MEP) − log(mean Null MEP). Thus a downward pointing bar indicated MEP reduction relative to the mean Null baseline. A “Good Job!” feedback message was only delivered if the cued (red) bar was downward pointing and more negative than the uncued (blue) bar. If not, a “Try Again” message was delivered. After feedback, the screen turned blank for 1 s before the next trial.
On all trials, pretrigger EMG was obtained for each muscle for 100 ms before TMS. If the root mean square for either trace exceeded 10 μV, a 1-s “No Tensing!” warning was delivered as feedback to prevent participants from prematurely activating any muscle. These trials were excluded from subsequent analysis.
Real-time TMS procedure details.
EMG for each muscle was amplified using a Grass QP511 Quad AC Amplifier System (Grass Technologies, West Warwick, RI). A 30-Hz to 1-kHz band-pass filter and a 60-Hz notch filter were applied. The amplifier output was then split (using T-shaped coaxial junctions) with one signal directed to an EMG recording computer (via a CED Micro1401 mk II acquisition system, sampled at 2 kHz) and the other to the participant's presentation computer (via analog inputs to a USB-1208FS data acquisition device; Measurement Computing, Norton, MA). On the presentation computer, the signal was read into MATLAB using the Psychtoolbox function DaqAInScan.m (Kleiner et al. 2007). The parameters for this function specified 1) the input leads of the two muscles, and 2) a sampling rate of 2,000/s.
TMS was delivered with a Magstim 2002 system (Magstim, Whitland, United Kingdom) and a figure-of-eight coil (7-cm diameter). The coil was initially placed 2 in. left and 1 in. anterior to the vertex to find the cortical representation of the resting right hand. The coil was incrementally repositioned, and the stimulus intensity was increased until an MEP was obtained in both the right FDI and ADM. The resting motor threshold (RMT) was the lowest stimulation level required to elicit MEPs of at least 0.05 mV in at least 5 of 10 trials in both recorded muscles. The experimental stimulation intensity was 110% of RMT rounded to the nearest percentage point, averaging 49.3 ± 8.7% of maximum stimulator output across participants.
TMS analysis.
Peak-to-peak MEP amplitude was calculated with custom MATLAB software. Trials were excluded when the root mean square of 100 ms of pre-TMS EMG trace exceeded 10 μV (8.7 ± 8.9 trials per subject). The distribution of MEP amplitudes following each cue (Suppress Index, Suppress Pinky, and Null) for the FDI (index) and ADM (pinky) were then respectively trimmed (to approximate normality of the distribution) by removing the upper and lower 10% of values (Wilcox 2001) and then averaged. MEP modulation of each muscle on a Suppress cue was calculated as the percentage change compared with the mean Null MEP, i.e., (Suppress cue MEP − Null MEP)/(Null MEP) × 100%.
Four TMS measures were calculated: 1) FDI modulation on Suppress Index trials (Cued Index Modulation); 2) ADM modulation on Suppress Pinky trials (Cued Pinky Modulation); 3) FDI modulation on Suppress Pinky trials (Uncued Index Modulation); and 4) ADM modulation on Suppress Index trials (Uncued Pinky Modulation). ANOVA was run with three factors, Session Half (Early vs. Late), Recorded Muscle (Index vs. Pinky), and Cued State (whether the muscle was cued for suppression or not). Similar analysis was done on the root mean square of EMG for 100 ms before TMS.
Results
For the MEP analysis, the 10 blocks of the experiment were divided into 2 halves (5 blocks each), and repeated-measures ANOVA was done with 3 factors [Session Half (Early vs. Late) × Recorded Muscle (Index vs. Pinky) × Cued State (the muscle was cued for suppression or not)]. There was a significant effect for Cued State (F1,13 = 7.711, d = 0.81, P = 0.016), no effect of Session Half [F1,13 = 1.434, d = 0.12, not significant (n.s.)], no effect for Recorded Muscle (F1,13 < 1, d = 0.37), no Cued State × Recorded Muscle interaction (F1,13 = 2.767, d = 0.08, n.s.), no Session Half × Recorded Muscle interaction (F1,13 < 1, d = 0.01), and no 3-way interaction (F1,13 < 1, d = 0.09). The Cued State × Session Half interaction trended toward significance (F1,13 = 3.969, d = 0.25, P = 0.068), suggesting a training effect in the ability to modulate MEPs to a cued stimulus (Fig. 2A; Table 1).
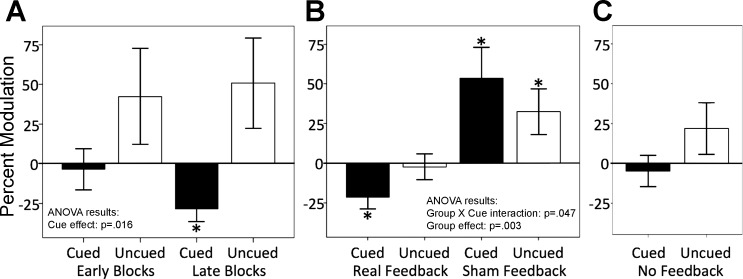
Fig. 2.
MEP results. A: experiment 1. There was a main effect of Cue, particularly seen in the later blocks. B: experiment 2 with real vs. sham feedback groups. The real feedback group suppressed the cued finger (as for experiment 1), whereas the sham feedback paradoxically activated both cued and uncued fingers. C: experiment 3. Participants showed no significant suppression of the cued finger. “Percent Modulation” represents the percentage change of the mean MEP amplitude on Suppression trials compared with that on Null trials: (Suppression cue MEP − Null MEP)/Null MEP × 100%. Asterisks indicate a significant change from the zero baseline (P < 0.05). Error bars represent 1 standard error of the mean.
Table 1.
Experiment 1 raw data (in microvolts)
| First 5 Blocks (Early Training) |
Last 5 Blocks (Late Training) |
|||
|---|---|---|---|---|
| Raw MEP Amplitude | Pre-TMS EMG | Raw MEP Amplitude | Pre-TMS EMG | |
| Index null baseline | 532 ± 295 | 1.7 ± 1.2 | 592 ± 396 | 1.6 ± 0.6 |
| Index when cued | 432 ± 352 | 2.1 ± 1.3 | 379 ± 334 | 1.7 ± 0.8 |
| Index when not cued | 610 ± 456 | 2.4 ± 1.6 | 655 ± 443 | 1.8 ± 0.9 |
| Pinky null baseline | 266 ± 187 | 1.5 ± 0.6 | 258 ± 175 | 1.4 ± 0.3 |
| Pinky when cued | 284 ± 253 | 1.9 ± 0.9 | 183 ± 120 | 1.4 ± 0.3 |
| Pinky when not cued | 333 ± 247 | 2.0 ± 0.8 | 346 ± 316 | 1.6 ± 0.6 |
Raw MEP amplitude is mean amplitude of the motor evoked potentials for a given condition. Pre-TMS EMG is the root-mean-square average of the electromyographic trace for 100 ms preceding transcranial magnetic stimulation delivery.
To examine pre-TMS excitability, the same ANOVA was run for the pre-TMS EMG. There was a significant effect of Session Half (F1,13 = 6.690, d = 0.50, P = 0.011), with EMG being greater for the first half than second half, no effect of Cue (F1,13 = 2.090, d = 0.27, n.s.), no effect of Recorded Muscle (F1,13 < 1, d = 0.09), and no interactions (all F1,13 < 1). This raised the possibility that differences in pre-TMS EMG may account for some of the above MEP effects.
To characterize the data further, we conducted post hoc tests on Cued vs. Uncued trials in the first and second halves of training separately. Since there were no effects of Recorded Muscle, the data from both muscles were pooled. In the 1st half (Early Training), there was no significant modulation of the Cued or Uncued conditions (Cued Modulation: −3.5 ± 48.5%, 1-sample t13 < 1, d = 0.07; Uncued Modulation: 42.3 ± 113.1%, 1-sample t13 = 1.40, d = 0.37, n.s.) and no significant difference (paired t13 = 1.961, d = 0.52, n.s.). By contrast, pre-TMS EMG did show significant activity in both the Cued (1-sample t13 = 2.332, d = 0.62, P = 0.036) and Uncued (t13 = 2.936, d = 0.78, P = 0.012) conditions and a condition difference (Uncued greater than Cued, paired t13 = 3.074, d = 0.82, P = 0.009). In the 2nd half (Late Training), MEPs were significantly below baseline in the Cued condition for 10 out of 14 participants (−28.4 ± 29.9%, 1-sample t13 = 3.563, d = 0.95, P = 0.003) but not the Uncued condition (50.7 ± 106.2%, 1-sample t13 = 1.785, d = 0.48, n.s.), with a significant difference between conditions (paired t13 = 3.244, d = 0.87, P = 0.006). There was no significant pre-TMS EMG activity for Cued (1-sample t13 < 1) or Uncued (1-sample t13 = 1.837, n.s.) conditions and no condition difference (paired t13 = 1.143, n.s.).
In summary, participants could indeed selectively reduce the excitability of a muscle, as manifest in the significant main effect of Cue. Moreover, this effect was particularly apparent in the last half of the experiment (as would be expected of a training effect), and, importantly, this was unconfounded by pre-TMS EMG activation.
When queried about effective mental suppression strategies, participants reported imagining the particular muscle “going numb” or “going on ice” or “thinking away” from the cued muscle to an alternative muscle (e.g., thinking about moving the pinky when told to suppress index).
Although the results of this study were striking, the question arises whether the proactive selective suppression was specifically due to the veridical feedback procedure. In experiment 2, we performed a double-blind study comparing groups with veridical and sham feedback.
EXPERIMENT 2
Methods
Participants.
Thirty healthy, right-handed participants were randomly assigned to either a real feedback group or sham feedback group (see below for sex and age distributions). Consenting, screening, and payment were the same as above.
Behavioral task and mental procedures.
A written list of strategies was provided from the outset, derived from the anecdotal responses from experiment 1.
1) Divert your attention away from the cued finger by thinking about moving the other.
2) Divert your attention to another part of the body.
3) Think about tensing the cued finger so it remains still.
4) Think about that muscle going numb or going on ice.
5) Think about suddenly stopping a movement of the cued finger.
6) Use any other strategy you come up with.
Participants then engaged in 10–12 blocks (depending on fatigue or time limitations) of the suppression task, identical in presentation and timing to experiment 1. A key difference, however, was that the unknowing participant was randomly assigned to either a real feedback group, for whom the feedback accurately reflected hand MEPs on a particular trial (as in experiment 1), or a sham feedback group. Feedback for each participant in the sham feedback group was matched to a previous participant in the real feedback group such that each group experienced the same frequency of positive (Good Job!) and negative (Try Again) feedback. The experimenter was also blind to group assignment except for the first participant (who must necessarily be in the real feedback group) and the last participant (who must necessarily be in the sham feedback group). A mixed-model ANOVA was run with the factors Cued State (whether a muscle was cued for suppression or not) and Group. We treated the two groups as independent rather than paired samples when doing t-tests.
After task completion, all participants were asked what strategies they had used and whether they felt they improved in their suppression ability.
TMS procedure details and analysis.
The real-time TMS procedure was identical to experiment 1. Since there was now no difference between index and pinky in suppression of MEPs, we pooled these into two conditions, Cued Modulation (FDI modulation on Suppress Index trials and ADM modulation on Suppress Pinky trials) and Uncued Modulation (FDI modulation on Suppress Pinky trials and ADM modulation on Suppress Index trials). Trials were excluded if the pre-TMS EMG trace exceeded 10 μV (10.6 ± 11.6 trials per subject).
Standard box-plot analysis of the group data revealed two extreme values in the real feedback group. Since this may have affected the behavior of the two matched sham feedback participants, these sham feedback participants were also excluded. This left 26 participants in the analysis (real feedback group: n = 13, 6 men, 7 women, mean age = 20.9 ± 2.4 yr; sham feedback group: n = 13, 6 men, 7 women, mean age = 20.7 ± 2.3 yr, no statistical difference in age). The experimental stimulation intensity was also comparable between groups (real: 48.3 ± 9.5% vs. sham: 49.8 ± 8.5%, t < 1).
Results
We now asked if it was specifically the feedback that enabled participants to develop a mental strategy for motor suppression. A mixed-model ANOVA with Group (real vs. sham feedback) × Cued State (Cued vs. Uncued) revealed a significant Group × Cued State interaction (F1,24 = 4.394, d = 0.86, P = 0.047; Fig. 2B; Table 2). There was also a significant effect of Group (F1,24 = 11.313, d = 1.37, P = 0.003) but no effect of Cued State (F1,24 < 1, d = 0.04). There were no significant effects or interactions when the above mixed-model ANOVA was applied to the pre-TMS EMG (all F1,24 < 1), showing that preparatory activation cannot account for the above results.
Table 2.
Experiments 2 and 3 raw data (in microvolts)
| Real Feedback Group (Experiment 2, n = 13) |
Sham Feedback Group (Experiment 2, n = 13) |
No Feedback Group (Experiment 3, n = 14) |
||||
|---|---|---|---|---|---|---|
| Raw MEP Amplitude | Pre-TMS EMG | Raw MEP Amplitude | Pre-TMS EMG | Raw MEP Amplitude | Pre-TMS EMG | |
| Index null baseline | 657 ± 174 | 1.8 ± 1.2 | 676 ± 295 | 1.6 ± 0.9 | 664 ± 363 | 0.9 ± 0.2 |
| Index when cued | 459 ± 266 | 1.8 ± 1.0 | 758 ± 541 | 1.7 ± 0.9 | 492 ± 247 | 0.9 ± 0.3 |
| Index when not cued | 618 ± 276 | 1.8 ± 1.0 | 694 ± 396 | 1.7 ± 0.9 | 632 ± 340 | 0.9 ± 0.3 |
| Pinky null baseline | 266 ± 221 | 2.0 ± 1.2 | 240 ± 147 | 1.9 ± 0.9 | 286 ± 158 | 1.0 ± 0.3 |
| Pinky when cued | 198 ± 106 | 2.2 ± 1.3 | 397 ± 229 | 2.1 ± 1.0 | 324 ± 258 | 1.0 ± 0.4 |
| Pinky when not cued | 246 ± 136 | 2.2 ± 1.3 | 352 ± 273 | 2.2 ± 1.1 | 382 ± 305 | 1.0 ± 0.4 |
To follow up the Group × Cued State interaction, MEP analyses were done separately for each group. In the real feedback group, participants significantly reduced the Cued, but not the Uncued, finger below baseline (Cued Modulation: −21.3 ± 26.9%, t12 = 2.860, d = 0.79, P = 0.014; Uncued Modulation: −2.3 ± 29.2%, d = 0.11, t12 < 1; Cued vs. Uncued, t12 = 2.563, d = 0.48, P = 0.025). Importantly, there was no significant difference in pre-TMS EMG activity between the Cued and Uncued condition in the real feedback group (t12 = 1.042, n.s.). By contrast, MEP analysis showed that the sham feedback group did not reduce but rather activated both the Cued and Uncued fingers (sham feedback Cued Modulation: 53.4 ± 70.5%, t12 = 2.388, d = 0.76, P = 0.032; Uncued Modulation: 32.3 ± 52.1%, t12 = 2.233, d = 0.67, P = 0.045; Cued vs. Uncued, t12 = 1.199, d = 0.32, n.s.).
When directly comparing the real and sham feedback groups, there was a difference in both Cued (t24 = 3.574, d = 1.40, P = 0.002) and Uncued finger modulation (t24 = 2.089, d = 0.76, P = 0.047). Again, there were no differences between groups between pre-TMS EMG in either the Cued (t24 = 1.185, n.s.) or Uncued (t24 < 1) conditions.
In summary, the real feedback group was able to reduce MEPs below baseline in the cued condition (an effect seen in 11 out of 13 participants), whereas this was not the case for the sham feedback group (where only 4 of 13 participants showed the below-baseline effect).
In the real feedback group, 9 of the 13 participants reported trying to suppress by diverting attention away from the cued finger to another finger of the hand. The other 4 reported a mix of imagining the cued finger going on ice, commanding the cued finger to stop, tensing the cued finger, or daydreaming. Six felt they improved, 6 were not sure, and 1 felt there was no improvement. In the sham feedback group, 6 of the 13 participants reported trying to suppress by diverting attention away from the cued finger to another finger of the hand; 3 participants reported trying to tense the cued finger, 2 reported directly commanding the finger to stop, and 2 reported other strategies. Eight felt they improved, 3 were not sure, and 2 thought there was no improvement.
The results of the real feedback group in this study were striking in replicating those of experiment 1. However, an alternative possibility is that the proactive selective suppression is not due to veridical TMS feedback so much as the subject practicing mental imagery. For example, an earlier study showed that participants could dampen the excitability in the hand using motor imagery (Sohn et al. 2003). On this account, MEPs are reduced in the real feedback group in experiment 2 because of motor imagery, whereas in the sham feedback group the presentation of yoked feedback is confusing to the subject, leading to activation rather than suppression. In experiment 3, we therefore examined whether cued suppression could arise from mere mental imagery in the absence of any feedback.
EXPERIMENT 3
Methods
Participants.
Eighteen healthy, right-handed participants took part. Consenting and screening were the same as above.
Behavioral task and mental procedures.
Our prediction in this study was a null effect, i.e., that mental imagery alone would not be effective for proactive selective suppression even when incentivized by money. Accordingly, it was important that the experimenter was blind to the purpose of the study. We trained a research assistant (C. Lewis) for this purpose, and she acquired all of the data. Participants were given the same written list of strategies as in experiment 2 and then engaged in 12 blocks of the suppression task, as before. Now, however, trial-by-trial feedback was not provided. Instead, $0.05 was accumulated for each trial corresponding to a Good Job! trial in the feedback paradigm (i.e., cued finger excitability reduced below baseline and more negative than uncued finger excitability reduction). Participants were informed of total reward earned at the end of every 4 blocks. A repeated-measures t-test compared Cued State (whether a muscle was cued for suppression or not). After task completion, all participants were asked what strategies they had used and whether they felt they improved in their suppression ability.
TMS procedure details and analysis.
As in experiment 2, trials were pooled into Cued and Uncued Modulation conditions. Trials were excluded if the pre-TMS EMG trace exceeded 10 μV (5.0 ± 9.5 trials per subject). Standard box-plot analysis of the group data revealed 4 participants with extreme values. This left 14 participants in the analysis (3 men, 11 women, mean age = 19.4 ± 1.2 yr). The experimental stimulation intensity was 54.2 ± 10.4%.
Results
We now asked whether cued suppression could arise from mere mental imagery in the absence of any feedback (cf. Sohn et al. 2003). Participants underwent the same procedure but, instead of trial-by-trial feedback, accumulated $0.05 for each trial corresponding to a Good Job! The total reward was displayed at the end of each four-block interval (to encourage task engagement). Average subject compensation was $6.39 ± 1.82 out of a maximum of $14.40.
Consistent with our hypothesis that veridical trial-by-trial feedback is needed, there was now no significant reduction of Cued finger excitability below baseline (Cued Modulation: −4.7 ± 36.5%, t13 < 1, d = 0.13) nor was the Uncued finger excitability significantly different from baseline (Uncued Modulation: 21.7 ± 60.2%, t13 = 1.352, d = 0.36, n.s.). The null result for Cued finger suppression was unlikely due to weak power: we estimated that 375 participants would be needed to have 80% power to show a significant below-baseline Cued finger modulation reduction.
There was a trend toward selective control (Cued vs. Uncued, t13 = 2.104, d = 0.56, P = 0.06; Fig. 2C; Table 2); however, rather than reflecting selective suppression (as for experiments 1 and 2), this may have reflected the trend toward greater pre-TMS EMG in the Uncued condition compared with the Cued condition (t13 = 1.824, d = 0.49, P = 0.09).
When directly comparing the current subjects with the real feedback group in experiment 2, there was indeed a difference for the Cued condition with moderate effect size, although this was not significant (t25 = 1.335, d = 0.52, n.s.), reflecting relatively poor power for a between-group comparison. There was no significant Uncued finger modulation from baseline (t25 < 1, d = 0.23). There were no differences between groups between pre-TMS EMG in either the Cued (t25 < 1) or Uncued (t25 < 1) conditions.
In summary, when the participants underwent the very same procedure as in experiments 1 and 2 but now without trial-by-trial feedback, they could not learn to suppress a particular response even though they were motivated by monetary reward to do their best.
Thirteen of the fourteen participants reported trying to suppress by diverting attention away from the cued finger to another finger of the hand. The remaining participant reported attempting nonspecific relaxation techniques. Eight felt they improved through the training paradigm (perhaps on account of financial compensation).
Thus this experiment showed that that mere motor imagery was not sufficient to lead to cued suppression even when incentivized by monetary reward.
DISCUSSION
We tested whether real-time feedback of TMS-derived MEPs allows participants to discover a mental strategy that is effective for suppressing a particular muscle. Experiment 1 showed that by the latter half of the training period, participants could effectively reduce the motor excitability of a particular hand muscle below a resting baseline when cued to suppress that muscle. Since this reduction occurred only in the cued muscle in the absence of pre-TMS EMG activity, this reduction in excitability likely reflects a voluntary suppression of a particular finger representation. Experiment 2 replicated the suppression effect in a group that received veridical feedback while showing that this was not the case for a yoked sham feedback group. Notably, the choice of control (sham) group in this double-blind study is highly rigorous, as these participants received feedback that matched the real feedback experimental group, thus experiencing the same degree of positive and negative feedback in the same temporal progression. Experiment 3 went further by showing that mere motor imagery was not sufficient to lead to cued suppression even when incentivized by monetary reward. Taken together, the control experiments thus establish that veridical feedback, and not sham feedback or mere motor imagery, is necessary for cued motor suppression within the time constraints of 1 h of training.
Not Mere Motor Imagery
The absence of motor suppression in experiment 3 is at odds with the motor imagery study of Sohn et al. (2003). They demonstrated reduced hand muscle excitability when participants were instructed to engage in mental-relaxation techniques in the absence of visual feedback. However, a key difference in paradigms was that in ours, participants were required to engage in selective motor control (suppression of a specific cued finger rather than the hand in general), which prevented them from possibly relying on a strategy of nonspecifically increasing motor excitability on baseline trials (and thus leading to an apparent reduction of excitability on nonbaseline trials). We suppose that selective response suppression is more complicated mechanistically than generalized relaxation and may thus require training with veridical feedback. It is also noteworthy that Sohn et al. (2003) used TMS stimulation at 140% RMT during their mental-relaxation paradigm (much larger than the 110% RMT used here), which surely causes the fingers to “jump”; therefore, participants could use the perceived level of that sensation as a method for actual trial-by-trial feedback. In that sense, their result may not have been based on pure motor imagery. By contrast, although participants in the present study experienced a small twitch, the sham feedback and no feedback controls of experiments 2 and 3 demonstrate clearly that participants were unable to use the perceived sensation of this twitch as a form of trial-by-trial feedback.
Mental Strategies and Mechanism of Suppression
By what mental strategy did our participants achieve cued motor suppression? In the veridical feedback group of experiment 2, most eventually settled on thinking away from the cued muscle. Since such motor imagery would activate the M1 representations of alternative muscles (Sharma et al. 2006), one mechanistic possibility is that this activation in turn inhibits the M1 representation of the cued finger through a form of motor surround inhibition (Hallett 2003; Sohn and Hallett 2004).
Surround inhibition is a well-documented organizational principle of the sensory system (Hubel and Wiesel 1968) and was originally thought to be a function of corticocortical inhibition local to the cortex (Gilbert and Wiesel 1983). Analogously, corticocortical inhibition has also been suggested in the motor cortex (Hanajima et al. 1996; Kujirai et al. 1993) and could underlie the suppression seen here. Further work in sensory systems, however, has suggested that the actual locus of surround inhibition may be in lower level thalamic structures that in turn reduce excitatory drive to cortical sensory regions (Ozeki et al. 2004; Smith 2006). Similarly, surround inhibition of the motor cortex has been thought to be a function of the influence of inhibitory basal ganglia pathways, such as the indirect pathway, in reducing thalamocortical drive to the cortex (Mink 2003). Further neuroimaging studies may serve to elucidate better the locus of this training effect.
There are, however, some concerns with this motor surround explanation. Although this account predicts activation of the alternate finger, e.g., the index when suppressing the pinky, this was not clear from the data (i.e., Fig. 2 shows no significant elevation from baseline in the real feedback Uncued condition). However, we only recorded MEPs from the index and pinky, and it is possible that excitability in other motor effectors was elevated. Interestingly, participants in experiment 3 also mostly reported relying on thinking away from the cued muscle yet did not show reliable motor suppression. It is thus possible that the strategy expressed above does not correspond to the underlying physiological mechanism. Instead of using a motor surround inhibition mechanism, which relies first on activating an alternative motor effector to suppress another, participants might instead have directly targeted suppression at the cued effector. Such a mechanism could depend on corticostriatal signaling to bring about top-down (prefrontally driven) suppression of particular basal ganglia “motor channels” via the classic indirect pathway, leading to downstream suppression of the M1; such top-down control has been suggested by recent studies using action-stopping paradigms (Majid et al. 2013; Smittenaar et al. 2013; Vink et al. 2014).
Study Novelty and Future Directions
Our method contrasts with earlier studies that have attempted to train IC using paradigms where people must rapidly stop in response to an external signal. One such study showed a modest 20-ms improvement of stopping speed over the course of 10 sessions of stop-signal task training (Berkman et al. 2014), whereas another showed that practicing motoric stopping (as well as detecting occasional signals and responding to them) also led to faster stopping speed compared with a control group (Chevalier et al. 2014). However, other stop-signal training studies report null findings (Cohen and Poldrack 2008; Enge et al. 2014; Logan and Burkell 1986; and reviewed by Spierer et al. 2013). It is possible that these effects are inconsistent (and only modest when they occur) because these studies engage a reactive form of IC where sudden stopping in response to an external signal is already too rapid to allow for much improvement with training (Spierer et al. 2013). Moreover, even if that system can be trained, the behavioral benefit of training may not be generalizable to real-world situations that require IC in the absence of clear external signals to stop. Rather, these real-world situations may benefit from training a more proactive form of IC (as we use here) where one suppresses the motor system before any need arises. Such proactive IC training may be more feasible because participants must consciously apply mental strategies (also known as implementation intentions; see Burkard et al. 2013) before overt behavior in a way that is not under speed pressure.
Further work is necessary to establish the functional benefit of training using this paradigm. This could be attempted, for instance, by coupling the selective training of motor suppression described here with behavioral tasks of selective stopping for which advanced motor suppression of a particular effector might facilitate subsequent complex responses to a stop-signal (for a task example, see Cai et al. 2011; Majid et al. 2013). More ambitiously, training motor suppression of a particular movement (such as index finger grasping) in the context of a provocative stimulus (a cigarette, for instance) might lead to reduced urges for that stimulus (cf. Freeman et al. 2014).
The motor suppression trained in this present study also differs from phenomena observed in other TMS studies that have previously shown suppression of a muscle when cued to stop possibly later in a trial or when preparing to select a response (Cai et al. 2011; Duque and Ivry 2009; Labruna et al. 2014; Majid et al. 2013). Whereas those effects arose in highly specific task contexts, participants in this study achieved motor suppression using a verbalizable mental strategy without overt behavior. This raises the interesting prospect that the mental suppression strategies could be flexibly translated to other motor effectors such as the leg or face. A limitation of the current study is that it did not test this generalizability of mental strategy, but if this held true, it would have high clinical relevance for Tourette's disorder, for instance, where targeted suppression may be of benefit against motor tics that affect and migrate between a number of regions (Shahana and Gilbert 2013). Another potential clinical application is with regard to focal hand dystonia where impairments of motor inhibition are known (Shahana and Gilbert 2013; Stinear and Byblow 2004). Here, suppression or activation training could be used to help patients better individuate particular movements.
In summary, we developed a new methodology of trial-by-trial real-time feedback of MEPs, and we show that this is effective in helping participants discover a mental strategy for selective motor suppression.
GRANTS
We thank the National Institutes of Health (Grants DA-026452 and F31-NS-077560) for financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.S.A.M. and A.R.A. conception and design of research; D.S.A.M. and C.L. performed experiments; D.S.A.M. analyzed data; D.S.A.M. and A.R.A. interpreted results of experiments; D.S.A.M. prepared figures; D.S.A.M. drafted manuscript; D.S.A.M., C.L., and A.R.A. edited and revised manuscript; D.S.A.M., C.L., and A.R.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jan Wessel for help with the real-time feedback method, Melissa Aguilar for data acquisition, Mark Appelbaum for statistical advice, David Linderman for participant recruitment, and Scott Freeman and Melissa Burney for helpful comments.
REFERENCES
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69: e55–e68, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18: 177–185, 2014. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108: 44–79, 2013. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Kahn LE, Merchant JS. Training-induced changes in inhibitory control network activity. J Neurosci 34: 149–157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard C, Rochat L, Van der Linden M. Enhancing inhibition: how impulsivity and emotional activation interact with different implementation intentions. Acta Psychol (Amst) 144: 291–297, 2013. [DOI] [PubMed] [Google Scholar]
- Cai W, Oldenkamp C, Aron AR. A proactive mechanism for selective suppression of response tendencies. J Neurosci 31: 5965–5969, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20: 255–261, 2007. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33: 631–646, 2009. [DOI] [PubMed] [Google Scholar]
- Channon S, Drury H, Martinos M, Robertson MM, Orth M, Crawford S. Tourette's syndrome (TS): inhibitory performance in adults with uncomplicated TS. Neuropsychology 23: 359–366, 2009. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Chatham CH, Munakata Y. The practice of going helps children to stop: the importance of context monitoring in inhibitory control. J Exp Psychol Gen 143: 959–965, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci 29: 15870–15877, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Poldrack RA. Automaticity in motor sequence learning does not impair response inhibition. Psychon Bull Rev 15: 108–115, 2008. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Stop and go: the neural basis of selective movement prevention. J Cogn Neurosci 21: 1193–1203, 2009. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex 19: 2013–2024, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge S, Behnke A, Fleischhauer M, Küttler L, Kliegel M, Strobel A. No evidence for true training and transfer effects after inhibitory control training in young healthy adults. J Exp Psychol Learn Mem Cogn 40: 987–1001, 2014. [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Huster RJ, Herrmann CS. Boosting brain functions: improving executive functions with behavioral training, neurostimulation, and neurofeedback. Int J Psychophysiol 88: 1–16, 2013. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Razhas I, Aron AR. Top-down response suppression mitigates action tendencies triggered by a motivating stimulus. Curr Biol 24: 212–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Clustered intrinsic connections in cat visual cortex. J Neurosci 3: 1116–1133, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Surround inhibition. Suppl Clin Neurophysiol 56: 153–159, 2003. [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci 140: 109–116, 1996. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol 195: 215–243, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Verbruggen F, Frank MJ, Waldorp LJ, Colzato L, Ridderinkhof KR, Forstmann BU. How preparation changes the need for top-down control of the basal ganglia when inhibiting premature actions. J Neurosci 32: 10870–10878, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard DH, Pelli D. What's new in Psychtoolbox-3? Perception 36: 2007. [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci 26: 269–278, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol 114: 216–222, 2005. [DOI] [PubMed] [Google Scholar]
- Logan GD, Burkell J. Dependence and independence in responding to double stimulation: a comparison of stop, change, and dual-task paradigms. J Exp Psychol 12: 549–563, 1986. [Google Scholar]
- Majid DS, Cai W, Corey-Bloom J, Aron AR. Proactive selective response suppression is implemented via the basal ganglia. J Neurosci 33: 13259–13269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 60: 1365–1368, 2003. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Sadakane O, Akasaki T, Naito T, Shimegi S, Sato H. Relationship between excitation and inhibition underlying size tuning and contextual response modulation in the cat primary visual cortex. J Neurosci 24: 1428–1438, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapkin SA, Falkenstein M, Marks A, Griefahn B. Practice-related effects in a Go-Nogo task. Percept Mot Skills 105: 1275–1288, 2007. [DOI] [PubMed] [Google Scholar]
- Shahana N, Gilbert DL. Tourette syndrome. Handb Clin Neurol 116: 631–642, 2013. [DOI] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke 37: 1941–1952, 2006. [DOI] [PubMed] [Google Scholar]
- Smith MA. Surround suppression in the early visual system. J Neurosci 26: 3624–3625, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smittenaar P, Guitart-Masip M, Lutti A, Dolan RJ. Preparing for selective inhibition within frontostriatal loops. J Neurosci 33: 18087–18097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn YH, Dang N, Hallett M. Suppression of corticospinal excitability during negative motor imagery. J Neurophysiol 90: 2303–2309, 2003. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res 158: 397–404, 2004. [DOI] [PubMed] [Google Scholar]
- Spierer L, Chavan CF, Manuel AL. Training-induced behavioral and brain plasticity in inhibitory control. Front Hum Neurosci 7: 427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Impaired modulation of intracortical inhibition in focal hand dystonia. Cereb Cortex 14: 555–561, 2004. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev 33: 647–661, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, van den Wildenberg WP, Du Plessis S, Kahn RS. Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum Brain Mapp 35: 4415–4427, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR. Fundamentals of Modern Statistical Methods: Substantially Improving Power and Accuracy. New York: Springer, 2001. [Google Scholar]