Abstract
Background
Exposure to medium or high doses of ionizing radiation is a known risk factor for cancer in children. The extent to which low-dose radiation from natural sources contributes to the risk of childhood cancer remains unclear.
Objectives
In a nationwide census-based cohort study, we investigated whether the incidence of childhood cancer was associated with background radiation from terrestrial gamma and cosmic rays.
Methods
Children < 16 years of age in the Swiss National Censuses in 1990 and 2000 were included. The follow-up period lasted until 2008, and incident cancer cases were identified from the Swiss Childhood Cancer Registry. A radiation model was used to predict dose rates from terrestrial and cosmic radiation at locations of residence. Cox regression models were used to assess associations between cancer risk and dose rates and cumulative dose since birth.
Results
Among 2,093,660 children included at census, 1,782 incident cases of cancer were identified including 530 with leukemia, 328 with lymphoma, and 423 with a tumor of the central nervous system (CNS). Hazard ratios for each millisievert increase in cumulative dose of external radiation were 1.03 (95% CI: 1.01, 1.05) for any cancer, 1.04 (95% CI: 1.00, 1.08) for leukemia, 1.01 (95% CI: 0.96, 1.05) for lymphoma, and 1.04 (95% CI: 1.00, 1.08) for CNS tumors. Adjustment for a range of potential confounders had little effect on the results.
Conclusions
Our study suggests that background radiation may contribute to the risk of cancer in children, including leukemia and CNS tumors.
Citation
Spycher BD, Lupatsch JE, Zwahlen M, Röösli M, Niggli F, Grotzer MA, Rischewski J, Egger M, Kuehni CE, for the Swiss Pediatric Oncology Group and the Swiss National Cohort. 2015. Background ionizing radiation and the risk of childhood cancer: a census-based nationwide cohort study. Environ Health Perspect 123:622–628; http://dx.doi.org/10.1289/ehp.1408548
Introduction
Ionizing radiation is a known risk factor for cancer [United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2006]. For a given radiation dose, children are at a greater risk than adults (UNSCEAR 2013). Ionizing radiation is the only established environmental risk factor for childhood leukemia and tumors of the central nervous system (CNS), the two most common tumor types in childhood (Belson et al. 2007; Wiemels 2012; Wrensch et al. 2002). Evidence for the carcinogenic effects of ionizing radiation in children comes mainly from studies of exposure to moderate or high doses from atomic bombs or therapeutic radiation (Wakeford 2013; Wrensch et al. 2002). It remains unclear whether dose–response relationships observed in these study populations extend to lower doses from more widespread exposures such as diagnostic radiology or natural background radiation (Wakeford 2013).
Natural background radiation is ubiquitous and, for most people, the main source of radiation exposure (UNSCEAR 2000). About a third of this is attributable to cosmic rays and terrestrial gamma radiation whereas the rest is due to inhalation (mainly indoor radon) and ingestion of radionuclides (UNSCEAR 2000). Whereas the effective dose from radon is delivered primarily to the respiratory system, terrestrial gamma and cosmic rays dominate doses to the red bone marrow (Kendall et al. 2009), the primary site of leukemia initiation. In Switzerland, exposure levels of the resident population to background radiation vary considerably due to the relatively high radioactivity of crystalline rocks of the central Alpine massif compared with the sedimentary northern Alpine Foreland (Jura, Molasse Basin) (Rybach et al. 1996, 2002).
Most previous studies on the risk of childhood cancer and background ionizing radiation from terrestrial gamma or cosmic rays were ecological. Results from these studies were heterogeneous. Most of them showed little or no evidence of an association (Auvinen et al. 1994; Evrard et al. 2006; Mason and Miller 1974; Muirhead et al. 1992; Richardson et al. 1995; Tirmarche et al. 1988), and others suggested a positive (Hatch and Susser 1990; Knox et al. 1988) or even a negative association (Frigerio et al. 1973). Few studies to date have used individual data (Axelson et al. 2002; Kendall et al. 2013; UK Childhood Cancer Study Investigators 2002b). A case–control study from the United Kingdom using measurements made in children’s homes found no evidence of an association (UK Childhood Cancer Study Investigators 2002b). Recently, a record-based case–control study from the United Kingdom found evidence of an increasing risk with cumulative gamma-ray dose for childhood leukemia but not for other cancer types (Kendall et al. 2013).
Given the limited and conflicting evidence from previous studies, we used a nationwide cohort study to investigate the association between external ionizing radiation from cosmic and terrestrial sources and incidence of childhood cancer and its major diagnostic groups in Switzerland. Geocoded residential locations at census time points were available for the entire population, and a spatial model with separate components for terrestrial gamma radiation and the directly ionizing component of cosmic radiation was used for exposure assessment. Cases were identified from the Swiss Childhood Cancer Registry (SCCR; http://www.childhoodcancerregistry.ch/) (Michel et al. 2008).
Methods
Population. Our study included the Swiss resident population < 16 years of age. Data collected on these children during national censuses in 1990 and 2000, including georeferenced residential locations and demographic and socioeconomic information, were obtained from the Swiss National Cohort (SNC; http://www.swissnationalcohort.ch/) (Bopp et al. 2009). The SNC is a research platform based on nationwide individual record linkage between different censuses and mortality and migration records. This linkage allows calculating follow-up time for all individuals registered in the two censuses (Bopp et al. 2009; Spoerri et al. 2010). Birth weight and birth order were obtained through record linkage with the national birth registry. We excluded children whose residential locations were unknown or uncertain or could not be georeferenced to within 100 m.
Cases of childhood cancer were identified from the SCCR. The SCCR has an estimated completeness of > 90% for cancers in children < 16 years old diagnosed in Switzerland since 1985 (Michel et al. 2008). We included all cases with a tumor classified according to the International Classification of Childhood Cancer, Third Edition (ICCC-3; http://seer.cancer.gov/iccc/) (Steliarova-Foucher et al. 2005). The SCCR collects residential address histories of patients from diagnosis back to birth, allowing us to obtain residence at census. Addresses were geocoded using a list of georeferenced building addresses from the Swiss postal system (GeoPost) or manually using the geoportal maintained by the Federal Office of Topography (swisstopo) at http://map.geo.admin.ch. We used probabilistic record linkage (G-LINK 2.3; Statistics Canada; http://www5.statcan.gc.ca/olc-cel/olc.action?lang=en&ObjId=10H0036&ObjType=22) to link cases with children from the SNC based on the variables sex, date of birth, maternal and paternal dates of birth, geocoded residence at census, municipality of residence at census and at birth, and nationality. This study is based on register data, and informed consent was not required. The SNC was approved by the ethics committees of the Cantons of Bern and Zurich and by the Federal Data Protection Office (http://www.edoeb.admin.ch/?lang=en).
Outcomes. We limited analyses to major diagnostic categories: any cancer (all ICCC-3 diagnostic groups), leukemias (ICCC-3 diagnostic group I), acute lymphoblastic leukemias (ALL) (I.a), lymphomas (II), and tumors of the CNS tumors (III), which include malignant and nonmalignant intracranial and intraspinal tumors. We also analyzed other malignant tumors comprising all remaining ICCC-3 diagnostic groups (IV–XII).
Exposure assessment. We estimated exposure to external background radiation as total dose rates at children’s homes from cosmic and terrestrial sources based on a previously developed exposure model (Rybach et al. 1996, 2002). This model estimates total dose rates for each cell of a 2 km × 2 km grid as the sum of three separately estimated components: the directly ionizing component of cosmic radiation, natural terrestrial gamma radiation, and artificial terrestrial radiation. The cosmic dose rate is calculated as a function of altitude. Grid values were obtained by averaging topographic altitude within grid cells using a digital terrain model. The natural terrestrial component combines airborne gamma-ray spectrometry (about 10% of the country’s surface surveyed by helicopter), in situ gamma-ray spectrometry (166 sites), in situ dose rate measurements using ionization chambers (837 sites), and laboratory measurements of rock and soil samples from 612 sites. These measurements span the time period from the early 1960s to mid-1990s. In addition to airborne measurements, a total of 1,615 ground data points were available; these correspond to about 1 point per 25 km2. The model did not account for temporal variations in natural radiation, for example, due to snow cover or sun activity. The artificial terrestrial component mainly reflects 137Cs (cesium) deposition originating from the Chernobyl accident and is based on airborne and in situ measurements taken after 1987. Grid cell dose rates for the terrestrial components were interpolated from the available data points using the inverse distance method and a search radius of 12 km. More details on measurements and calibration procedures are provided elsewhere (Rybach et al. 1996).
Potential confounders. We considered the following potential confounding factors: traffic-related air pollution (proxied by distance to nearest highway), electromagnetic fields from radio and TV transmitters (field strength based on a geographic model) (Hauri et al. 2014), and from high-voltage power lines (distance to nearest 380 kV or 220 kV power line), degree of urbanization of municipality (urban, peri-urban, rural), socioeconomic status based on the Swiss neighborhood index of socioeconomic position (Swiss-SEP) (Panczak et al. 2012), education of household reference person (compulsory, secondary, tertiary), and crowding (number of persons per room), birth weight, and birth order of the child.
Statistical analyses. We investigated incidence of childhood cancer by total dose rate using time to event analyses with age as the underlying time scale. Follow-up time began at the first census in which a child was recorded (entry time) and ended on the earliest of following events: diagnosis, death, emigration, the child’s 16th birthday, administrative censoring on 31 December 2008. Exposure was based on residential location at census. For a child appearing in both censuses but living at a different location in 2000 than in 1990, the 1990 exposure was updated in 1995 or 2000 depending on whether or not the child lived at the new location 5 years before census 2000 (information from census questionnaire). Total dose rate was categorized into regular intervals of 50 nSv/hr with subsequent regrouping such that no interval contained < 1% of the census populations. This resulted in the following categorization: < 100 nSv/hr, 100 to < 150, 150 to < 200, ≥ 200 nSv/hr. We estimated hazard ratios (HR) for different exposure categories using Cox proportional hazards models. All models were adjusted for sex and birth year, and in separate models we adjusted for the other potential confounders. We also ran trend analyses using a linear exposure term. To explore potential effects of misclassification due to residential mobility, we restricted time-to-event analyses to children who had a stable place of residence up to entry into the cohort, defined as those reporting at entry to have had the same residence 5 years earlier or, if these data were missing (e.g., for children < 5 years of age at census), reporting to have lived in the same municipality at birth.
We repeated the trend analyses using cumulative dose instead of dose rate. Cumulative dose was calculated by integrating dose rate over time since birth. To conduct these analyses, we created a nested case–control data set by randomly sampling 100 controls per case from among those at risk at the time of the case’s failure. We then calculated cumulative doses and fitted conditional logistic regression models conditioning on case–controls sets. This procedure is asymptotically equivalent to Cox proportional hazards regression using the full cohort (Goldstein and Langholz 1992). All analyses were done using Stata version 12.1 (StataCorp, College Station, TX, USA).
Results
Characteristics of the study population. Of 3,502 eligible patients in the SCCR who were diagnosed between the census in 1990 and the end of 2008, 1,782 could be included in time-to-event analyses, and of these 1,311 belonged to the subcohort of children with stable place of residence (Figure 1). The distribution of diagnoses among eligible and included cases is shown in Supplemental Material, Table S1. Because included cases were required to be without diagnosis at census, they tended to be older at diagnosis and hence included slightly fewer leukemia and more lymphoma and CNS cases compared with all eligible cases.
Figure 1.
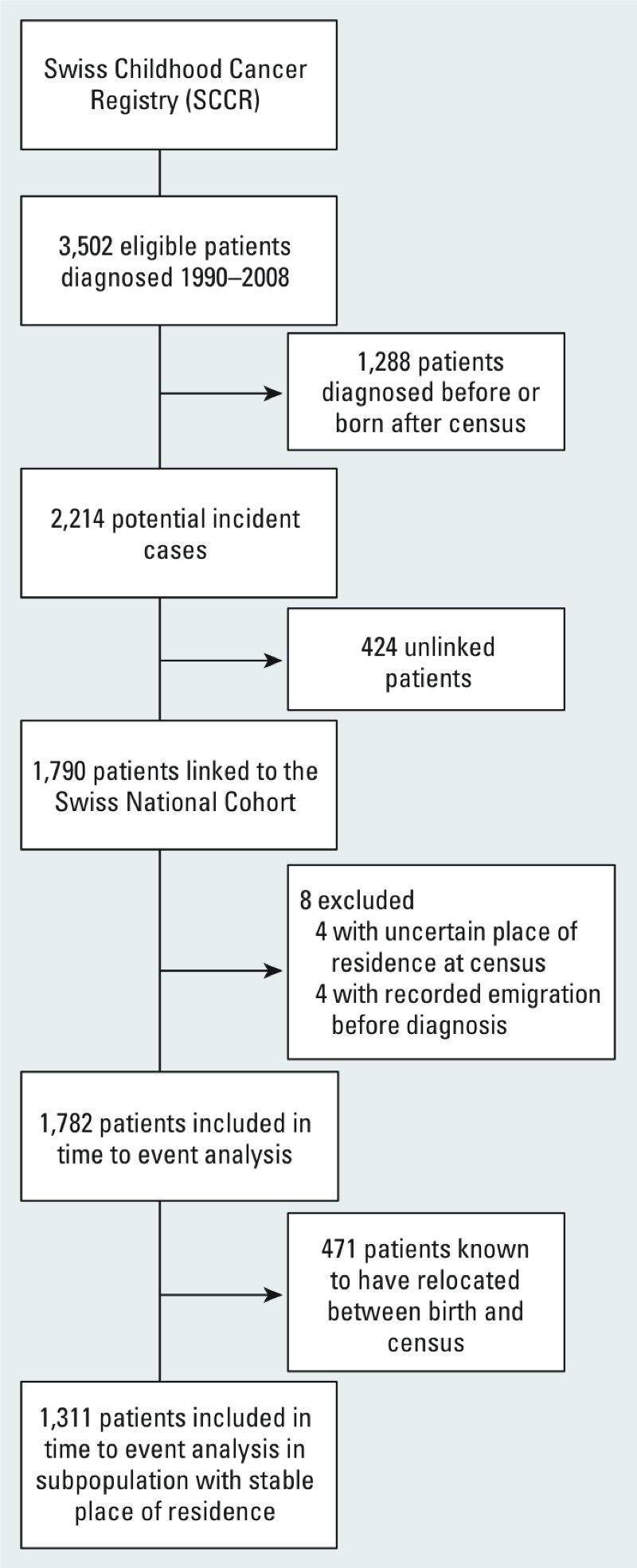
Flow chart of childhood cancer cases included in analyses.
The SNC included 2,129,264 children < 16 years of age at census. Of these, 34,371 were excluded due to uncertain residence and 1,233 did not contribute person time (mostly because they were linked to an SCCR case diagnosed before census) leaving 2,093,660 (98.3%) for time-to-event analyses. These children had a mean age of 7.0 years at entry into the cohort, that is, the first census they were registered in. They were followed-up for a mean of 7.7 years and accrued 16.1 million person-years at risk. Follow-up time ended due to emigration or death in 47,119 children (2.6% of those included). At child’s entry into the cohort, the mean dose rate of external background radiation was 109 nSv/hr (median, 103 nSv/hr; range, 55–383 nSv/hr; interquartile range, 95–112 nSv/hr). On average, natural terrestrial radiation contributed 54 nSv/hr, cosmic radiation 45 nSv/hr, and artificial terrestrial radiation 8 nSV/hr. In terms of exposure variability, natural terrestrial radiation is most relevant (see Supplemental Material, Figure S1). Table 1 reports other characteristics of the study population at entry into the cohort according to categories of total external background radiation. Compared with the least exposed group, highly exposed children tended to live in more rural areas and in neighborhoods of lower SEP. For instance, 53% of children exposed to a dose rate of ≥ 200 nSv/hr belonged to the lowest SEP quintile compared with 26% of those exposed to < 100 nSv/hr. Children with higher exposure also tended to be more exposed to highways and electromagnetic fields from high voltage power lines and broadcast transmitters.
Table 1.
Characteristics of study population by exposure to external background radiation.
| Characteristic | Exposure category [n (%)] | p-Valuea | |||
|---|---|---|---|---|---|
| < 100 nSv/hrn = 806,450 (100.0%) | 100 to < 150 nSv/hrn = 1,146,470 (100.0%) | 150 to < 200 nSv/hrn = 119,245 (100.0%) | ≥ 200 nSv/hrn = 21,495 (100.0%) | ||
| Sex | |||||
| Male | 413,816 (51.3) | 587,551 (51.2) | 61,002 (51.2) | 10,980 (51.1) | 0.620 |
| Female | 392,634 (48.7) | 558,919 (48.8) | 58,243 (48.8) | 10,515 (48.9) | |
| Year of birth | |||||
| 1974–1979 | 143,378 (17.8) | 206,716 (18.0) | 22,720 (19.1) | 3,884 (18.1) | < 0.001 |
| 1980–1984 | 147,055 (18.2) | 210,097 (18.3) | 21,658 (18.2) | 4,072 (18.9) | |
| 1985–1989 | 171,858 (21.3) | 247,084 (21.6) | 24,959 (20.9) | 4,636 (21.6) | |
| 1990–1994 | 167,075 (20.7) | 234,456 (20.5) | 24,091 (20.2) | 4,398 (20.5) | |
| 1995–2000 | 177,084 (22.0) | 248,117 (21.6) | 25,817 (21.7) | 4,505 (21.0) | |
| Degree of urbanization | |||||
| Urban | 142,366 (17.7) | 335,881 (29.3) | 22,915 (19.2) | 913 (4.2) | < 0.001 |
| Peri-urban | 366,564 (45.5) | 526,711 (45.9) | 52,251 (43.8) | 7,214 (33.6) | |
| Rural | 297,520 (36.9) | 283,878 (24.8) | 44,079 (37.0) | 13,368 (62.2) | |
| Swiss-SEP index | |||||
| 1st quintile (low SEP) | 210,686 (26.1) | 297,632 (26.0) | 56,522 (47.4) | 11,423 (53.1) | < 0.001 |
| 2nd quintile | 172,663 (21.4) | 227,926 (19.9) | 30,711 (25.8) | 5,006 (23.3) | |
| 3rd quintile | 161,461 (20.0) | 214,197 (18.7) | 18,456 (15.5) | 3,098 (14.4) | |
| 4th quintile | 146,832 (18.2) | 209,000 (18.2) | 10,398 (8.7) | 1,568 (7.3) | |
| 5th quintile (high SEP) | 112,837 (14.0) | 194,929 (17.0) | 2,687 (2.3) | 351 (1.6) | |
| Missing | 1,971 (0.2) | 2,786 (0.2) | 471 (0.4) | 49 (0.2) | |
| Education level of head of household | |||||
| Compulsory or less | 138,987 (17.2) | 210,295 (18.3) | 24,486 (20.5) | 3,502 (16.3) | < 0.001 |
| Secondary level | 407,213 (50.5) | 555,474 (48.5) | 64,151 (53.8) | 13,056 (60.7) | |
| Tertiary level | 235,179 (29.2) | 341,226 (29.8) | 27,434 (23.0) | 4,390 (20.4) | |
| Not known | 25,071 (3.1) | 39,475 (3.4) | 3,174 (2.7) | 547 (2.5) | |
| Persons per room (tertiles) | |||||
| < 0.82 | 297,857 (36.9) | 405,454 (35.4) | 39,882 (33.4) | 7,372 (34.3) | < 0.001 |
| 0.82 to < 1.08 | 296,570 (36.8) | 417,782 (36.4) | 44,575 (37.4) | 7,929 (36.9) | |
| ≥ 1.08 | 212,023 (26.3) | 323,234 (28.2) | 34,788 (29.2) | 6,194 (28.8) | |
| Birth weight [g (tertiles)] | |||||
| < 3,152 | 152,649 (18.9) | 216,703 (18.9) | 24,864 (20.9) | 4,945 (23.0) | < 0.001 |
| 3,152 to < 3,541 | 152,019 (18.9) | 209,712 (18.3) | 22,582 (18.9) | 4,406 (20.5) | |
| ≥ 3,541 | 156,445 (19.4) | 212,081 (18.5) | 20,558 (17.2) | 3,844 (17.9) | |
| Missing | 345,337 (42.8) | 507,974 (44.3) | 51,241 (43.0) | 8,300 (38.6) | |
| Birth sequence | |||||
| 1st | 193,045 (23.9) | 272,626 (23.8) | 29,287 (24.6) | 5,581 (26.0) | < 0.001 |
| 2nd | 168,655 (20.9) | 233,509 (20.4) | 25,130 (21.1) | 4,825 (22.4) | |
| 3rd or later | 85,261 (10.6) | 109,151 (9.5) | 11,207 (9.4) | 2,343 (10.9) | |
| Missing | 359,489 (44.6) | 531,184 (46.3) | 53,621 (45.0) | 8,746 (40.7) | |
| Distance to nearest highway (m) | |||||
| < 100 | 10,756 (1.3) | 16,325 (1.4) | 1,317 (1.1) | 397 (1.8) | < 0.001 |
| 100 to < 250 | 28,945 (3.6) | 47,372 (4.1) | 5,318 (4.5) | 1,072 (5.0) | |
| 250 to < 500 | 62,115 (7.7) | 97,023 (8.5) | 11,792 (9.9) | 1,578 (7.3) | |
| ≥ 500 | 704,634 (87.4) | 985,750 (86.0) | 100,818 (84.5) | 18,448 (85.8) | |
| Distance to high-voltage power line (m) | |||||
| < 100 | 6,066 (0.8) | 7,198 (0.6) | 2,203 (1.8) | 588 (2.7) | < 0.001 |
| 100 to < 250 | 17,212 (2.1) | 19,262 (1.7) | 5,536 (4.6) | 2,183 (10.2) | |
| 250 to < 500 | 41,979 (5.2) | 52,866 (4.6) | 11,736 (9.8) | 3,281 (15.3) | |
| ≥ 500 | 741,193 (91.9) | 1,067,144 (93.1) | 99,770 (83.7) | 15,443 (71.8) | |
| EMF from broadcast transmitters (V/m)b | |||||
| < 0.05 | 719,804 (89.3) | 953,221 (83.1) | 87,381 (73.3) | 18,990 (88.3) | < 0.001 |
| 0.05 to < 0.2 | 67,901 (8.4) | 142,217 (12.4) | 19,705 (16.5) | 1,019 (4.7) | |
| ≥ 0.2 | 17,390 (2.2) | 48,744 (4.3) | 11,900 (10.0) | 1,454 (6.8) | |
| Missing | 1,355 (0.2) | 2,288 (0.2) | 259 (0.2) | 32 (0.1) | |
| Abbreviations: SEP, socioeconomic position; EMF, electromagnetic fields.aFrom chi-square tests. bModeled field strength from radio and TV transmitters. | |||||
Association between childhood cancer and dose rate. Tables 2 and 3 and Figure 2 show results of analyses using dose rate as exposure. We found a markedly increased risk among children exposed to a dose rate ≥ 200 nSv/hr compared with those exposed to < 100 nSv/hr for any cancer [hazard ratio (HR) = 1.64; 95% confidence interval (CI): 1.13, 2.37], leukemia (HR = 2.04; 95% CI: 1.11, 3.74), ALL (HR = 2.12; 95% CI: 1.09, 4.16), and CNS tumors (HR = 1.99; 95% CI: 0.98, 4.05) (Table 2). For intermediate exposure levels, HRs tended to be close to 1. Adjusting for potential confounders did not materially alter results (see Supplemental Material, Figure S2). In trend analyses using a linear exposure term, HRs per increase of 100 nSv/hr in dose rate were between 1.2 and 1.4 for all diagnostic groups except lymphoma, where it was close to 1. The lower confidence limit exceeded 1 only for all cancers (HR = 1.27; 95% CI: 1.06, 1.52 per 100 nSv/hr) (Table 3, Figure 2). When we restricted analyses to children with stable residence before entry into the cohort (66.5% of the entire cohort), results remained similar with somewhat larger effect estimates (Table 3; see also Supplemental Material, Table S2).
Table 2.
Association between childhood cancer and dose rate of external background radiation in the Swiss National Cohort.
| Outcome | Dose rate (nSv/hr) | Cases (n) | IRa | HR (95% CI)b |
|---|---|---|---|---|
| All cancers | < 100 | 659 | 10.56 | 1.00 (reference) |
| 100 to < 150 | 982 | 11.16 | 1.06 (0.96, 1.17) | |
| 150 to < 200 | 112 | 12.32 | 1.17 (0.96, 1.43) | |
| ≥ 200 | 29 | 17.22 | 1.64 (1.13, 2.37) | |
| Leukemia | < 100 | 201 | 3.22 | 1.00 (reference) |
| 100 to < 150 | 288 | 3.27 | 1.02 (0.85, 1.22) | |
| 150 to < 200 | 30 | 3.30 | 1.03 (0.70, 1.51) | |
| ≥ 200 | 11 | 6.53 | 2.04 (1.11, 3.74) | |
| ALL | < 100 | 158 | 2.53 | 1.00 (reference) |
| 100 to < 150 | 225 | 2.56 | 1.01 (0.82, 1.24) | |
| 150 to < 200 | 24 | 2.64 | 1.05 (0.68, 1.61) | |
| ≥ 200 | 9 | 5.34 | 2.12 (1.09, 4.16) | |
| Lymphoma | < 100 | 122 | 1.96 | 1.00 (reference) |
| 100 to < 150 | 186 | 2.11 | 1.08 (0.86, 1.36) | |
| 150 to < 200 | 17 | 1.87 | 0.96 (0.58, 1.59) | |
| ≥ 200 | 3 | 1.78 | 0.91 (0.29, 2.86) | |
| CNS tumors | < 100 | 150 | 2.40 | 1.00 (reference) |
| 100 to < 150 | 239 | 2.72 | 1.13 (0.92, 1.39) | |
| 150 to < 200 | 26 | 2.86 | 1.19 (0.79, 1.81) | |
| ≥ 200 | 8 | 4.75 | 1.99 (0.98, 4.05) | |
| Other malignant tumors | < 100 | 186 | 2.98 | 1.00 (reference) |
| 100 to < 150 | 269 | 3.06 | 1.03 (0.85, 1.24) | |
| 150 to < 200 | 39 | 4.29 | 1.44 (1.02, 2.04) | |
| ≥ 200 | 7 | 4.16 | 1.39 (0.66, 2.97) | |
| Abbreviations: ALL, acute lymphoblastic leukemia; CNS, central nervous system; HR, hazard ratio; IR, incidence rate.aPer 100,000 person-years at risk. bFrom Cox proportional hazards models adjusting for sex and birth year. | ||||
Table 3.
Hazard ratios for childhood cancer per 100 nSv/hr dose rate of external radiation in the Swiss National Cohort.
| Outcome | Entire cohort | Subcohort with stable place of residence before entrya | ||
|---|---|---|---|---|
| HR (95% CI)b | p-Value | HR (95% CI)b | p-Value | |
| All cancers | 1.27 (1.06, 1.52) | 0.011 | 1.33 (1.08, 1.62) | 0.006 |
| Leukemia | 1.25 (0.90, 1.75) | 0.186 | 1.31 (0.90, 1.92) | 0.159 |
| ALL | 1.23 (0.84, 1.81) | 0.278 | 1.31 (0.86, 2.01) | 0.205 |
| Lymphoma | 1.06 (0.68, 1.67) | 0.788 | 1.16 (0.70, 1.92) | 0.558 |
| CNS tumors | 1.32 (0.91, 1.91) | 0.139 | 1.42 (0.96, 2.12) | 0.081 |
| Other malignant tumors | 1.37 (0.98, 1.91) | 0.064 | 1.36 (0.93, 1.98) | 0.110 |
| Abbreviations: ALL, acute lymphoblastic leukemia; CNS, central nervous system; HR, hazard ratio.aChildren with same residence 5 years before entry into the cohort or, if this information was lacking, lived in the same municipality at birth. bFrom Cox proportional hazards models using a linear exposure term adjusting for sex and birth year. | ||||
Figure 2.
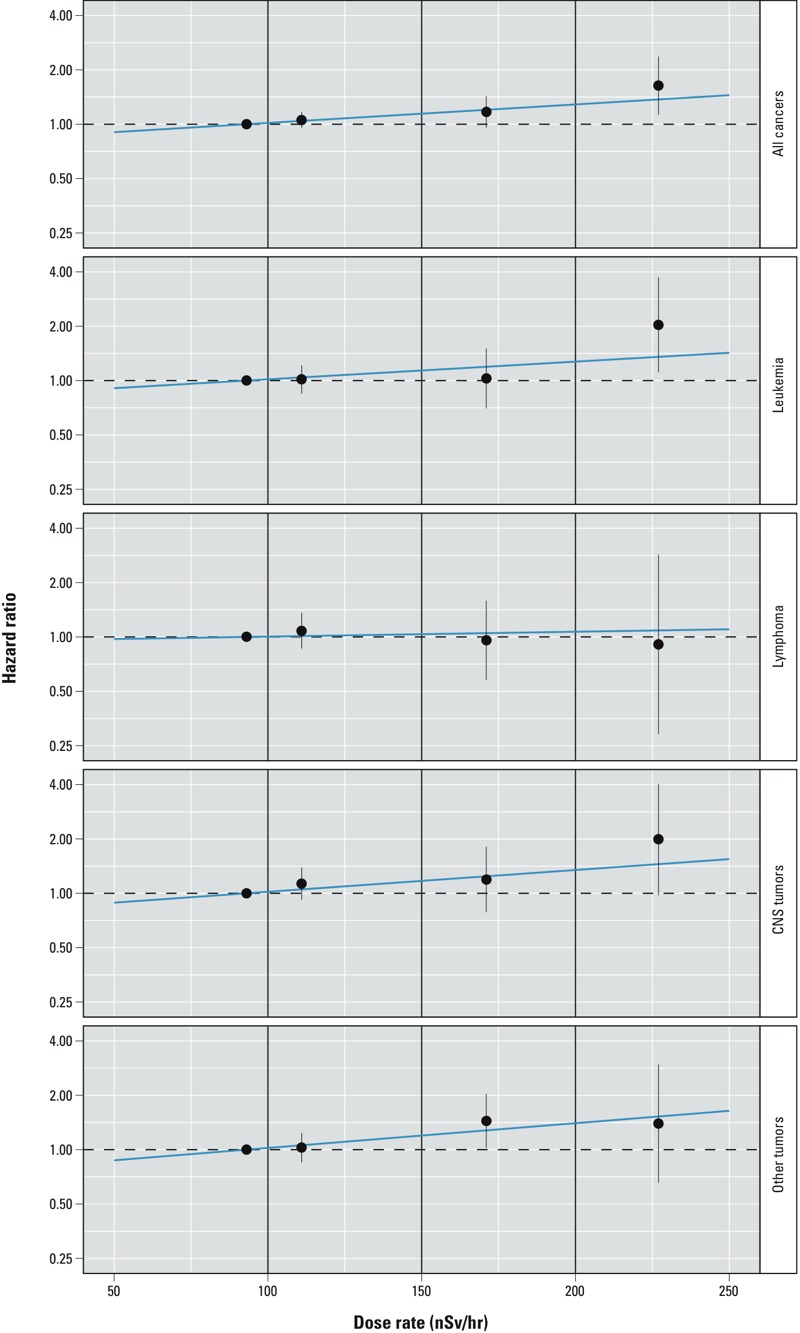
Hazard ratios for cancer by dose rate of external ionizing radiation among children < 16 years of age in the Swiss National Cohort. Results from Cox proportional hazards models adjusting for sex and birth year using a categorized exposure [points and bars (95% CIs) placed along the x-axis at mean dose rates within categories; categories delineated by vertical lines] and a linear exposure term (blue line). Dose rates < 100 nSv/hr are the reference category.
Trend analyses using cumulative dose. Calculated cumulative dose had a mean of 9.06 mSv (median, 9.12 mSv; range, 0.03–49.4 mSv; interquartile range, 5.55–12.1 mSv) and, as expected, correlated strongly with age (see Supplemental Material, Table S3). Trend analyses using cumulative dose showed a similar pattern as for dose rate with stronger evidence of a trend for all cancers (HR = 1.028; 95% CI: 1.008, 1.048 per mSv cumulative dose), leukemia (HR = 1.036; 95% CI: 0.997, 1.077), and CNS tumors (HR = 1.042; 95% CI: 1.002, 1.084) (Table 4). Restricting to the subcohort with stable place of residence before census again resulted in larger effect estimates, particularly for CNS tumors (HR = 1.060; 95% CI: 1.015, 1.106 per mSv) (Table 4).
Table 4.
Hazard ratios for childhood cancer per mSv cumulative dose of external radiation in the Swiss National Cohort.
| Outcome | Entire cohort | Subcohort with stable placeof residence before entrya | ||
|---|---|---|---|---|
| HR (95% CI)b | p-Value | HR (95% CI)b | p-Value | |
| All cancers | 1.028 (1.008, 1.048) | 0.006 | 1.040 (1.017, 1.064) | < 0.001 |
| Leukemia | 1.036 (0.997, 1.077) | 0.075 | 1.046 (0.999, 1.096) | 0.054 |
| ALL | 1.037 (0.990, 1.086) | 0.124 | 1.049 (0.994, 1.107) | 0.084 |
| Lymphoma | 1.007 (0.964, 1.052) | 0.746 | 1.022 (0.973, 1.073) | 0.386 |
| CNS tumors | 1.042 (1.002, 1.084) | 0.041 | 1.060 (1.015, 1.106) | 0.008 |
| Other malignant tumors | 1.025 (0.989, 1.063) | 0.177 | 1.034 (0.991, 1.078) | 0.121 |
| Abbreviations: ALL, acute lymphoblastic leukemia; CNS, central nervous system; HR, hazard ratio. aChildren with same residence 5 years before entry into the cohort or, if this information was lacking, lived in the same municipality at birth. bFrom conditional logistic regression in nested case–control sample (equivalent to Cox proportional hazards regression) adjusting for sex and birth year. | ||||
Discussion
This nationwide census-based cohort study in Switzerland found evidence of an increased risk of cancer among children exposed to external dose rates of background ionizing radiation of ≥ 200 nSv/hr compared with those exposed to < 100 nSv/hr. Trend analyses showed an increasing risk with cumulative dose received since birth for all cancers taken together, and for leukemia and CNS tumors.
Although the effects of acute exposure to moderate or high doses (> 100 mSv) of ionizing radiation on the risk of childhood cancer have been clearly demonstrated, the evidence for protracted exposure to low-dose radiation is still scarce (Wakeford 2013). Studies from high-risk groups, including atomic bomb survivors and groups exposed to therapeutic radiation, report relative risks in the order of about 5–8 and 2–5 per Sv for leukemia and CNS tumors, respectively, among subjects exposed at < 20 years of age (UNSCEAR 2013). For leukemia, estimated relative risks exceed 50 per Sv shortly after exposure among those exposed in early life (UNSCEAR 2013; Wakeford 2013). Extrapolating from models calibrated to risks observed in atomic bomb survivors, the excess fraction of childhood leukemia cases due to natural background radiation has been estimated to be up to about 20% in France (Laurent et al. 2013) and the United Kingdom (Little et al. 2009). However, there are great uncertainties attached to such estimates.
A recent register-based case–control study from the United Kingdom including 27,447 cases (of whom 9,058 had leukemia and 6,585 had CNS tumors) and 36,793 matched controls found a relative risk of 1.03 (95% CI: 1.00, 1.07) for all childhood cancer, 1.09 (95% CI: 1.02, 1.17) for leukemia, 1.10 (95% CI: 1.02, 1.19) for ALL, 1.01 (95% CI: 0.93, 1.09) for lymphoma, and 1.02 (95% CI: 0.96, 1.08) for CNS tumors for each milligray increase in cumulative indoor gamma-ray exposure since birth (Kendall et al. 2013). Given the rarity of childhood cancer, the hazard ratios per millisievert increase in cumulative dose of outdoor radiation found in our study (Table 4) can be interpreted as risk ratios (Symons and Moore 2002). Assuming a 20% reduction of doses due to the shielding effect of buildings (UNSCEAR 2000), an estimated difference of 1 mSv cumulative dose in our data relates to a difference indoors of 0.8 mSv, and the results in the first column of Table 4 translate to relative risks of 1.03 (95% CI: 1.01, 1.06) for all childhood cancers, 1.05 (95% CI: 1.00, 1.10) for leukemia, 1.05 (95% CI: 0.99, 1.11) for ALL, 1.01 (95% CI: 0.96, 1.07) for lymphoma, and 1.05 (95% CI: 1.00, 1.11) for CNS tumors. Compared to the UK study, our point estimates are smaller for leukemia and larger for CNS tumors. However, the wide overlap of confidence intervals for corresponding outcomes demonstrates good agreement between the studies. The effect estimates observed in the population with stable residence before entry into the cohort (Table 4) suggest that failure to account for residential mobility results in a downward bias and that relative risks in both studies were underestimated. Somewhat surprisingly, our confidence intervals are narrower despite the much smaller number of cases included in the study. This could have several reasons: For a given number of cases, a cohort study such as ours has larger statistical power than a case–control study with only a single control per case (Little et al. 2010); both the case–control pairs in the UK study and the risk sets in our study were age-matched; and, conditional on age, variability of cumulative exposure was considerably larger in our study [compare Supplemental Material, Table S3, with Table S7 in Kendall et al. (2013)]; furthermore, the UK study assessed exposures with a lower spatial resolution, and, consequently, almost half of the cases shared the same exposure level as their controls.
Few other studies have used individual data to investigate a potential link between childhood cancer and radiation from natural gamma or cosmic rays (Axelson et al. 2002; UK Childhood Cancer Study Investigators 2002b) (see Supplemental Material, Table S4). The UK Childhood Cancer Study—a case–control study that used gamma dose rates (including the cosmic component of penetrating radiation) measured in children’s homes at the time of diagnosis—found no evidence of an association for leukemia. As in our analysis, effect estimates for CNS tumors were elevated in the highest exposure categories, but confidence intervals were wide and included one. A case–control study from Sweden reported an odds ratio of 1.4 (95% CI: 1.0, 2.0) for ALL among subjects < 20 years of age, comparing those living in buildings made of alum shale concrete with those living in other houses (Axelson et al. 2002). Overall, studies using individual data suggest an increasing risk for ALL with cumulative dose of natural gamma radiation (see Supplemental Material, Table S4). In contrast to this, an investigation in high background radiation areas in China and India found no indication of increased risks of childhood leukemia (Akiba et al. 2002).
We did not adjust for domestic radon exposure because this information was available only for the 2000 census, and our recent study on domestic radon and childhood cancer found no evidence of an association in the SNC (Hauri et al. 2013). The UK record-based case–control study by Kendall et al. and the UK Childhood Cancer Study also found little indication of an increased risk of childhood cancers due to radon (Kendall et al. 2013; UK Childhood Cancer Study Investigators 2002a). However, a Danish record-based case–control study and most ecologic studies reported positive associations between childhood leukemia and domestic radon (Raaschou-Nielsen 2008; Raaschou-Nielsen et al. 2008; Tong et al. 2012).
Exposure assessment in our study was based on a geographic model rather than on actual measurements at children’s homes. Although the model was based on a dense net of measurements covering the entire country, methods of interpolation and calibration, measurement error, and the neglect of exposure variability due to natural factors such as snow cover or sun activity are likely to have caused some exposure misclassification. Calculated doses were based on outdoor dose rates, although children spend most of their time indoors. Unfortunately we did not have address histories for the entire population and could therefore not fully account for residential mobility in our calculation of cumulative dose. However, for some children (21%) residential locations were known at two time points. Outcome assessment was based on probabilistic record linkage between the SCCR and SNC and is likely to have resulted in some misclassification of the outcomes. Based on linkage results, we judge that at least 93% of the linked SCCR–SNC pairs represent true matches. These either had residential locations matching to within 50 m combined with perfect matches on date of birth, sex, and municipality of residence at birth or had more convincing similarities. Assuming that half of the remaining pairs are false matches, < 4% of those classified as having cancer were false positives. Conversely, > 400 potentially incident cases were not linked to the SNC, likely resulting in false negatives. But we found no indication that these differed from linked cases in radiation exposure, suggesting that the risk of bias due to linkage errors was small.
Major strengths of our study are its cohort design and the use of nationwide routine data. The cohort design maximizes statistical power for a given number of cases and accounts for lost to follow-up by migration or death. Assessment of both exposures and outcomes were based on routine data of nationwide coverage essentially eliminating the risk of selection bias. Our study was able to include a wide range of potential confounding factors. Furthermore, our study was based on a relatively wide range of exposure levels.
It is plausible that the observed associations between background radiation and childhood cancer reflect a causal relationship: Ionizing radiation is known to cause childhood cancer at high doses and dose rates. Associations were stronger for outcomes previously linked to radiation, such as leukemia and CNS tumors, although no evidence of an association was found for lymphoma where such links have been less demonstrable (UNSCEAR 2006). Our findings were little affected by adjustments for a number of potentially confounding factors. We found evidence of a dose response, and this evidence was strongest in a subcohort of children with stable residence before entry into the cohort, that is, with less exposure misclassification due to residential mobility. We cannot, however, exclude biases due to inaccurate exposure measurement.
In conclusion, our study suggests that background radiation may contribute to the risk of cancer in children. Results suggest that risks for leukemia and CNS tumors are similarly affected. Future research in this field could greatly benefit from improved exposure assessment.
Supplemental Material
Acknowledgments
The members of the Swiss Pediatric Oncology Group Scientific Committee: R.A. Ammann (Bern), R. Angst (Aarau), M. Ansari (Geneva), M. Beck Popovic (Lausanne), E. Bergstraesser (Zurich), P. Brazzola (Bellinzona), J. Greiner (St. Gallen), M. Grotzer (Zurich), H. Hengartner (St. Gallen), T. Kuehne (Basel), K. Leibundgut (Bern), F. Niggli (Zurich), J. Rischewski (Lucerne), and N. von der Weid (Basel). The members of the Swiss National Cohort Study Group: F. Gutzwiller (Chairman of the Executive Board, Zurich), M. Bopp (Zurich), M. Egger (Chairman of the Scientific Board, Bern), A. Spoerri (Bern), M. Zwahlen (Bern), N. Künzli (Basel), F. Paccaud (Lausanne), M. Oris (Geneva).
This study was supported by the Swiss Federal Office of Public Health (grants 08.001616, 10.002946, 12.008357), Swiss Cancer League (grant 02224-03-2008), Swiss Cancer Research (grants 3049-08-2012, 3515-08-2014), and Swiss National Science Foundation (grant 33CS30-134273). B.D.S. was supported by a Swiss National Science Foundation fellowship (PZ00P3_147987). The work of the Swiss Childhood Cancer Registry is supported by the Swiss Pediatric Oncology Group (http://www.spog.ch), Schweizerische Konferenz der kantonalen Gesundheitsdirektorinnen und –direktoren (http://www.gdk-cds.ch), Swiss Cancer Research (http://www.krebsforschung.ch), Kinderkrebshilfe Schweiz (http://www.kinderkrebshilfe.ch), Ernst-Göhner Stiftung, Stiftung Domarena, and National Institute of Cancer Epidemiology and Registration (http://www.nicer.ch).
The authors declare they have no actual or potential competing financial interests.
References
- Akiba S, Sun QF, Tao ZF, Gabgadharan P. Child cancer risk in high-background radiation areas. Int Congr Ser. 2002;1225:283–287. [Google Scholar]
- Auvinen A, Hakama M, Arvela H, Hakulinen T, Rahola T, Suomela M, et al. Fallout from Chernobyl and incidence of childhood leukaemia in Finland, 1976–92. BMJ. 1994;309:151–154. doi: 10.1136/bmj.309.6948.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson O, Fredrikson M, Akerblom G, Hardell L. Leukemia in childhood and adolescence and exposure to ionizing radiation in homes built from uranium-containing alum shale concrete. Epidemiology. 2002;13:146–150. doi: 10.1097/00001648-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Belson M, Kingsley B, Holmes A.2007Risk factors for acute leukemia in children: a review. Environ Health Perspect 115138–145.; 10.1289/ehp.9023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp M, Spoerri A, Zwahlen M, Gutzwiller F, Paccaud F, Braun-Fahrländer C, et al. Cohort Profile: the Swiss National Cohort—a longitudinal study of 6.8 million people. Int J Epidemiol. 2009;38:379–384. doi: 10.1093/ije/dyn042. [DOI] [PubMed] [Google Scholar]
- Evrard AS, Hémon D, Billon S, Laurier D, Jougla E, Tirmarche M, et al. Childhood leukemia incidence and exposure to indoor radon, terrestrial and cosmic gamma radiation. Health Phys. 2006;90:569–579. doi: 10.1097/01.HP.0000198787.93305.35. [DOI] [PubMed] [Google Scholar]
- Frigerio NA, Eckerman KF, Stowe RS. Argonne Radiological Impact Program (ARIP). Part I. Carcinogenic Hazards from Low-Level, Low-Rate Radiation. ANL/ES-26 Part I. Argonne, IL:Argonne National Laboratory. 1973. Available: http://www.osti.gov/scitech/biblio/4368021 [accessed 20 January 2015]
- Goldstein L, Langholz B. Asymptotic theory for nested case-control sampling in the Cox regression-model. Ann Stat. 1992;20:1903–1928. [Google Scholar]
- Hatch M, Susser M. Background gamma radiation and childhood cancers within ten miles of a US nuclear plant. Int J Epidemiol. 1990;19:546–552. doi: 10.1093/ije/19.3.546. [DOI] [PubMed] [Google Scholar]
- Hauri D, Spycher B, Huss A, Zimmermann F, Grotzer M, von der Weid N, et al. 2013Domestic radon exposure and risk of childhood cancer: a prospective census-based cohort study. Environ Health Perspect 1211239–1244.; 10.1289/ehp.1306500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri DD, Spycher B, Huss A, Zimmermann F, Grotzer M, von der Weid N, et al. Exposure to radio-frequency electromagnetic fields from broadcast transmitters and risk of childhood cancer: a census-based cohort study. Am J Epidemiol. 2014;179:843–851. doi: 10.1093/aje/kwt442. [DOI] [PubMed] [Google Scholar]
- Kendall GM, Fell TP, Harrison JD. Dose to red bone marrow of infants, children and adults from radiation of natural origin. J Radiol Prot. 2009;29:123–138. doi: 10.1088/0952-4746/29/2/001. [DOI] [PubMed] [Google Scholar]
- Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JC, Vincent TJ, et al. A record-based case–control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia. 2013;27:3–9. doi: 10.1038/leu.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox EG, Stewart AM, Gilman EA, Kneale GW. Background radiation and childhood cancers. J Radiol Prot. 1988;8:9–18. [Google Scholar]
- Laurent O, Ancelet S, Richardson DB, Hémon D, Ielsch G, Demoury C, et al. Potential impacts of radon, terrestrial gamma and cosmic rays on childhood leukemia in France: a quantitative risk assessment. Radiat Environ Biophys. 2013;52:195–209. doi: 10.1007/s00411-013-0464-y. [DOI] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Kendall GM. Updated estimates of the proportion of childhood leukaemia incidence in Great Britain that may be caused by natural background ionising radiation. J Radiol Prot. 2009;29:467–482. doi: 10.1088/0952-4746/29/4/001. [DOI] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Lubin JH, Kendall GM. The statistical power of epidemiological studies analyzing the relationship between exposure to ionizing radiation and cancer, with special reference to childhood leukemia and natural background radiation. Radiat Res. 2010;174:387–402. doi: 10.1667/RR2110.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TJ, Miller RW. Cosmic radiation at high altitudes and U.S. cancer mortality, 1950–1969. Radiat Res. 1974;60:302–306. [PubMed] [Google Scholar]
- Michel G, von der Weid NX, Zwahlen M, Redmond S, Strippoli MP, Kuehni CE, et al. Incidence of childhood cancer in Switzerland: the Swiss Childhood Cancer Registry. Pediatr Blood Cancer. 2008;50:46–51. doi: 10.1002/pbc.21129. [DOI] [PubMed] [Google Scholar]
- Muirhead CR, Butland BK, Green BMR, Draper GJ. An analysis of childhood leukemia and natural radiation in Britain. Radiat Prot Dosimetry. 1992;45:657–660. [Google Scholar]
- Panczak R, Galobardes B, Voorpostel M, Spoerri A, Zwahlen M, Egger M, et al. A Swiss neighbourhood index of socioeconomic position: development and association with mortality. J Epidemiol Community Health. 2012;66:1129–1136. doi: 10.1136/jech-2011-200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O. Indoor radon and childhood leukaemia. Radiat Prot Dosimetry. 2008;132:175–181. doi: 10.1093/rpd/ncn288. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Andersen CE, Andersen HP, Gravesen P, Lind M, Schüz J, et al. Domestic radon and childhood cancer in Denmark. Epidemiology. 2008;19:536–543. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Richardson S, Monfort C, Green M, Draper G, Muirhead C. Spatial variation of natural radiation and childhood leukaemia incidence in Great Britain. Stat Med. 1995;14:2487–2501. doi: 10.1002/sim.4780142116. [DOI] [PubMed] [Google Scholar]
- Rybach L, Bachler D, Bucher B, Schwarz G. Radiation doses of Swiss population from external sources. J Environ Radioact. 2002;62:277–286. doi: 10.1016/s0265-931x(01)00169-2. [DOI] [PubMed] [Google Scholar]
- Rybach L, Medici F, Schwarz GF. Construction of radioelement and dose rate baseline maps by combining ground and airborne radiometric data. In: Uranium Exploration Data and Techniques Applied to the Preparation of Radioelement Maps. Proceedings of a technical committee meeting held in Vienna, 13–17 May 1996. IAEA-Tecdoc-980. Vienna, Austria:International Atomic Energy Agency (IAEA), 33–44. 1996. Available: http://www-pub.iaea.org/books/IAEABooks/5635/Uranium-Exploration-Data-and-Techniques-Applied-to-the-Preparation-of-Radioelement-Maps [accessed 20 January 2015]
- Spoerri A, Zwahlen M, Egger M, Bopp M. The Swiss National Cohort: a unique database for national and international researchers. Int J Public Health. 2010;55:239–242. doi: 10.1007/s00038-010-0160-5. [DOI] [PubMed] [Google Scholar]
- Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55:893–899. doi: 10.1016/s0895-4356(02)00443-2. [DOI] [PubMed] [Google Scholar]
- Tirmarche M, Rannou A, Mollie A, Sauve A. Epidemiological study of regional cancer mortality in France and natural radiation. Radiat Prot Dosimetry. 1988;24:479–482. [Google Scholar]
- Tong J, Qin L, Cao Y, Li J, Zhang J, Nie J, et al. Environmental radon exposure and childhood leukemia. J Toxicol Environ Health B Crit Rev. 2012;15:332–347. doi: 10.1080/10937404.2012.689555. [DOI] [PubMed] [Google Scholar]
- UK Childhood Cancer Study Investigators. The United Kingdom Childhood Cancer Study of exposure to domestic sources of ionising radiation: I: radon gas. Br J Cancer. 2002a;86:1721–1726. doi: 10.1038/sj.bjc.6600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Childhood Cancer Study Investigators. The United Kingdom Childhood Cancer Study of exposure to domestic sources of ionising radiation: 2: gamma radiation. Br J Cancer. 2002b;86:1727–1731. doi: 10.1038/sj.bjc.6600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). Annex B: Exposures from natural radiation sources. In: UNSCEAR Report 2000, Sources and Effects of Ionizing Radiation, Vol. I. New York:United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), United Nations. 2000. Available: http://www.unscear.org/docs/reports/annexb.pdf [accessed 20 January 2015]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). Annex A: Epidemiological studies of radiation and cancer. In: UNSCEAR Report 2006, Effects of Ionizing Radiation, Vol. I. New York:United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), United Nations. 2006. Available: http://www.unscear.org/docs/reports/2006/07-82087_Report_Annex_A_2006_Web_corr.pdf [accessed 20 January 2015]
- UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). Annex B: Effects of radiation exposure of children. In: UNSCEAR Report 2013, Sources, Effects and Risks of Ionizing Radiation, Vol. II. New York:United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), United Nations. 2013. Available: http://www.unscear.org/docs/reports/2013/UNSCEAR2013Report_AnnexB_Children_13-87320_Ebook_web.pdf [accessed 20 January 2015]
- Wakeford R. The risk of childhood leukaemia following exposure to ionising radiation—a review. J Radiol Prot. 2013;33:1–25. doi: 10.1088/0952-4746/33/1/1. [DOI] [PubMed] [Google Scholar]
- Wiemels J. Perspectives on the causes of childhood leukemia. Chem Biol Interact. 2012;196:59–67. doi: 10.1016/j.cbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


