Abstract
Purpose of review
Stroke rehabilitation needs to take major steps forward to reduce functional disability for survivors. In this article, we suggest that spatial retraining might greatly increase the efficiency and efficacy of motor rehabilitation, directly addressing the burden and cost of paralysis after stroke.
Recent findings
Combining motor and cognitive treatment may be practical, as well as addressing needs after moderate–to-severe stroke. Spatial neglect could suppress motor recovery and reduce motor learning, even when patients receive appropriate rehabilitation to build strength, dexterity, and endurance. Spatial neglect rehabilitation acts to promote motor as well as visual-perceptual recovery. These findings, and previous underemphasized studies, make a strong case for combining spatial neglect treatment with traditional exercise training. Spatial neglect therapies might also help people who cannot participate in intensive movement therapies because of limited strength and endurance after stroke.
Summary
Spatial retraining, currently used selectively after right brain stroke, may be broadly useful after stroke to promote rapid motor recovery.
Keywords: spatial neglect, prism adaptation, motor rehabilitation, spatial cognition
Introduction
Stroke is a major public health problem in the USA, and globally. Annually, about 795,000 people in the United States have a stroke, and stroke costs the nation $38.6 billion, including the cost of health care services, medications, and lost productivity (1). Of the 15 million people worldwide who suffer a stroke annually, at least 5 million are permanently disabled, placing a burden on family and community (2). Stroke incidence is declining in many developed countries, largely as a result of better blood pressure control and reduced smoking, and the age-standardized rates of stroke mortality decreased worldwide in the past two decades. However, the personal and social cost of stroke is still increasing because of population aging (2). Thus, year by year, the number of stroke survivors and the overall global burden of stroke are increasing (3).
When we consider the resources needed to reduce the burden of stroke, it is helpful to understand impairments that are strongly associated with functional limitations. Certainly, paralysis is a major reason for activity and social/vocational limitation after stroke. For this reason, a large research investment has been devoted to studying the mechanisms of motor recovery, and to scientifically developing interventions to address these mechanisms in rehabilitation (4). However, this research outlay has not yet resulted in major changes to the paradigm for stroke rehabilitation to increase return of function after stroke.
In this article, we will provide an overview of articles from recent, emerging literature, as well as classic studies that are key to understanding innovation in spatial retraining. We will argue that integrating specific spatial cognition techniques as part of routine rehabilitation could result in greater, motor-based, functional recovery.
We will first present evidence that combined cognitive and motor rehabilitation may be beneficial. A knowledge gap may exist between motor and functional recovery after stroke, and the missing element to consider may be spatial: higher-order, brain-based mental function that coordinates with, and modulates, the corticospinal tract, motor cortex, basal ganglia, and other primary motor systems.
Next, we will describe how spatial problems, common after right brain stroke, adversely affect successful motor and functional recovery. We will lastly present evidence suggesting that treatments currently used to improve visual-perceptual function in spatial neglect could be prescribed for post-stroke paralysis, as augmentative or even primary motor rehabilitation treatment. Using rehabilitation approaches that activate both cognitive and motor systems is efficient, addresses areas of great need, and could help improve the feasibility of intensive treatment in stroke rehabilitation.
Motor function and spatial recovery
If cognitive treatments indirectly stimulate the motor system and facilitate motor recovery, this could be important for several reasons. Stroke survivors too weak to perform repetitive movements are likely to be excluded from current intensive, exercise-based care options. These survivors of moderate to severe stroke are more than three times less likely to return home after stroke, and their care, requiring skilled personnel and inpatient/residential settings, comes at greatly increased cost (5). Providing treatments to survivors of moderate to severe stroke not only serves social justice by addressing healthcare disparity, but could also reduce the social cost of stroke by reducing needs for skilled and caregiver assistance among some of the most disabled survivors. However, alternate intensive methods of stimulating the motor system to restore function are not widely available. Spatial cognitive treatment could “work around” damaged corticospinal systems (6). Visual-motor integration tasks might be practiced as part of spatial cognitive therapy (7), using different movements than those affected by stroke, or using unaffected limb(s).
Another reason for using spatial cognitive approaches to augment motor rehabilitation concerns the frequency with which cognitive problems affect people with moderate to severe stroke. These events usually cause both cognitive and motor impairments, which interact (8). It is widely acknowledged that cognitive problems caused by stroke are strongly associated with later daily life disability such as limitations to community and social participation (9,10), and even minor strokes are associated with disabling hidden memory, spatial and mood disorders (11). Moderate to severe strokes almost always induce symptoms in both cognitive and motor domains (12–15). In particular, spatial deficits probably occur in > 50% of moderate-severe right brain stroke survivors (16).
Cognitive deficits of all kinds are associated with limitations of intellectual capability, and also with mobility problems, poor motor recovery, and falls (17). The intimate relationship between cognitive and motor capability inspired the use of physical exercise to enhance cognitive function (18–20). However, cognitive therapies are not yet being used to stimulate the motor system. This is a lost opportunity, since cognitive-motor interactions powerfully activate and influence motor performance (21–23). When current rehabilitation programs provide both cognitive and motor training in stroke care, cognitive and motor interventions are usually separately-administered, based on pragmatic factors such as the availability of personnel, reimbursement provided by insurers, or convenience. It might also possible for a single, therapeutic, cognitive-motor intervention to replace two, separately-administered, cognitive and motor therapies. This could decrease the total time needed for treatment, and thus the cost of care. It is also possible that using interventions based on scientific knowledge of how cognitive and motor brain systems interact would improve outcomes of stroke rehabilitation.
Knowledge gap between motor and functional recovery
Reports suggest that recovering function after stroke requires more than the successful return of strength, dexterity, and endurance. In Lang et al.’s (24) study examining the extent to which paralysis explained post-stroke functional variance, although motor dysfunction at hospital admission was a good predictor of functional recovery at 3 weeks (88% of total variance), 3 months (80% of total variance) and 6 months (73% of total variance), it did not account for all of the individual variance in functional recovery. An important factor to consider is that balance, an important factor in motor performance, is strongly related to spatial function and body awareness. Fong et al. (25) found that balance on admission accounts for the greatest total variance of patients’ functional performance at hospital discharge, as contrasted with poor predictive ability of strength or speed of movements. These authors reported that a model integrating only balance and cognitive judgment scores on hospital admission significantly predicted discharge functional ability, accounting for 51% of total variance. Löfgren et al. (26) also found postural stability highly predictive of return home after stroke, combined in a model with age and perceptual function. Patterson et al. (27) found that in stroke survivors able to walk, who were more impaired (slower walkers), spatial, balance and body awareness factors predict mobility over and above muscle strength. In this study, there was a strong correlation of balance performance with 6-minute walking performance in slower walkers, but leg strength was not correlated with walking performance.
Two studies explicitly examined the relative predictive ability of motor function and neglect in motor recovery, and found spatial neglect to be a powerful and separate predictor of stroke outcomes. Giaquinto et al. (28) reported that motor function was not a significant predictor of functional outcome post-stroke. In this study, spatial neglect, cognitive ability to performed skilled limb movements (limb apraxia), age, and cognitive and sphincter performance predicted 72% of total variance of recovery on the Functional Independence Measure (FIM; 29). The FIM strongly emphasizes the impact of motor recovery, and assesses the burden of care post-stroke. Oh-Park et al. (30) reported that spatial dysfunction at admission predicted community mobility at six months after stroke (University of Alabama at Birmingham Study of Aging Life-Space Assessment), over and above motor performance of functional activities assessed by the Barthel Index.
Post-stroke spatial neglect
Spatial computations and actions, interacting with strength, deftness, and endurance of movement systems, give us exactly-specified movements in three-dimensional space. Spatial function is also critical to make continuous adjustments as we move, relative to a rapidly-moving world: walking, reading signs while we drive, and avoiding obstacles as we carry out hundreds of daily-life tasks. Spatial neglect is a disabling post-stroke syndrome causing failure to report, respond or orient to stimuli on the side of space opposite a brain lesion (31, 32). Evidence suggests that spatial neglect delays motor recovery, even when survivors receive appropriate strength and exercise training (33–35). Three-dimensional visual-motor integration is obviously relevant to gait, balance and reaching. Thus, a possible effect of spatial neglect on balance is not surprising: falls are much more common in people with spatial neglect (36). However, other components of spatial neglect such as abnormal sustained visual and auditory attention (37) also strongly influence motor and functional recovery. In this study, an auditory attention task (a tone-counting procedure) best predicted hand and arm dexterity performance and functional outcomes at two years. Lastly, spatial neglect can affect the motor preparatory system, and movement computations, making it more difficult to move toward the neglected side, or use the neglected arm or leg, a deficit called “Aiming” motor-intentional spatial neglect (38). Although right brain stroke survivors are commonly affected by spatial neglect, left brain stroke survivors may also suffer from this spatial-motor disorder. Coslett (39) reviewed linguistic and motor performance in a group of patients with left brain stroke, and found that about half of those with parietal lesions had less-capable motor performance in right-sided, contralesional space, as compared with their performance in left space. This was true even when they performed motor tasks with the unaffected left hands, consistent with right-sided “Aiming” spatial motor neglect.
Jehkonen et al. (40) strongly supported the role of spatial neglect in predicting stroke recovery, in a review of 26 studies examining the relationship of spatial dysfunction with functional outcome of stroke. In 25/26 studies, spatial neglect predicted the ability to regain daily life function, and in 11 studies, spatial neglect was an independent predictor of functional outcomes. Spatial neglect predicted functional outcome in combination with other variables in 14 studies: motor function was one of these additional factors in only 4 (41–44). In the other 10 studies, other cognitive factors or other variables (e.g. age) combined with spatial neglect to predict functional disability.
Nijboer et al. (45) recently performed a careful study examining the longitudinal relationship between arm/hand paralysis and spatial neglect, over the first post-stroke year (101 survivors). They found that spatial neglect appeared to suppress arm/hand motor recovery over the first 10 weeks post-stroke. In the 51 patients with evidence of spatial neglect (42 right-sided and 9 left-sided stroke), the time over which motor gains occurred was also delayed in people with spatial neglect. Oh-Park et al. (30) also emphasized the long-term impact of spatial neglect. In their study of 31 right brain stroke survivors, greater spatial neglect severity within 2 months of stroke predicted less community mobility at six months post-stroke. Spatial neglect severity accounted for about 56% of the total variance in reported community mobility (area of daily life movement in the home, neighborhood, community and region). This was true regardless of the degree of spatial neglect recovery that occurred between initial neglect assessment and six months post-stroke.
Toward a new paradigm for stroke treatment: spatial retraining
Spatial retraining is not currently part of routine stroke rehabilitation; its potential value to activate motor brain systems and stimulate motor learning has not been fully explored. In this section we will discuss several studies that support the use of spatial cognitive treatments to promote better three-dimensional perceptual-motor integration. These treatments are generally prescribed to improve visual and perceptual function in people with post-stroke spatial neglect. However, here we advocate the use of spatial retraining to promote motor recovery: spatial cognitive stimulation may improve dexterity and movement coordination, increase strength, and help develop adaptive body movements during ambulation, transfers, self-care, and other functional activities.
Vallar et al. (46) and Paolucci et al (47) reported that interventions to improve visual-perceptual orienting in spatial neglect also improved post-stroke paralysis and motor recovery. Vallar et al. (46) had two patients with left-sided weakness and spatial neglect after right brain stroke view dots moving 45 degrees/second leftward. These optokinetic stimuli induce an illusory sensation of body movement, previously demonstrated to reduce left spatial neglect, via asymmetric vestibular-spatial stimulation (48). The investigators demonstrated that during the period the patients with left neglect viewed optikinetic stimuli, grip strength in the left, paretic hand improved; there was no change in left grip strength with a control stimulus, however (rightward optikinetic movement; see Figure 1). Paolucci et al (47) demonstrated that stroke patients randomly assigned to spatial retraining with visual scanning, reading/copying, drawing and visual scene description demonstrated more improvement of motor impairment than did patients assigned to slightly fewer hours (3 hours versus 5 hours weekly) of general cognitive stimulation (puzzles, games, conversation).
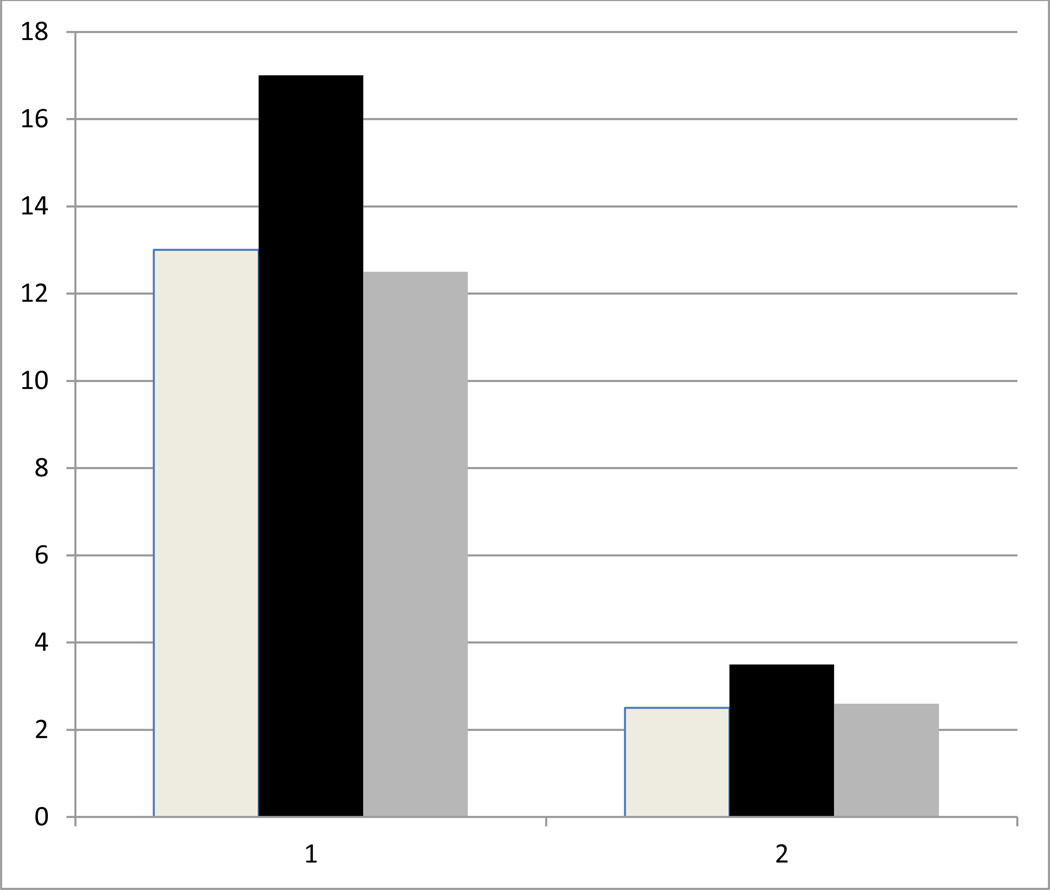
Figure 1.
Results of Vallar et al. (1997), based on Figures 2 and 3 of that publication. Two patients with right brain stroke and spatial neglect (x axis, left hand group of bars = patient 1, right hand group of bars = patient 2) performed a hand grip before (white bar), during (black bar) and after (gray bar) optokinetic stimulation with leftward-moving dots, intended to reduce left neglect. In both patients, grip strength (in kg, y axis) increased during optokinetic stimulation.
Two additional, recent studies specifically demonstrated that optical prism treatment with intensive visual-motor training, to improve spatial neglect (prism adaptation training; 7) resulted in motor gains. In both Goedert et al. (49) and Mizuno et al. (35), patients with spatial neglect demonstrated significant motor improvement (FIM) after 10 days (two weeks, 5 sessions/week) of prism adaptation treatment. Mizuno et al. observed greater improvement in motor functional ability after prism adaptation treatment in patients with milder stroke syndromes. Goedert et al. (49) observed no difference based on stroke or neglect severity, but observed that patients with Aiming, motor-intentional spatial deficits responded better than those with spatial neglect restricted to perceptual-attentional, Where spatial systems.
Spatial retraining has been reported to improve motor function in patients who have no spatial neglect (50), although this may not be uniform across stroke patients or spatial interventions (46). Even if spatial retraining only improves motor recovery in patients with spatial neglect, it still has significant potential, because spatial neglect is likely under-identified (51; Figure 2). It is not surprising that spatial stimulation affects motor systems; visual-motor, integrative brain activity stimulates beneficial reorganization (52). Thus, routine augmentative spatial retraining as part of motor therapy could result in a sharp improvement in the efficacy of in-hospital stroke rehabilitation.
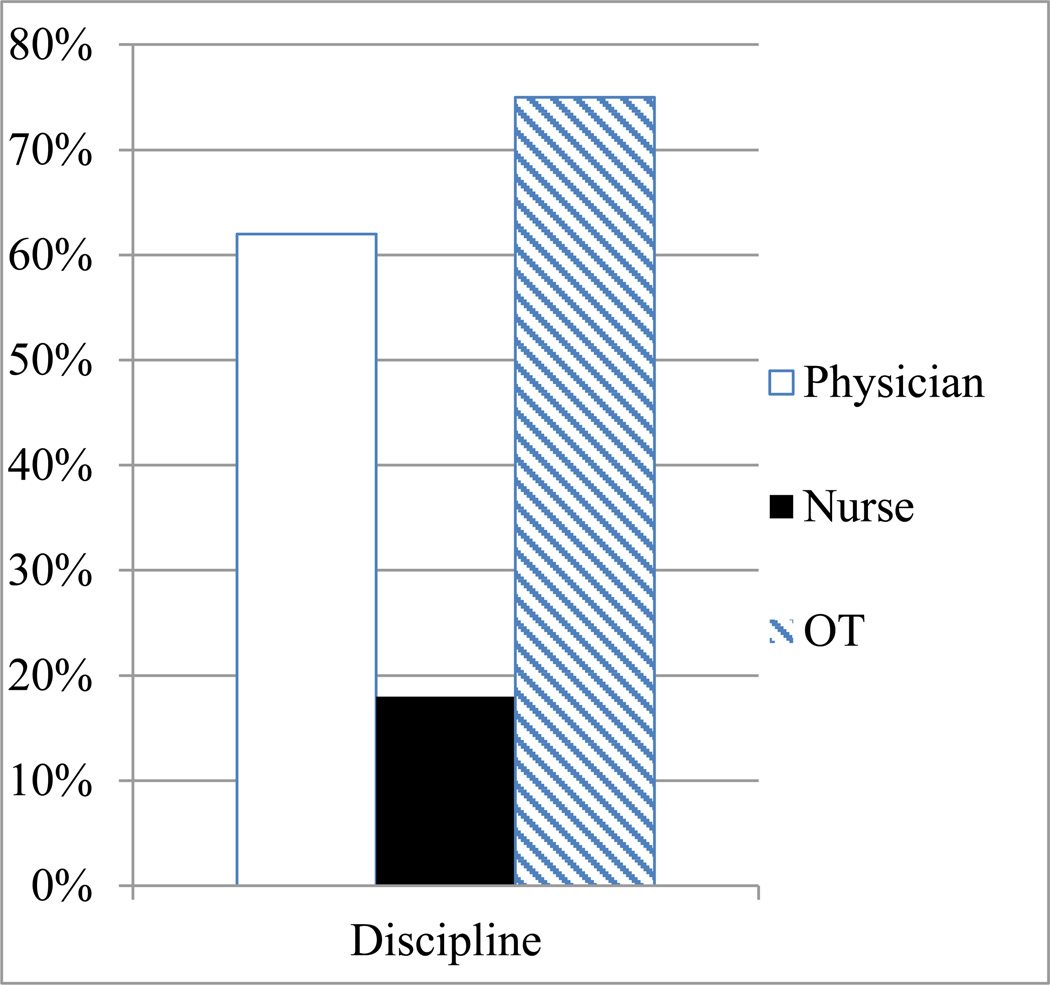
Figure 2.
Results of Chen et al. (2013), revised based on Figure 2 in that publication. Under-documentation of spatial neglect occurred across disciplines, including failure to document neglect in ¼ of patients by occupational therapists (striped bar), with nurses (black bar) and physicians (white bar) documenting the diagnosis of spatial neglect at even lower levels.
Conclusion
In this article, we discussed how combining cognitive and motor treatment could save time and address patient needs. We reviewed key studies suggesting that spatial neglect influences motor recovery after stroke. Spatial retraining could reduce the burden of care in patients having both spatial neglect and hemiparesis, by stimulating beneficial brain network interaction and reorganization. Further research evaluating short- and long-term benefits of routine spatial retraining as part of motor rehabilitation after acute stroke, is needed.
Key points.
Cognitive and motor deficits may interact, especially with regard to balance and spatially-directed movements.
Spatial neglect adversely affects motor and functional recovery after stroke, and increases the cost of stroke care.
Spatial neglect treatment with prism adaptation therapy improves motor as well as visual-perceptual stroke recovery, and could potentially augment motor rehabilitation.
Acknowledgements
Funded by the Kessler Foundation, the National Institutes of Health (K24HD062647; PI Barrett) and the Department of Education/NIDRR (H133G120203; PI Barrett). Study contents do not necessarily reflect the policy of the Department of Education, and one should not assume endorsement by the federal government.
Footnotes
Conflicts of interest: The authors declare no scientific or financial conflicts of interest.
Contributor Information
A.M. Barrett, Stroke Rehabilitation Research, Kessler Foundation, West Orange, NJ, USA.
Tufail Muzaffar, Department of Physical Medicine and Rehabilitation, Vardhman Mahavir Medical College and Safdarjang Hospital, New Delhi, India.
References
- 1. American Heart Association. Heart Disease and Stroke Statistics 2014 Update. Dallas, TX: American Heart Association; 2014. [Accessed August 1, 2014]. Available from: http://circ.ahajournals.org/lookup/doi/10.1161/01.cir.0000441139.02102.80. **invaluable information on the demographics and impact of stroke, updated annually.
- 2.World Health Organization, regional office for the Eastern Mediterranean. [Accessed August 1, 2014];Stroke, cerebrovascular accident. http://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html.
- 3.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Lancet. 2014;383(9913):245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer S, Duncan P, Barrett A. [Accessed August 1, 2014];(co-chairs). Report of the NIH Stroke Progress Review Group (SPRG): Recovery and Rehabilitation. 2012 Jan; http://www.ninds.nih.gov/about_ninds/groups/stroke_prg/01-2012-stroke-prg-report.htm#RR.
- 5.Rundek T, Mast H, Hartmann A, et al. Predictors of resource utilization after acute hospitalization: the Northern Manhattan Stroke Study. Neurology. 2000;55:1180–1187. doi: 10.1212/wnl.55.8.1180. [DOI] [PubMed] [Google Scholar]
- 6.Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- 7.Rossetti Y, Rode G, Pisella L, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166–169. doi: 10.1038/25988. [DOI] [PubMed] [Google Scholar]
- 8.Whyte J, Barrett AM. Advancing the evidence base of the rehabilitation treatments: a developmental approach. Arch Phys Med Rehabil. 93(8, Suppl 2):S101–S110. doi: 10.1016/j.apmr.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown T, Mapleston J, Naim A, Molloy A. Relationship of cognitive and perceptual abilities to functional independence in adults who have had a stroke. Occup Ther Int. 2013;20:11–22. doi: 10.1002/oti.1334. [DOI] [PubMed] [Google Scholar]
- 10.Wagle J, Farner L, Flekkøy K, et al. Early Post-Stroke Cognition in Stroke Patients Predicts Functional Outcome at 13 Months. Dement Geriatr Cogn Disord. 2011;31:379–387. doi: 10.1159/000328970. [DOI] [PubMed] [Google Scholar]
- 11.Blum S, Luchsinger JA, Manly JJ, et al. Memory after silent stroke: hippocampus and infarcts both matter. Neurology. 2012;78:38–46. doi: 10.1212/WNL.0b013e31823ed0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatemichi TK, Desmond DW, Stern Y, et al. Cognitive impairment after stroke: frequency, patterns, & relationship to functional abilities. J Neurol Neuro Psychiatr. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srikanth VK, Thrift AG, Saling M, et al. Increased Risk of Cognitive Impairment 3 Months After Mild to Moderate First-Ever Stroke: A Community-Based Prospective Study of Nonaphasic English-Speaking Survivors. Stroke. 2003;34:1136–1143. doi: 10.1161/01.STR.0000069161.35736.39. [DOI] [PubMed] [Google Scholar]
- 14.Cumming TB, Bernhardt J, Linden T. The Montreal Cognitive Assessment: Short Cognitive Evaluation in a Large Stroke Trial. Stroke. 2011;42:2642–2644. doi: 10.1161/STROKEAHA.111.619486. [DOI] [PubMed] [Google Scholar]
- 15. Dong Y, Slavin MJ, Chan BPL, et al. Cognitive screening improves the predictive value of stroke severity scores for functional outcome 3-6 months after mild stroke and transient ischaemic attack: an observational study. BMJ Open. 2013;3:e003105. doi: 10.1136/bmjopen-2013-003105. *Key to understand the importance of considering deficits affecting brain areas outside the motor and sensory system when predicting chronic functional performance after stroke.
- 16.Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62:749–756. doi: 10.1212/01.wnl.0000113730.73031.f4. [DOI] [PubMed] [Google Scholar]
- 17.Hwang S, Woo Y, Kim KH, Ki KI. Effects of falls on cognitive functions and physical activities in community-dwelling individuals with chronic stroke. Int J Rehabil Res. 2013;36:134–139. doi: 10.1097/MRR.0b013e32835b667e. [DOI] [PubMed] [Google Scholar]
- 18.Marzolini S, Oh P, McIlroy W, Brooks D. The effects of an aerobic and resistance exercise program on cognition following stroke. Neurorehabil Neural Repair. 2013;27:392–402. doi: 10.1177/1545968312465192. [DOI] [PubMed] [Google Scholar]
- 19.Weuve J, Kang JH, Manson J, et al. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 20.Colcombe S, Kramer AF. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 21.Ifejika-Jones NL, Barrett AM. Rehabilitation—emerging technologies, innovative therapies, and future objectives. Neurotherapeutics. 2011;8:452–462. doi: 10.1007/s13311-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett AM, Buxbaum LJ, Coslett HB, et al. Cognitive rehabilitation interventions for neglect and related disorders: moving from bench to bedside in stroke patients. J Cogn Neurosci. 2006;18:1223–1236. doi: 10.1162/jocn.2006.18.7.1223. [DOI] [PubMed] [Google Scholar]
- 23.Barrett AM, Foundas AL. Apraxia. In: Rizzo M, Eslinger PJ, editors. Principles & Practice of�Behavioral Neurology and Neuropsychology. Philadelphia: Saunders/Churchill Livingstone/Mosby; 2004. pp. 409–422. [Google Scholar]
- 24. Lang CE, Bland MD, Bailey RR, et al. Assessment of upper extremity impairment, function, and activity after stroke. J Hand Ther. 2013;26(2):104–114. doi: 10.1016/j.jht.2012.06.005. *An important article for understanding the trajectory of arm/hand recovery after stroke.
- 25.Fong K, Chan CC, Au DK. Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Inj. 2001;15(5):443–453. doi: 10.1080/02699050010005940. [DOI] [PubMed] [Google Scholar]
- 26.Löfgren B, Nyberg L, Österlind P, et al. Stroke rehabilitation—Discharge predictors. Cerebrovasc Dis. 1997;7:168–174. [Google Scholar]
- 27.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of Walking Function After Stroke: Differences by Deficit Severity. Arch Phys Med Rehabil. 2007;88(1):115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Giaquinto S, Buzzelli S, Di Francesco L, et al. On the prognosis of outcome after stroke. Acta Neurol Scand. 1999;100:202–208. doi: 10.1111/j.1600-0404.1999.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 29.Guide for the Uniform Data Set for Medical Rehabilitation (including the FIM™ instrument), version 5.1. Buffalo, NY: Uniform Data System for Medical Rehabilitation; 1997. [Google Scholar]
- 30. Oh-Park M, Hung C, Chen P, Barrett AM. Severity of spatial neglect during acute inpatient rehabilitation predicts community mobility post stroke. PM&R. 2014;6(8):716–722. doi: 10.1016/j.pmrj.2014.01.002. *This article convincingly presents a strong association between spatial-motor problems in the weeks after stroke and community mobility at chronic recovery stages.
- 31.Heilman KM. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. first edition. NY: Oxford; 1979. pp. 268–307. [Google Scholar]
- 32.Barrett AM, Burkholder S. Monocular patching in subjects with right hemisphere stroke affects perceptual-attentional bias. J. Rehabil. Res. Devel. 2006;43:337–346. doi: 10.1682/jrrd.2005.01.0015. [DOI] [PubMed] [Google Scholar]
- 33.Jackson D, Thornton H, Turner-Stokes L. Can young severely disabled stroke patients regain the ability to walk independently more than three months post stroke? Clin Rehabil. 2000;14:538–547. doi: 10.1191/0269215500cr358oa. [DOI] [PubMed] [Google Scholar]
- 34.Smania N, Aglioti SM, Girardi F, et al. Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurology. 2006;67:2050–2052. doi: 10.1212/01.wnl.0000247279.63483.1f. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno K, Tsuji T, Takebayashi T, et al. Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: a randomized, controlled trial. Neurorehabil. Neur. Rep. 2011;25:711–720. doi: 10.1177/1545968311407516. [DOI] [PubMed] [Google Scholar]
- 36.Teasell R, Bitensky J, Salter K, Bayona NA. The role of timing and intensity of rehabilitation therapies. Top Stroke Rehabil. 2005;12:46–57. doi: 10.1310/ETDP-6DR4-D617-VMVF. [DOI] [PubMed] [Google Scholar]
- 37.Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: a 2-year follow-up study. Neuropsychology. 1997;11:290–295. doi: 10.1037//0894-4105.11.2.290. [DOI] [PubMed] [Google Scholar]
- 38.Adair JC, Barrett AM. Spatial neglect clinical and neuroscience review: a wealth of information on the poverty of attention. Ann NY Acad Sci. 2008;1142:21–43. doi: 10.1196/annals.1444.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coslett HB. Spatial influences on motor and language function. Neuropsychologia. 1999;37(6):695–706. doi: 10.1016/s0028-3932(98)00116-x. [DOI] [PubMed] [Google Scholar]
- 40.Jehkonen M, Laihosalo M, Kettunen JE. Impact of neglect on functional outcome after stroke: A review of methodological issues and recent research findings. Rest Neurol Neurosci. 2006;24:209–215. [PubMed] [Google Scholar]
- 41.Jehkonen M, Ahonen JP, Dastidar P, et al. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol Scand. 2000;101:195–201. doi: 10.1034/j.1600-0404.2000.101003195.x. [DOI] [PubMed] [Google Scholar]
- 42.Jehkonen M, Ahonen JP, Dastidar P, et al. Predictors of discharge to home during the first year after right hemisphere stroke. Acta Neurol Scand. 2001;104:136–141. doi: 10.1034/j.1600-0404.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 43.Mercier L, Audet T, Hébert R, et al. Impact of motor, cognitive and perceptual disorders on ability to perform activities of daily living after stroke. Stroke. 2001;32:2602–2608. doi: 10.1161/hs1101.098154. [DOI] [PubMed] [Google Scholar]
- 44.Young J, Bogle S, Forster A. Determinants of social outcome measured by the Frenchay Activities Index at one year after stroke onset. Cerebrovasc Dis. 2001;12:114–120. doi: 10.1159/000047690. [DOI] [PubMed] [Google Scholar]
- 45. Nijboer TCW, Kollen BJ, Kwakkel G. The impact of recovery of visual-spatial neglect on motor recovery of the upper paretic limb after stroke. PLOS ONE. 2014;9(6):e100584. doi: 10.1371/journal.pone.0100584. **Convincing demonstration of cognitive-motor interaction during the longitudinal process of stroke recovery.
- 46.Vallar G, Guariglia C, Nico D, Pizzamiglio L. Motor deficits and optokinetic stimulation in patients with left hemineglect. Neurology. 1997;49(5):1364–1370. doi: 10.1212/wnl.49.5.1364. [DOI] [PubMed] [Google Scholar]
- 47.Paolucci S, Antonucci G, Guariglia C, et al. Facilitatory effect of neglect rehabilitation on the recovery of left hemiplegic stroke patients: a cross-over study. J Neurol. 1996;243:308–314. doi: 10.1007/BF00868403. [DOI] [PubMed] [Google Scholar]
- 48.Pizzamiglio L, Frasca R, Guariglia C, et al. Effect of optokinetic stimulation in patients with visual neglect. Cortex. 1990;26:535–540. doi: 10.1016/s0010-9452(13)80303-6. [DOI] [PubMed] [Google Scholar]
- 49. Goedert KM, Chen P, Boston RC, et al. Presence of motor-intentional aiming deficit predicts functional improvement of spatial neglect with prism adaptation. Neurorehabil Neural Rep. 2013;28(5):483–493. doi: 10.1177/1545968313516872. *Conclusively demonstrates benefit of spatial retraining administered during acute stroke rehabilitation, and points out the importance of assessing non-visual spatial symptoms.
- 50.Fong KN, Yang NY, Chan MK, et al. Combined effects of sensory cueing and limb activation on unilateral neglect in subacute left hemiplegic stroke patients: a randomized controlled pilot study. Clin Rehabil. 2013;27(7):628–637. doi: 10.1177/0269215512471959. [DOI] [PubMed] [Google Scholar]
- 51.Chen P, McKenna C, Kutlik AM, Frisina PG. Interdisciplinary communication in inpatient rehabilitation facility: Evidence of under-documentation of spatial neglect after stroke. Disability and Rehabilitation. 2013;35(12):1033–1038. doi: 10.3109/09638288.2012.717585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nudo RJ. Postinfarct cortical plasticity and stroke recovery. Stroke. 2007;38(Part 2):840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]