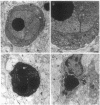
Abstract
We have previously observed that an axon-sparing injury to the developing striatum induced by the excitotoxin quinolinate results in a decrease in dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the adult. This decrease occurs in the absence of direct injury to the SNpc. As the striatum is a major target for the SNpc dopaminergic system, we have hypothesized that a decrease in the size of the striatal target during development may result in an induced regressive event in the SNpc, similar to what has been described for many developing neural systems with peripheral targets. We have examined by morphologic and biochemical means the time course and character of cell death in SN following a unilateral striatal lesion with quinolinate in immature rats. The striatal lesion is associated with an induced cell death event in the ipsilateral SN, observed first in SNpc and then in SN pars reticulata. The morphologic characteristics of the dying cells were typical of apoptosis. Immunostaining for tyrosine hydroxylase indicated that some of the apoptotic cells in the SNpc were dopaminergic. We conclude that developmental striatal excitotoxic injury is associated with induced apoptotic cell death in SN.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Macaya A., DeVivo D., Kenyon N., Janec E. M. Neonatal hypoxic-ischemic or excitotoxic striatal injury results in a decreased adult number of substantia nigra neurons. Neuroscience. 1992 Oct;50(3):559–569. doi: 10.1016/0306-4522(92)90447-a. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I. W., Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. I. A light and electron microscopic study of naturally occurring and induced cell loss during development. J Comp Neurol. 1978 Jan 1;177(1):33–57. doi: 10.1002/cne.901770105. [DOI] [PubMed] [Google Scholar]
- Clarke P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Cunningham T. J. Naturally occurring neuron death and its regulation by developing neural pathways. Int Rev Cytol. 1982;74:163–186. doi: 10.1016/s0074-7696(08)61172-9. [DOI] [PubMed] [Google Scholar]
- Gallyas F., Wolff J. R., Böttcher H., Zaborszky L. A reliable and sensitive method to localize terminal degeneration and lysosomes in the central nervous system. Stain Technol. 1980 Sep;55(5):299–306. doi: 10.3109/10520298009067258. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmendinger L. M., Garber B. B., Hoffmann P. C., Heller A. Target neuron-specific process formation by embryonic mesencephalic dopamine neurons in vitro. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1264–1268. doi: 10.1073/pnas.78.2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K., Sunter K. Numerical matching during cerebellar development: quantitative analysis of granule cell death in staggerer mouse chimeras. J Neurosci. 1987 Mar;7(3):829–836. doi: 10.1523/JNEUROSCI.07-03-00829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer E. B. Anterograde and transsynaptic degeneration 'en cascade' in basal ganglia induced by intrastriatal injection of kainic acid: an animal analogue of Huntington's disease. Brain Res. 1980 Aug 25;196(1):209–221. doi: 10.1016/0006-8993(80)90727-1. [DOI] [PubMed] [Google Scholar]
- Lauder J. M., Bloom F. E. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974 Jun 15;155(4):469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., di Porzio U., Kato A., Berger B., Glowinski J. In vitro maturation of mesencephalic dopaminergic neurons from mouse embryos is enhanced in presence of their striatal target cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5387–5391. doi: 10.1073/pnas.76.10.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S., Dean E., Neubort S. Electron microscopic analysis of adrenalectomy-induced hippocampal granule cell degeneration in the rat: apoptosis in the adult central nervous system. J Comp Neurol. 1993 Apr 15;330(3):337–351. doi: 10.1002/cne.903300305. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S., Sollas A. L., Dean E., Neubort S. Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: characterization of an in vivo model of controlled neuronal death. J Comp Neurol. 1993 Apr 15;330(3):324–336. doi: 10.1002/cne.903300304. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y., Appel S. H. Soluble striatal extracts enhance development of mesencephalic dopaminergic neurons in vitro. Brain Res. 1986 Dec 3;399(1):111–124. doi: 10.1016/0006-8993(86)90605-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Zakeri Z. F., Quaglino D., Latham T., Lockshin R. A. Delayed internucleosomal DNA fragmentation in programmed cell death. FASEB J. 1993 Mar;7(5):470–478. doi: 10.1096/fasebj.7.5.8462789. [DOI] [PubMed] [Google Scholar]