Summary
Agents that have shown improvements in survival in mCRPC now include abiraterone, enzalutamide, docetaxel, cabazitaxel and radium-223. Bone supportive agents and palliative radiation continue to play an important role in the overall management of mCRPC. Given the complexity, variety and importance of optimizing the use of these agents, a multidisciplinary team approach is highly recommended.
Introduction
Castration-resistant prostate cancer (CRPC) is defined by disease progression despite castrate levels of testosterone and may present as either a continuous rise in serum prostate-specific antigen (PSA) levels, the progression of preexisting disease, and/or the appearance of new metastases.
Advanced prostate cancer has been known under a few names over the years, including hormone-resistant prostate cancer (HRPC) and androgen-insensitive prostate cancer (AIPC). Most recently, the terms castration-resistant prostate cancer or castration-recurrent prostate cancer were introduced with the realization that extra-testicular androgen production plays a significant role in the resistance of prostate cancer cells to medical or surgical castration therapy.1
In their second publication, the Prostate Cancer Working Group defined CRPC as a continuum on the basis of whether metastases are detectable (clinically or by imaging) and whether the serum testosterone is in the castrate range by surgical orchidectomy or medical therapy.2 This definition creates a clinical-states model where patients can be classified. The rising PSA states (castrate and non-castrate) indicates that no detectable (measurable or non-measurable) disease has ever been found. The clinical metastases states (castrate and non-castrate) signify that disease was detectable at some point in the past, regardless of whether it is detectable now.3
Prognosis is associated with several factors that go beyond PSA levels. These include performance status, presence of visceral metastases, presence of bone pain, extent of disease on bone scan, and serum lactate dehydrogenase and alkaline phosphatase levels. Bone metastases will occur in 90% of men with CRPC and can produce significant morbidity, including pain, pathologic fractures, spinal cord compression and bone marrow failure. Paraneoplastic effects, including anemia, weight loss, fatigue, hypercoagulability and increased susceptibility to infection, are also common.
CRPC includes patients without metastases or symptoms with rising PSA levels despite androgen deprivation therapy (ADT) to patients with metastases and significant debilitation due to cancer symptoms. Clinical scenarios are outlined in Table 1.
Table 1.
Clinical scenarios and management options for patients with CRPC
| CRPC includes a wide range of disease types: from patients without metastases or symptoms with rising PSA levels despite ADT to patients with metastases and significant debilitation due to cancer symptoms. The panel recommends that ADT be continued in the presence of CRPC. |
|
1. Rising PSA non metastatic CRPC There is no standard of care and no approved regimen in M0 CRPC. AA therapy should be discontinued if patients are receiving these agents. Secondary hormonal treatments (excluding abiraterone or enzalutamide) may be attempted (Level 3, Grade C). Detection of metastases and imaging It is suggested to screen for bone metastases with bone scans and monitor for lymph node and visceral metastases/progression with periodic imaging of the abdomen/pelvis and chest. Patients with a rapid PSADT (<8 months) are at risk for developing earlier metastases. Imaging in these patients should be performed every 3 to 6 months. Patients with slower PSADT (>12 months) should be screened every 6 to 12 months (Grade C). |
|
2. Metastatic CRPC without symptoms or minimally symptomatic (defined as pain that is relieved by acetaminophen or a non-steroidal anti-inflammatory) AA therapy should be discontinued if patients are on it to test for an AA withdrawal response. Introduction of, or changes to, a first-generation AA or the use of corticosteroids with or without ketoconazole may be considered (Level 3, Grade C). Abiraterone acetate 1000 mg/day plus prednisone 5 mg/twice daily is recommended as first-line therapy (Level 1, Grade A). Abiraterone acetate significantly improved overall survival, radiographic progression-free survival, time to pain progression and time to chemotherapy initiation; it also delayed ECOG performance status deterioration. The study did not include patients with visceral metastases. Enzalutamide 160 mg/day is recommended as first-line therapy* (Level 1, Grade A). Enzalutamide significantly improved overall survival, progression free survival, time to pain progression, time to chemotherapy initiation and delayed ECOG performance status deterioration. The study included patients with visceral metastases. Treatment with docetaxel 75 mg/m2 every 3 weeks plus 5 mg oral prednisone twice daily can be offered (Level 1, Grade A). Docetaxel has been shown to improve overall survival, disease control, symptom palliation and quality of life. The timing of docetaxel therapy in men with evidence of metastases, but without symptoms, should be discussed with the patient and therapy should be individualized based on the patient’s clinical status and preference. |
|
3. Metastatic CRPC with symptoms Treatment with docetaxel 75 mg/m2 every 3 weeks plus 5 mg oral prednisone twice daily is recommended (Level 1, Grade A). Docetaxel has been shown to improve overall survival, disease control, symptom palliation and quality of life. Radium-223 every 4 weeks for 6 cycles is recommended in patients with pain due to bone metastases and who do not have visceral metastases (Level 1, Grade A). Radium-223 significantly improved overall survival and reduced symptomatic skeletal related events in patients with symptomatic mCRPC who had previously received docetaxel chemotherapy or were deemed unfit for docetaxel. Abiraterone acetate 1000 mg/day plus prednisone 5 mg twice daily or enzalutamide 160 mg/day may be considered as first-line therapy in patients who cannot receive or refused docetaxel (Expert opinion). The studies in chemotherapy-naïve patients did not include patients with moderate or severe pain; therefore, the efficacy is not well-documented in patients with significant symptoms. |
|
4. Metastatic CRPC who progress after docetaxel-based chemotherapy Options with survival benefit
|
|
5. Patients with CRPC and bone metastases (includes the pre or post chemotherapy settings) In men with CRPC and bone metastases, denosumab (120 mg subcutaneous) or zoledronic acid (4 mg intravenous) every 4 weeks, along with daily calcium and vitamin D supplementation, is recommended to prevent disease-related skeletal complications (Level 1, Grade A). |
Pending Health Canada Approval in the chemo-naïve setting. CRPC: castration-resistant prostate cancer; ADT: androgen deprivation therapy; mCRPC: metastatic CRPC; PSA: prostate-specific antigen; AA: anti-androgen; PSADT: PSA doubling time; ECOG: Eastern Cooperative Oncology Group.
Management of CRPC
First- and second-line hormonal agents
Because the androgen receptor remains active in most patients who have developed castration-resistant disease, it is recommended that ADT be continued for the remainder of a patient’s life (Level 3, Grade C).
In patients who develop CRPC, secondary hormonal treatments may be attempted (Level 3, Grade C).
To this date, no study of secondary hormone treatment has shown survival benefits; most trials have been small and were not designed to evaluate overall survival and were heavily confounded by future treatments used. In patients treated with luteinizing hormone-releasing hormone (LHRH) agonist/antagonist monotherapy or who have had an orchidectomy, the addition of total androgen blockade (TAB) with androgen receptor antagonists, such as bicalutamide, can offer modest PSA responses that are short-lived in 30% to 35% of patients.4
For patients who have undergone TAB, the antiandrogen (AA) should be discontinued to test for an AA withdrawal response (AAWD). Introducing or changing AA or using corticosteroids with or without ketoconazole has been noted to cause transient PSA reductions in about 30% of patients (Level 3, Grade C).
Non-metastatic CRPC
There is no standard of care and no approved regimen in M0 CRPC. AA therapy should be discontinued if patients are receiving these agents. Secondary hormonal treatments may be attempted (Level 3, Grade C).
Detection of metastases and imaging
For patients who progress on ADT without evidence of distant metastases, it is suggested to screen for bone metastases with bone scans and monitor for lymph node and visceral metastases/progression with imaging of the abdomen/pelvis and chest.
Patients with a rapid PSA doubling time (PSADT (<8 months) are at risk for developing earlier metastases. Imaging in these patients should be performed every 3 to 6 months. Patients with a slower PSADT (>12 months) should be screened every 6 to 12 months (Expert Opinion). Imaging techniques most commonly used include nuclear bone scans and abdominal/pelvic computed tomography and chest X-ray. The role of magnetic resonance imaging (MRI) and positron-emission tomography is still unclear.
Treatment of metastatic CRPC (mCPRC)
Currently, only patients with CRPC who have detectable macroscopic metastatic disease should be considered for systemic therapy (i.e., new hormonal agents or chemotherapy) outside of a clinical trial. Patients with advanced prostate cancer should optimally receive multidisciplinary care to maximize survival and quality of life. Because any treatment for advanced disease remains non-curative, patients with advanced prostate cancer should be encouraged to participate in clinical trials.
1. Androgen receptor (AR) signaling therapeutic options
Novel agents that can affect the androgen receptor signaling have recently been developed and have renewed the enthusiasm for effective hormone manipulation. In men with CRPC, phase III clinical trials have evaluated the role of abiraterone acetate and enzalutmide in both the chemo-naïve and post-chemotherapy settings.
Abiraterone acetate
Abiraterone acetate is a potent and irreversible inhibitor of CYP-17, a critical enzyme in androgen biosynthesis.
Chemo-naïve setting
Abiraterone acetetate 1000 mg/day plus prednisone 5 mg twice daily is recommended for first-line therapy for asymptomatic or minimally symptomatic metastatic CRPC (Level 1, Grade A).
In asymptomatic or minimally symptomatic patients (defined as pain that is relieved by acetaminophen or a non-steroidal anti-inflammatory) without visceral metastases, abiraterone acetate significantly improved radiographic progression-free survival (PFS) (16.5 vs. 8.3 months) (hazard ratio [HR] 0.53; 95% confidence interval [CI] 0.45–0.62; p < 0.001). Abiraterone also significantly delayed time to pain progression, time to chemotherapy initiation, time to opiate initiation and deterioration of the Eastern Cooperative Oncology Group (ECOG) performance status. The final analysis of the study confirms a statistically significant 4.4 months improvement in overall survival (HR 0.81 p = 0.0033).5
Post-docetaxel setting
Abiraterone acetate (1000 mg per day) plus prednisone (5 mg twice daily) is recommended in patients progressing on or after docetaxel-based chemotherapy (Level 1, Grade A).
In the post-docetaxel setting, abiraterone-prednisone compared to placebo-prednisone has significantly prolonged median overall survival by 4.6 months (15.8 vs. 11.2 months; HR 0.74; p = 0.0001) in patients with mCRPC who had progressed after docetaxel treatment. Moreover, all secondary endpoints supported the superiority of abiraterone over placebo: median time to PSA progression (8.5 vs. 6.6 months; HR 0.63; p < 0.0001), radiographic PFS (5.6 vs. 3.6 months; HR 0.66; p < 0.0001), confirmed PSA response rate defined as ≥ 50% reduction in PSA from the pre-treatment baseline PSA (29% vs. 5.5%; p < 0.0001) and objective response by Response Evaluation Criteria in Solid Tumors (RECIST) (14.8% vs. 3.3%; p < 0.0001).6
Enzalutamide
Enzalutamide is a potent multi-targeted androgen signalling pathway inhibitor.
Chemo-naïve setting
Enzalutamide (160 mg per day) is recommended as first-line therapy for asymptomatic or minimally symptomatic metastatic CRPC (Level 1, Grade A). (Pending Health Canada approval in the chemo-naïve setting.)
In asymptomatic or minimally symptomatic patients (defined as pain that is relieved by acetaminophen or a nonsteroidal anti-inflammatory), enzalutamide decreased the risk of radiographic progression or death by 81% (HR 0.19; 95% CI 0.15–0.23; p < 0.001) and the risk of death by 29% (HR 0.71; 95% CI 0.60–0.84; p < 0.001) as compared with placebo. The benefit of enzalutamide was demonstrated for all secondary endpoints, including time to initiation of cytotoxic chemotherapy time to first skeletal-related event (SRE), best overall soft tissue response (59% vs. 5%; p < 0.001), time to PSA progression (HR 0.17; p < 0.001), and ≥50% PSA decline rate (78% vs. 4%; p < 0.001). Enzalutamide also significantly delayed time to pain progression, time to opiate initiation, and deterioration of the ECOG performance status.7
Post-docetaxel setting
Enzaluatmide (160 mg per day) is recommended in patients progressing on or after docetaxel-based chemotherapy (Level 1, Grade A).
In patients previously treated with docetaxel the AFFIRM trial compared enzalutamide and placebo.8,9 The study demonstrated a significant advantage in overall survival of 4.8 months (18.4 vs. 13.6 months; HR 0.62; p < 0.0001) and all secondary endpoints, including confirmed PSA response rate (54% vs. 2%, p < 0.001), soft-tissue response rate (29% vs. 4%, p < 0.001), the time to PSA progression (8.3 vs. 3.0 months; HR 0.25; p < 0.001), radiographic PFS (8.3 vs. 2.9 months; HR 0.40; p < 0.001), and the time to the first SRE (16.7 vs. 13.3 months; HR 0.69; p < 0.001).
The studies in the chemo-naïve setting did not include patients with moderate or severe symptoms; however abiraterone and enzalutamide may be potential therapeutic options in patients who are deemed chemotherapy ineligible (Expert Opinion).
2. Chemotherapy
First-line systemic chemotherapy
Docetaxel 75 mg/m2 intravenous (IV) every 3 weeks with 5 mg oral prednisone twice are recommended for patients with metastatic CRPC (Level 1, Grade A).
The TAX-327 study randomized 1006 patients to one of three treatment arms: (1) docetaxel (75 mg/m2 IV, every 3 weeks); (2) docetaxel (30 mg/m2, 5 times weekly for 5 of 6 weeks); or (3) control therapy with mitoxantrone.10,11 The study reported improved survival with docetaxel (every 3 weeks) compared with mitoxantrone-prednisone (median survival 18.9 vs. 16.5 months; HR 0.76 [95% CI 0.62–0.94], two-sided p = 0.009). No overall survival benefit was observed with docetaxel given on a weekly schedule (HR 0.91, [95% CI 0.75–1.11], two-sided p = 0.36). Significantly more patients treated with docetaxel (every 3 weeks) achieved a pain response compared with patients receiving mitoxantrone (35% vs. 22%, p = 0.01). Quality of life response, defined as a sustained 16-point or greater improvement from baseline on two consecutive measurements, was higher with docetaxel given every 3 weeks (22% vs. 13%, p = 0.009) or weekly (23% vs. 13%, p = 0.005) compared with mitoxantrone.
PSA response rates were also statistically significantly higher with docetaxel compared to mitoxantrone. In the 2 trials, 27% (n = 412) and 29% (n = 196) of patients had measurable disease.
Although patients received up to 10 cycles of treatment if no progression and no prohibitive toxicities were noted, the duration of therapy should be based on the assessment of benefit and toxicities. Rising PSA only should not be used as the sole criteria for progression; assessment of response should incorporate clinical and radiographic criteria.
Alternative therapies that have not demonstrated improvement in overall survival, but can provide disease control, palliation and improve quality of life, include weekly docetaxel plus prednisone, and mitoxantrone plus prednisone (Level 2, Grade B).12
The timing of docetaxel therapy in men with evidence of metastases, but without symptoms, should be discussed with patients and therapy should be individualized based on patients’ clinical status and preferences (Level 3, Grade C).
Use of estramustine in combination with other cytotoxic agents is not recommended due to the increased risk of clinically important toxicities. There is no evidence to support the use of this combination to improve survival or palliation (Level 2, Grade C).
Neuroendocrine differentiation may be present in patients who do not respond to first-line ADT or who progress clinically or radiologically without significant PSA elevations. Biopsy of accessible lesions should be considered to identify these patients; these patients should then be treated with combination chemotherapy, such as cisplatin/etoposide or carboplatin/etoposide (Level 3, Grade C).
Second-line systemic chemotherapy
Cabazitaxel is recommended for mCRPC patients progressing on or following docetaxel (Level 1, Grade A).
A phase 3 study comparing cabazitaxel to mitoxantrone in patients previously treated with docetaxel has shown a statistically significant survival advantage.13 This randomized, placebo-controlled trial recruited 755 docetaxel pre-treated CRPC patients. Overall survival was the primary endpoint of the study. Patients were randomized to receive prednisone 10 mg/day with 3-weekly mitoxantrone 12 mg/m2 or cabazitaxel 25 mg/m2. An advantage in survival emerged in favour of the cabazitaxel group, with a median survival of 15.1 months compared with 12.7 months in the mitoxantrone group (HR 0.70; 95% CI 0.59–0.83; p < 0.0001).
Other options
For patients who have had a good response to first-line docetaxel re-treatment with docetaxel can be considered (Expert Opinion).14–16
Mitoxantrone may be considered a therapeutic option in symptomatic patients with mCRPC in the first- or second-line setting. Mitoxantrone has not shown any survival advantage, but may give symptomatic relief. Of note in the second-line setting, mitoxantrone has limited activity and increased toxicity (Grade C).
3. Bone-targeted therapy
Life-prolonging therapy
Radium-223
Radium-223 every 4 weeks for 6 cycles is recommended in patients with pain due to bone metastases and who do not have visceral metastases (Level 1, Grade A).
Radium-223 (previously known as alpharadin) is an intravenous alpha-emitting agent that mimics calcium preferentially targeting bone metastases. In a randomized phase III study, radium-223 given every 4 weeks for 6 cycles was compared to placebo.17 Radium-223 demonstrated a significant improvement in overall survival and symptomatic SRE. Overall survival was improved by 3.6 months (HR 0.7, p < 0.0001) and SREs were delayed by 5.8 months (p < 0.0001). The study included patients with symptomatic bone metastases who were post-docetaxel or ineligible for docetaxel. The study excluded patients with visceral metastases. Compared to PSA, alkaline phosphatase appears to be a better marker of activity given the mechanism of action of radium-223.
Supportive agents
Denosumab and zoledronic acid
In men with CRPC and bone metastases, denosumab (120 mg subcutaneous [SC]) or zoledronic acid (4 mg IV) every 4 weeks are recommended to prevent disease-related SREs, including pathological fractures, spinal cord compression, surgery or radiation therapy to bone (Level 1, Grade A).
Bone loss associated with ADT has been shown to increase the risk of fracture.18–22 Moreover, about 90% of patients with mCRPC will develop bone metastases, which cause local decreases in bone integrity. Patients are at significant risk of SREs that include pathological fractures, debilitating bone pain requiring palliative radiation therapy and spinal cord compression. Quality of life is affected by these complications.
Zoledronic acid is a third-generation nitrogen containing bisphosphonate. Bisphosphonates other than zoledronic acid are not known to be effective to prevent disease-related SREs. In the placebo controlled zoledronic acid study, fewer men receiving zoledronic acid had SREs (38% vs. 49%, p = 0.02).23 Zoledronic acid also increased the median time to first SRE (488 vs. 321 days, p = 0.01). There was an overall 36% reduction in the rate of SREs in treated patients.
Treatment with zoledronic acid should not be used in men with baseline creatinine clearance <30 mL/min.
Denosumab is a fully humanized monoclonal antibody against RANK ligand. It has been shown to be effective in preventing bone loss and new vertebral fractures due to ADT.22 In the setting of mCRPC, denosumab (120 mg SC, every 4 weeks) compared to zoledronic acid (4 mg IV, every 4 weeks) has shown significant improvement in the time to the first SRE (20.7 vs. 17.1 months, p < 0.001 for non-inferiority; p = 0.008 for superiority), while overall survival and PFS were not different.24
No dose modification for renal function is necessary in the case of denosumab; however, the risk of hypocalcaemia is increased and calcium monitoring and supplementation (with calcium and vitamin D) is recommended for both denosumab and zoeldronic acid. Denosumab has not been studied in patients with severe renal impairment (glomerular filtration rate <30 mL/min).
Good oral hygiene, baseline dental evaluation for high-risk individuals, and avoidance of invasive dental surgery during therapy are recommended to reduce risk of osteonecrosis of the jaw (ONJ) for patients treated with bone-targeted therapies (Level 3, Grade C).25–27 Zoledronic acid and denosumab have been used in combination with all the agents presently in use for the treatment of mCRPC. To date there have been no additional safety issues of concern that have been reported.23,24
The optimal duration of zoledronic acid and denosumab in men with CRPC and bone metastases is undefined. The risk of ONJ appears to be related to time on bone-targeted therapy; therefore caution should be taken in using these agents more than 2 years.28
Denosumab and zoledronic acid are not approved and not indicated for bone metastases prevention in Canada.
4. Other supportive care therapies
Systemic corticosteroid therapy
Corticosteroid therapy with low-dose prednisone or dexamethasone may also offer improvements in PSA values and/ or palliative outcomes in up to 30% of patients in both symptomatic and asymptomatic men. Steroids may also exert an anti-neoplastic effect on prostate cancer (Level 3, Grade C).29,30
Palliative radiation
Bone metastases from prostate cancer are often radiosensitive and most men will experience partial or complete pain relief from external beam radiation to a specific lesion. Studies have shown that a single fraction of standard palliative radiotherapy is as effective as 5 or more fractions in providing palliation. However, more patients require re-treatment for pain recurrence with single fraction radiation (Level 2, Grade B). Stereotactic body radiotherapy is a more precise and may be a more effective form of palliation delivered in 5 or fewer treatments and may also be considered (Level 3, Grade C).
Radionuclide therapy, in the form of systemic strontium-89 therapy, may be useful in the palliation of CRPC when multiple skeletal sites are involved in carefully selected patients. Risks include severe prolonged myelosuppression and transfusion dependence. Strontium-89 may be associated with a worse overall survival as compared to external beam radiotherapy.31,32
Malignant spinal cord compression is an oncologic emergency that requires immediate diagnosis, if suspected, with an MRI. Options for treatment are debulking surgery + radiotherapy, vertebrectomy with stabilization and radiotherapy, or radiotherapy + steroids (Level 1, Grade A).33
Conclusion
Advances in treatment for men with CRPC have improved survival and quality of life, but most, if not all, patients eventually succumb to their disease. Several new agents are being studied in all states of CRPC and an increase in options is likely in the near future.9,25–27,34–36 Because CRPC remains an incurable and ultimately fatal illness, inclusion of patients in clinical trials remains paramount.
Fig. 1.
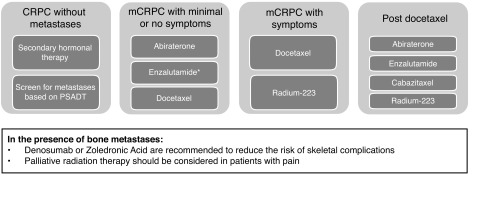
- The optimal sequence of available options remains unknown.
- Patients who have had little no response to hormonal agents OR who progress with minimal change in PSA or with significant visceral metastases should be considered for early chemotherapeutic options.
- Radium-223 is not approved for patients with visceral metastases.
- Whenever possible, clinical trials should remain the first choice in patients with CRPC.
Footnotes
Notes: MEDLINE search of the English language and conference proceedings were used to produce the present document. Wherever Level 1 evidence is lacking, the guideline attempts to provide expert opinion to aid in the management of patients. Levels of evidence and grades of recommendation employing the International Consultation on Urologic Disease (ICUD)/WHO modified Oxford Center for Evidence-Based Medicine grading system were applied.
This paper has been peer-reviewed.
Competing interests: No financial support was obtained for the work in preparing this document. Dr. Saad is a member of the advisory boards for Amgen, Astellas, Janssen, Abbott, Sanofi and Bayer. He has also received research grants and honoraria from Amgen, Astellas, Janssen, Abbott, Sanofi and Bayer. He has participated in clinical trials in the past 2 years for Amgen, Astellas, Janssen, Sanofi, and Bayer. Dr. Chi has received research funding from OncoGenex Technologies Inc., Astellas, Janssen, and Novartis and is a consultant for Janssen, Astellas, Amgen, Bayer, Millennium, Novartis, and Sanofi. Dr. Finelli has participated in clinical trials in the past 2 years for Amgen, Astellas, Janssen and Ferring. He is also Chair of the CUA Guidelines Committee. Dr. Izawa is a member of the advisory boards for Janssen and Actavis. He has also received a grant from Abbott. Dr. Kapoor is a member of the Speakers Bureau for Pfizer and Novartis. He has also received grants from Pfizer, GSK, Novartis, and Amgen. He is currently participating in a clinical trial with NCIC, Pfizer, GSK, Novartis, and Amgen. Dr, Kassouf is a member of the advisory boards and Speakers Bureau for Amgen and Astellas. He has also received grants from Amgen and Astellas. Dr, Loblaw is a member of the advisory board for GE Healthcare and has received grants from GE Healthcare. Dr. Rendon is a member of the advisory boards and Speakers Bureau for Astellas, Amgen, Janssen, and Ferring. He is currently participating in a clinical trial with Amgen. Dr. So is a member of the advisory boards for Amgen, Janssen, and Astellas. He also holds the patent on a product for CDRD. He is currently participating in a clinical trial with Amgen, Cougar, Janssen, Astellas. Dr. Usmani is a member of the advisory boards for Astellas, Johnson & Johnson, and Amgen. He has also received grants from Best Medical and Varian Medical Systems. Dr. Fleshner is a member of the advisory boards for Amgen, Janssen, Astellas and Eli Lily. He has received honoraria from Amgen, Janssen, Astellas, and Eli Lily. He is and has participated in clinical trials for Amgen, Janssen, Medivation, OICR, and Prostate Cancer Canada. Dr. Hotte, Dr. North, and Dr. Vigneault declare no competing financial or personal interests.
References
- 1.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.CCR-1146-03. . [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. . [DOI] [PubMed] [Google Scholar]
- 4.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: A phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. . [DOI] [PubMed] [Google Scholar]
- 5.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–25. doi: 10.1016/j.eururo.2014.02.056. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sternberg CN, Castellano D, Daugaard G, et al. Abiraterone acetate for patients with metastatic castration-resistant prostate cancer progressing after chemotherapy: Final analysis of a multicentre, open-label, early-access protocol trial. Lancet Oncol. 2014;15:1263–8. doi: 10.1016/S1470-2045(14)70417-6. . [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787. doi: 10.1126/science.1168175. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. . [DOI] [PubMed] [Google Scholar]
- 10.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. . [DOI] [PubMed] [Google Scholar]
- 11.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. . [DOI] [PubMed] [Google Scholar]
- 12.Michels J, Montemurro T, Murray N, et al. First- and second-line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer: Does sequence matter? Cancer. 2006;106:1041–6. doi: 10.1002/cncr.21695. . [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147. doi: 10.1016/S0140-6736(10)61389-X. . [DOI] [PubMed] [Google Scholar]
- 14.Oh WK, Manola J, Babcic V, et al. Response to second-line chemotherapy in patients with hormone refractory prostate cancer receiving two sequences of mitoxantrone and taxanes. Urology. 2006;67:1235–40. doi: 10.1016/j.urology.2006.01.006. . [DOI] [PubMed] [Google Scholar]
- 15.Jankovic B, Beardsley E, Chi KN. Rechallenge with docetaxel as second-line chemotherapy in patients with metastatic hormone refractory prostate cancer (HRPC) after previous docetaxel: A population based analysis [abstract #196]. 2008 ASCO Genitourinary Symposium; 2008. [Google Scholar]
- 16.Eymard J, Oudard S, Gravis G, et al. Docetaxel reintroduction in patients with metastatic castration-resistant docetaxel-sensitive prostate cancer: A retrospective multicentre study. BJU Int. 2010;106:974–8. doi: 10.1111/j.1464-410X.2010.09296.x. . [DOI] [PubMed] [Google Scholar]
- 17.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. . [DOI] [PubMed] [Google Scholar]
- 18.Diamond TH, Higano CS, Smith MR, et al. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 19.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. . [DOI] [PubMed] [Google Scholar]
- 20.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. . [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. . [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–82. doi: 10.1093/jnci/djh141. . [DOI] [PubMed] [Google Scholar]
- 24.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813. doi: 10.1016/S0140-6736(10)62344-6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreicer R, Agus DB, MacVicar GR, et al. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostate cancer: A phase I/II, open-label study. J Clin Oncol. 2010;28:S15. [Google Scholar]
- 26.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099. doi: 10.1200/JCO.2009.25.0597. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad F, Hotte SJ, North SA, et al. A phase II randomized study of custirsen (OGX-011) combination therapy in patients with poor-risk hormone refractory prostate cancer who relapsed on or within six months of 1st-line docetaxel therapy. Can Urol Assoc J. 2008;2:568. [Google Scholar]
- 28.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storlie JA, Buckner JC, Wiseman GA, et al. Prostate specific antigen levels and clinical response to low dose dexamethasone for hormone-refractory metastatic prostate carcinoma. Cancer. 1995;76:96–100. doi: 10.1002/1097-0142(19950701)76:1<96::AID-CNCR2820760114>3.0.CO;2-E. . [DOI] [PubMed] [Google Scholar]
- 30.Heng DY, Chi KN. Prednisone monotherapy in asymptomatic hormone refractory prostate cancer. Can J Urol. 2006;13:3335–9. [PubMed] [Google Scholar]
- 31.Porter AT, McEwan AJ. Strontium-89 as an adjuvant to external beam radiation improves pain relief and delays disease progression in advanced prostate cancer: Results of a randomized controlled trial. Semin Oncol. 1993;20:38–43. [PubMed] [Google Scholar]
- 32.Oosterhof GO, Roberts JT, de Reijke TM, et al. Strontium (89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: A phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. Eur Urol. 2003;44:519–26. doi: 10.1016/S0302-2838(03)00364-6. . [DOI] [PubMed] [Google Scholar]
- 33.Loblaw DA, Mitera G, Ford M, et al. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84:312–7. doi: 10.1016/j.ijrobp.2012.01.014. . [DOI] [PubMed] [Google Scholar]
- 34.Saad F. Src as a therapeutic target in men with prostate cancer and bone metastases. BJU Int. 2009;103:434. doi: 10.1111/j.1464-410X.2008.08249.x. . [DOI] [PubMed] [Google Scholar]
- 35.Araujo J, Armstrong AJ, Braud EL, et al. Dasatinib and docetaxel combination treatment for patients with castration-resistant progressive prostate cancer: A phase I/II study (CA180086) J Clin Oncol. 2009;27:5061. http://meeting.ascopubs.org/cgi/content/short/27/15S/5061. [Google Scholar]
- 36.Chi KN, Hotte JS, Yu EY, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:4247–54. doi: 10.1200/JCO.2009.26.8771. . [DOI] [PubMed] [Google Scholar]


