Abstract
Introduction:
We report the incidence of stent failure, defined as the need for salvage percutaneous nephrostomy (PCN) placement following the placement of a ureteral stent, in patients with infection of an obstructed urinary tract secondary to urolithiasis. We also sought to identify risk factors associated with ureteral stent failure.
Methods:
Using the Nationwide Inpatient Sample, we used time trend analysis to examine the incidence of ureteral stent failure for infected urolithiasis, as well as the estimated annual percent change (EAPC) from 1998 to 2010. Logistic regression was performed to estimate the odds of stent failure based on patient and hospital characteristics.
Results:
A total of 164 546 stents were placed during the study period. Of these, 97.8% resulted in successful decompression. The rates of successful stent decompression and stent failure increased over time (EAPC 14.05%, p < 0.001; EAPC 11.61%, p < 0.001). Middle-aged males with renal stones and acute kidney failure had higher odds of stent failure (p < 0.05). Salvage percutaneous nephrostomies were performed most frequently in urban teaching institutions (odds ratio [OR] 1.98, p = 0.001; OR 1.83, p < 0.001).
Conclusions:
Ureteral stent decompression for an infected obstructed urinary tract secondary to urolithiasis is almost always effective. For a small proportion of patients, stent failure will occur and will require the placement of a nephrostomy tube. Stent failure is associated with male gender, stone location, and renal failure. Salvage percutaneous nephrostomies for these patients occur most frequently in urban teaching hospitals. Of note, this study was limited by the presumption that coding for a PCN after stent placement indicated stent failure, which could not be verified because of the inherent limitations of the dataset.
Introduction
The incidence of urolithiasis and associated sepsis in the United States is increasing,1,2 yet there remains a debate over which form of management is ideal for these types of patients.3 The two options for decompression of an infected urinary system include placement of a ureteral stent or percutaneous nephrostomy (PCN). Although both approaches have theoretical advantages, a well-powered comparison of the two procedures has not been performed. Nonetheless, a recent population-based study has shown an overall decline in the use of PCN in the United States.2 Given that ureteral stents are becoming increasingly favoured, determining the frequency of stent failure requiring PCN placement and associated risk factors for failure is of renewed importance.
In a hydronephrotic system, PCN placement has been shown to have success rates between 95% and 100%.4–7 The true frequency of stent failure itself is not well-established. Previous studies have shown a 0 to 20% rate of failure to place a ureteral stent in patients with infections related to stone obstruction,5,7,8 while a rate of 12% to 23% has been reported for stent failure after placement for intrinsic ureteral obstructions.9,10 These studies had very small study populations. Understanding why stent failure occurs may result in more expeditious and efficacious treatment of a potentially life-threatening condition.
The aim of this study was to investigate the incidence of stent failure requiring PCN placement in patients with obstructed infected upper tract stones in the United States. A second objective was to elucidate risk factors for stent failure. Ultimately, we hope these findings will help guide practitioners in the early identification of patients at risk for stent failure.
Methods
In accordance with use of de-identified administrative data, an IRB exemption was obtained. We used the Nationwide Inpatient Sample (NIS). The NIS is a set of longitudinal hospital inpatient databases included in the Healthcare Cost and Utilization Project (HCUP) family, created by the Agency for Healthcare Research and Quality through a Federal-state partnership. Data are ascertained by a 20% stratified probability sample of non-federal hospitals in the United States. The database includes discharge abstracts from 8 million hospital stays and is the sole hospital database in the United States with charge information on all patients regardless of payer, including persons covered by Medicare, Medicaid, private insurance, and the uninsured. Each discharge includes up to 15 inpatient diagnostic and 15 procedural codes. All procedures and diagnoses are coded using the International Classification of Diseases, 9th revision, Clinical Modification (ICD–9–CM).
Study population
All hospital discharges in the United States between January 1, 1998 and December 31, 2010, aged ≥18 years, with a primary or secondary diagnosis code 592.x (Calculus of kidney or ureter) were abstracted and assessed for concomitant urinary tract infection (UTI) or pyelonephritis (ICD-9 codes in Appendix 1).2 Of the patients with concomitant urolithiasis and infection, those who underwent ureteral stent placement (ICD-9: 59.8) on the first or second day of admission served as our study cohort. Patients who underwent an attempted ureteral stent placement but who were unsuccessful were not included in this study as these patients could not be identified from this database.
Covariates
Independent variables included age, gender, race, and insurance status. Baseline patient comorbidities were determined using the Charlson comorbidity index (CCI),11 as adapted by Deyo and colleagues for use in administrative datasets12 and dichotomized as low (0–1) versus high (≥2). Additionally, we included potential predictors of stent failure, such as obesity (ICD-9: V77.8, 278.00, 278.01), cancer (ICD-9: 140–239), renal failure (ICD-9: 584, 580, 585, 3995, 3996), and sepsis (ICD-9: 020.0, 038.x, 785.52, 790.7, 995.91, 995.92). We also distinguished patients with renal stones (ICD-9: 592.0) from those with ureteral stones (ICD-9: 592.1). Hospital region (Northeast, Midwest, South, West) was obtained from the American Hospital Association Annual Survey of Hospitals and defined by the United States Census Bureau.13 Hospitals were divided into academic and non-academic institutions; their status was obtained from the American Hospital Association Annual Survey of Hospitals.14
Endpoints
To determine stent failure, we examined patients with initial ureteral stent placement who subsequently underwent nephrostomy placement (55.02) or percutaneous nephrostomy without fragmentation (55.03) during the same hospitalization. Patients undergoing transurethral removal of obstruction from ureter and renal pelvis (56.0), percutaneous nephrostomy with fragmentation (55.04) or nephroscopy (55.21) were excluded from analysis. Patients who underwent stent placement that preceded nephrostomy placement were ascertained using the NIS variable for the date of each procedure (PRDAYn), and were considered stent failures. Relying on the assumption that stent placement after nephrostomy in the context of infected stone disease was a rare event, patients who underwent stenting and nephrostomy on the same day were also included in the stent failure group.
Statistical analyses
Weighted estimates were calculated by uniformly applying stratum weights to the discharges according to the stratum from which the discharge was drawn.15 Incidences were normalized to population estimates from census data from the 2000 United States census and intercensal population estimates. Temporal trends in rates were quantified by estimated annual percent change (EAPC) using the least squares linear regression methodology advanced by Anderson and colleagues.16 Subsequently, frequencies and proportions were generated for categorical variables and the chi-square and Mantel-Haenszel tests were used to assess the statistical significance of differences in populations and odds ratios of outcome measures, respectively. Finally, logistic regression was used to predict failed stenting and relied on generalized estimating equations (GEE-models) to adjust for clustering among hospitals.12 All tests were two-sided with a statistical significance set at p < 0.05 and were weighted to reflect national estimates between 1998 and 2010. Analyses were conducted using the R statistical package (the R foundation for Statistical Computing, version 3.0.1).
Results
During our study period, 164 546 patients (weighted) underwent ureteral stenting. The rate of ureteral stent failure was 2.2% versus a success rate of 97.8% (Table 1). Most (76%) of the salvage PCNs were placed on hospital day zero and one. An overall increase in the number of stent placements per year was observed, with an estimated annual increase of 11.61% for stent failure (p < 0.001, 95% confidence interval [CI] 9.21–14.06) and 14.05% for stent success (95% CI, p < 0.001, 95% CI 12.82–15.28) (Fig. 1).
Table 1.
Weighted descriptive characteristics of 164 546 patients treated with ureteral stents for infected upper tract urolithiasis, stratified according to successful/failed stent, Nationwide Inpatient Sample (1998–2010)
| Characteristics | Successful stent decompression | Stent failure after placement | Overall | p value† |
|---|---|---|---|---|
| n (%) | 160 918 (97.8) | 3628 (2.2) | 164 546 (100) | |
| Age, categorical | ||||
| <18 | 0 | 0 | 0 | <0.001 |
| 18–40 | 27.5 | 21.4 | 27.4 | |
| 41–50 | 17.7 | 17.9 | 17.7 | |
| 51–60 | 18.8 | 18.8 | 18.8 | |
| 61–70 | 16.3 | 17.0 | 16.3 | |
| 71–80 | 12.6 | 16.8 | 12.7 | |
| >80 | 7.1 | 8.1 | 7.1 | |
| Gender | ||||
| Male | 27.6 | 34.2 | 27.8 | <0.001 |
| Female | 72.4 | 65.8 | 72.2 | |
| Race | ||||
| Caucasian | 66.9 | 59.5 | 66.7 | <0.001 |
| African American | 6.0 | 9.5 | 6.1 | |
| Other | 13.7 | 16.8 | 13.8 | |
| Unknown | 13.4 | 14.2 | 13.4 | |
| BMI | ||||
| Non-obese (<30) | 91.0 | 92.6 | 91.0 | 0.001 |
| Obese (>30) | 9.0 | 7.4 | 9.0 | |
| CCI | ||||
| 0–1 | 89.8 | 85.7 | 89.7 | <0.001 |
| ≥2 | 10.2 | 14.3 | 10.3 | |
| Stone location | ||||
| Kidney | 16.6 | 52.0 | 17.4 | <0.001 |
| Ureter | 83.4 | 48.0 | 82.6 | |
| Sepsis | ||||
| No | 79.5 | 79.3 | 79.5 | 0.821 |
| Yes | 20.5 | 20.7 | 20.5 | |
| Renal failure | ||||
| No | 84.8 | 77.9 | 84.7 | <0.001 |
| Yes | 15.2 | 22.1 | 15.3 | |
| Renal malignancy | ||||
| No | 96.8 | 95.4 | 96.7 | <0.001 |
| Yes | 3.2 | 4.6 | 3.3 | |
| Insurance status | ||||
| Private | 44.2 | 35.2 | 44.0 | |
| Medicaid | 11.5 | 14.6 | 11.6 | <0.001 |
| Medicare | 31.6 | 41.0 | 31.8 | |
| Other | 12.7 | 9.2 | 12.6 | |
| Median household income | ||||
| 1st quartile | 19.7 | 21.2 | 19.7 | 0.007 |
| 2nd quartile | 26.1 | 27.4 | 26.1 | |
| 3rd quartile | 25.3 | 23.4 | 25.2 | |
| 4th quartile | 26.9 | 25.8 | 26.9 | |
| Unknown | 2.0 | 2.2 | 2.1 | |
| Hospital location | ||||
| Rural | 13.5 | 6.3 | 13.4 | <0.001 |
| Urban | 86.5 | 93.7 | 86.6 | |
| Hospital teaching status | ||||
| Non-teaching | 60.8 | 41.2 | 60.3 | <0.001 |
| Teaching | 39.2 | 58.8 | 39.7 | |
| Hospital region | ||||
| Northeast | 22.4 | 29.1 | 22.5 | <0.001 |
| Midwest | 15.6 | 10.2 | 15.5 | |
| South | 44.3 | 42.2 | 44.2 | |
| West | 17.7 | 18.5 | 17.8 |
chi2 test; BMI: body mass index; CCI: Charlson comorbidity index.
Fig. 1.
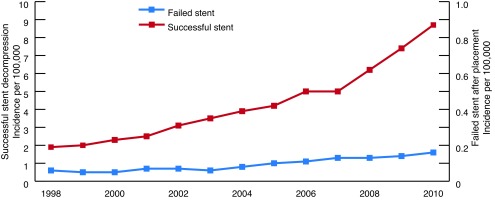
Temporal trend in the incidence of successful and failed decompression following ureteral stent placement for infected upper tract urolithiasis.
Women made up the majority of the population (72.4%), and they had a reduced risk of stent failure when compared to the male cohort (OR 0.779 [0.66–0.919], p < 0.003) (Table 2). The risk of stent failure was highest in the 41–50 age range (OR 1.3, p = 0.043). Overall, most patients had a low CCI and were not obese (Table 1); further, neither obesity nor comorbidity affected successful decompression with a ureteral stent (OR 0.826, p = 0.171 and OR 1.088, p = 0.458, respectively) (Table 2).
Table 2.
Multivariable logistic regression analyses of predictors of failure in ureteral stent placement for infected upper tract urolithiasis, Nationwide Inpatient Sample (1998–2010)
| Characteristics | OR (95% CI) | p value |
|---|---|---|
| Age, categorical | ||
| <18 | - | |
| 18–40 | 1.0 (ref.) | |
| 41–50 | 1.285 (1.007–1.638) | 0.043 |
| 51–60 | 1.223 (0.959–1.56) | 0.105 |
| 61–70 | 1.111 (0.832–1.485) | 0.475 |
| 71–80 | 1.338 (0.957–1.869) | 0.088 |
| >80 | 1.093 (0.734–1.629) | 0.661 |
| Gender | ||
| Male | 1.0 (ref.) | |
| Female | 0.779 (0.66–0.919) | 0.003 |
| Race | ||
| Caucasian | 1.0 (ref.) | |
| African American | 1.332 (1.014–1.748) | 0.039 |
| Other | 1.224 (0.988–1.516) | 0.065 |
| Unknown | 1.378 (1.087–1.747) | 0.008 |
| BMI | ||
| Non-obese (<30) | 1.0 (ref.) | |
| Obese (>30) | 0.826 (0.629–1.086) | 0.171 |
| CCI | ||
| 0–1 | 1.0 (ref.) | |
| ≥2 | 1.088 (0.871–1.358) | 0.458 |
| Stone location | ||
| Kidney | 1.0 (ref.) | |
| Ureter | 0.204 (0.174 – 0.239) | <0.001 |
| Sepsis | ||
| No | 1.0 (ref.) | |
| Yes | 0.968 (0.788–1.19) | 0.758 |
| Renal failure | ||
| No | 1.0 (ref.) | |
| Yes | 1.488 (1.209–1.831) | <0.001 |
| Renal malignancy | ||
| No | 1.0 (ref.) | |
| Yes | 1.147 (0.798–1.649) | 0.46 |
| Insurance Status | ||
| Private | 1.0 (ref.) | |
| Medicaid | 1.329 (1.046–1.688) | 0.02 |
| Medicare | 1.38 (1.081–1.762) | 0.01 |
| Other | 0.866 (0.656–1.142) | 0.308 |
| Median household income | ||
| 1st quartile | 1.0 (ref.) | |
| 2nd quartile | 1.061 (0.853–1.32) | 0.593 |
| 3rd quartile | 0.891 (0.702–1.131) | 0.343 |
| 4th quartile | 0.873 (0.684–1.114) | 0.275 |
| Unknown | 0.952 (0.585–1.55) | 0.843 |
| Hospital location | ||
| Rural | 1.0 (ref.) | |
| Urban | 1.983 (1.34–2.935) | 0.001 |
| Hospital teaching status | ||
| Non-teaching | 1.0 (ref.) | |
| Teaching | 1.836 (1.547–2.179) | <0.001 |
| Hospital region | ||
| Northeast | 1.0 (ref.) | |
| Midwest | 0.537 (0.401–0.719) | <0.001 |
| South | 0.895 (0.726– 1.103) | 0.298 |
| West | 0.854 (0.668–1.092) | 0.21 |
OR: odds ratio; CI: confidence interval; ref.: referent; CCI: Charlson comorbidity index; BMI: body mass index.
Caucasian patients (66.7%) underwent ureteral stent placement most frequently. Of the patients who underwent ureteral stenting, African American patients made up only a small portion of them (6.1%); however, they had a higher risk of stent failure compared to their Caucasian counterparts (OR 1.332 [1.014–1.748], p = 0.039). Nearly half of the patient population had private insurance (44%), while 32% had Medicare and 11.6% Medicaid coverage (Table 1). Those patients with Medicare or Medicaid had significantly higher odds of stent failure (OR 1.329 [1.046–1.688], p = 0.02 and OR1.38 [1.081–1.762], p = 0.01, respectively) (Table 2). Median household income was relatively well-distributed within the population and did not appear to influence the success of ureteral stent placement.
Eighty-three percent of the population had ureteral stones, and among those, 83.4% had successful ureteral stenting. The odds of a successful decompression were higher for patients with ureteral stones as compared to those with renal stones (OR 0.204 [0.174–0.239], p < 0.001) (Table 2).
A minority of the study population (21%) met the previously described criteria for sepsis, while sepsis itself was not associated with stent failure (OR 0.968, p = 0.758). About 15% of the study population had concomitant acute renal failure, which portended significantly higher odds of undergoing a salvage PCN (OR 1.488 [1.209–1.831], p < 0.001) (Table 2).
Most of our patients underwent their procedures in urban non-teaching hospitals and almost half of the procedures were performed in southern states. (See Appendix 2 for a list of states corresponding to each region). Patients in urban, teaching hospitals were much more likely to undergo salvage PCN (OR 1.983 [1.34–2.935], p = 0.001 and OR 1.836 [1.547–2.179], p < 0.001, respectively).
Discussion
Without adequate decompression, urinary tract infections associated with stone obstruction can rapidly lead to pyelonephritis, urosepsis, and potentially death.17 Time to decompression of the urinary tract is therefore critical in the management of these patients. During the last decade, an increasing number of patients in the United States were decompressed with ureteral stent rather than percutaneous drainage.2 Given this increase in the utilization of ureteral stent, as well as the emergent nature of the condition, understanding the efficacy of this particular intervention is essential for expedient clinical decision-making.
At present, there is a debate over which type of decompression, ureteral stent versus PCN, is superior for managing infected obstructed urolithiasis.3 As such, one of our most notable findings is that for the vast majority of cases (98%), ureteral stenting is successful at decompressing the urinary tract (i.e., did not require PCN placement) – only 2.2% (3628) of patients required salvage PCN after stenting. Reported rates of successful ureteral stenting in the setting of an intrinsic obstruction have been between 77 and 88%.9,10 These studies did not focus solely on stones or stones associated with infection; other causes of obstruction, such as ureteral strictures, ureterovesical and ureteropelvic junction obstructions, contributed to the lower success rates. This study is the first to report stent failure rates specifically in patients with infected urolithiasis who are at a high risk for morbid sequelae if not adequately decompressed. Additionally, this is the first study to examine this subject in a large nationwide population. Most other studies had a limited study population (40–150 patients).
Despite the low rates of salvage PCN in our study population, we found that stent failure occurred in 2.2% of patients. Further, the incidence of stent failure increased during the study period (EAPC 12%, p < 0.001) at a pace near to that of successful stent placement (EAPC 14%, p < 0.001), suggesting that stent failure is an adverse event inherent to stent placement. As such, stent failure is a veritable, albeit unusual, problem that practitioners must consider when a patient does not clinically improve after ureteral stenting.
In particular, renal failure was the clinical sign associated with increased odds of stent failure in our study population (OR 1.5, p < 0.001). We propose three reasons for this finding. First, persistent inadequate drainage is likely to cause post-obstructive acute kidney injury. Second, a patient who is inadequately decompressed and therefore inadequately treated is at risk for pre-renal kidney injury secondary to hypovolemia and renal hypoperfusion. Finally, a lack of improvement may increase the patient’s risk of exposure to nephrotoxins (e.g., antibiotics and intravenous contrast). In concordance with these assumptions, both elevated creatinine and severity of hydronephrosis have previously been linked to stent failure.9,10
Stent failure requiring salvage PCN was significantly less likely to occur in patients with ureteral stones (OR 0.2; p < 0.001). Although this dataset does not include details regarding stone size, PCN utilization has been associated with increased renal stone burden.18 We propose that the physical space for a stent to appropriately function is increasingly compromised as renal stone size increases; as such, large renal stones, including staghorn calculi, may account for a proportion of stent failure in our population. Further, ureteral stones that cause an obstruction significant enough for stent failure may be those for which a stent cannot be placed in the first place. In two studies evaluating failure to place stents for the acute stone, problems were encountered solely in the ureter.7,8
As demonstrated previously by Sammon and colleagues,2 although men are more frequently affected by symptomatic urolithiasis, women suffer more frequently from infection and infection associated with urolithiasis. The finding that males are at higher odds for ureteral stent failure is interesting and perplexing. Of the two previously mentioned studies evaluating stent failure in intrinsic obstruction, one found stent success in only 69% of males compared to 100% of females,9 while the other found no significant difference in stent failure between men and women.10 One possible explanation is the increased incidence of stent migration in taller individuals.19 Given that females are on average shorter than men, there may be less of a risk of stent migration and subsequent dysfunction in this group. Further, in a typical case of stent migration, endoscopic stent retrieval would be performed. In a patient with an infect urinary tract, however, attempting retrieval may be viewed as less ideal than percutaneous decompression. Another possibility is the effect of a large prostate on stent function. Stent migration related to intramural ureteral distortion or local ureteral vesical junction obstruction from an enlarged prostate could potentially contribute to ureteral stent failure.
Finally, our data suggest that stent failure requiring salvage PCN occurred most frequently in urban teaching hospitals (OR 1.98, p < 0.001; OR 1.84, p < 0.001). We believe this finding is partly related to PCN utilization patterns in the United States. A recent population-based study found that most PCNs placed for infected urolithiasis occurred in urban (94.8%), teaching (61.2%) hospitals.2 Similar trends have been seen for percutaneous nephrolithotomy (PCNL), where PCNL utilization occurred most frequently at high-volume, urban teaching hospitals.20,21 Given this pattern, we infer that one reason why a higher rate of stent failure was observed in urban teaching hospitals reflects access to interventional radiology teams or stone specialists who place PCNs. We do not feel that stent failure rarely occurred in rural non-teaching hospitals, but rather that our study population did not include transfers to tertiary care centers after stent placement. We acknowledge, however, that transfers alone do not account entirely for our findings. Urban teaching hospitals are more likely to receive complex urologic patients (e.g., patients with urinary diversions); therefore, our rate of stent failure in urban teaching hospitals may also reflect a higher number of complex patients seen within those institutions.
Given the morbid nature of an infected obstructed urinary tract, it is essential to select the most expedient form of decompression. In many cases, this depends on timing and access to the specific procedure rather than patient characteristics.3 We have demonstrated that ureteral stents are effective at decompression in 98% of patients. For patients who undergo ureteral stenting but do not have improvements in renal function, early evaluation for stent malposition and hydronephrosis should be performed as stent failure does occur in a small subset of patients with infected obstructing stones.
The limitations inherent to using administrative data include the possibility of coding errors, misclassification, and selection bias. By cross-referencing diagnosis and procedure codes, we attempted to minimize the risk of misclassification. Additionally, there is an apparent selection bias as all patients in this study underwent ureteral stent placement. In regards to our method of patient abstraction, it relied on an assumption that stenting performed on the same day as a PCN represented stent failure. We cannot verify this assumption was true for all cases, but given the known success rates of PCN we believe this to be a reliable assumption. It is plausible that some PCNs were placed due to poorly tolerated ureteral stents, or possibly in preparation for future stone removal procedures. We also presume that infection was the impetus for ureteral stent placement; however, we are unable to discern whether infection was a presenting diagnosis or occurred as a result of stent placement. In addition, our study likely underestimates the number of failed stents because patients with stent failure who were transferred to tertiary centres for PCN placement would not fit our selection criteria as the procedures were performed at different hospitals. Additionally, a limitation of this study is that it is assumed that patients coded for urolithiasis and UTI or pyelonephritis and who underwent ureteral stent placement had obstructed stones. Finally, as discussed above, we were unable to evaluate specific patient details, such as stone size, laterality, and collecting system abnormalities, which would provide additional valuable information about patients who fail ureteral stenting.
Conclusion
In patients with obstructed infected stones, ureteral stenting is successful in most cases. However, failure of ureteral stent requiring salvage nephrostomy does occur. Patients with higher odds of stent failure include middle-aged males with upper tract stones in acute renal failure. Patients requiring salvage PCN are more likely to be managed in urban teaching institutions where interventional radiologists and stone experts are readily available.
Appendix 1. International Classification of Diseases, 9th revision, codes of urinary tract infection or pyelonephritis
| 595.0 | Acute cystitis |
| 595.9 | Cystitis unspecified |
| 599.0 | Urinary tract infection, site not specified; infections affecting structures participating in the secretion and elimination of urine: the kidneys, ureters, urinary bladder, and urethra |
| 590.00 | Chronic pyelonephritis without lesion of renal medullary necrosis |
| 590.01 | Chronic pyelonephritis with lesion of renal medullary necrosis |
| 590.1 | Acute pyelonephritis |
| 590.1 | Acute pyelonephritis without lesion of renal medullary necrosis |
| 590.11 | Acute pyelonephritis with lesion of renal medullary necrosis |
| 590.2 | Renal and perinephric abscess |
| 590.3 | Pyeloureteritis cystica |
| 590.8 | Other pyelonephritis or pyonephrosis not specified as acute or chronic |
| 590.8 | Pyelonephritis; unspecified inflammation of the kidney and its pelvis due to infection |
| 590.81 | Pyelitis or pyelonephritis in diseases classified elsewhere |
Appendix 2. All states, by region
| Region | States |
| 1: Northeast | Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont |
| 2: Midwest | Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, Wisconsin |
| 3: South | Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, West Virginia |
| 4: West | Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, Wyoming |
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sammon JD, Ghani KR, Karakiewicz PI, et al. Temporal trends, practice patterns, and treatment outcomes for infected upper urinary tract stones in the United States. Eur Urol. 2013;64:85–92. doi: 10.1016/j.eururo.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Matlaga BR. How do we manage infected, obstructed hydronephrosis? Eur Urol. 2013;64:93–4. doi: 10.1016/j.eururo.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Lee WJ, Patel U, Patel S, et al. Emergency percutaneous nephrostomy: Results and complications. J Vasc Interv Radiol. 1994;5:135–9. doi: 10.1016/s1051-0443(94)71470-6. [DOI] [PubMed] [Google Scholar]
- 5.Pearle MS, Pierce HL, Miller GL, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160:1260–4. [PubMed] [Google Scholar]
- 6.Ramchandani P, Cardella JF, Grassi CJ, et al. Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2003;14:S277–81. [PubMed] [Google Scholar]
- 7.Mokhmalji H, Braun PM, Martinez Portillo FJ, et al. Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: A prospective, randomized clinical trial. J Urol. 2001;165:1088–92. [PubMed] [Google Scholar]
- 8.Sivalingam S, Tamm-Daniels I, Nakada SY. Office-based ureteral stent placement under local anesthesia for obstructing stones is safe and efficacious. Urology. 2013;81:498–502. doi: 10.1016/j.urology.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Wenzler DL, Kim SP, Rosevear HM, et al. Success of ureteral stents for intrinsic ureteral obstruction. J Endourol. 2008;22:295–9. doi: 10.1089/end.2007.0201. [DOI] [PubMed] [Google Scholar]
- 10.Yossepowitch O, Lifshitz DA, Dekel Y, et al. Predicting the success of retrograde stenting for managing ureteral obstruction. J Urol. 2001;166:1746–9. doi: 10.1016/S0022-5347(05)65666-2. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Census Bureau, Census 2000. https://www.census.gov/prod/2002pubs/c2kprof00-us.pdf. Accessed April 2, 2015.
- 14.American Hospital Association Annual Survey of Hospitals AHA Annual Survey Online. 2013 http://www.ahadataviewer.com. Accessed April 2, 2015. [Google Scholar]
- 15.Healthcare Cost and Utilization Project (HCUP) 2007. –2009https://www.hcup-us.ahrq.gov/. Accessed April 2, 2015
- 16.Anderson WF, Camargo MC, Fraumeni JF, Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–8. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagenlehner FM, Weidner W, Naber KG. Optimal management of urosepsis from the urological perspective. Int J Antimicrob Agents. 2007;30:390–7. doi: 10.1016/j.ijantimicag.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura K, Utsunomiya N, Ichioka K, et al. Emergency drainage for urosepsis associated with upper urinary tract calculi. J Urol. 2005;173:458–62. doi: 10.1097/01.ju.0000150512.40102.bb. [DOI] [PubMed] [Google Scholar]
- 19.Breau RH, Norman RW. Optimal prevention and management of proximal ureteral stent migration and remigration. J Urol. 2001;166:890–3. [PubMed] [Google Scholar]
- 20.Morris DS, Taub DA, Wei JT, et al. Regionalization of percutaneous nephrolithotomy: Evidence for the increasing burden of care on tertiary centers. J Urol. 2006;176:242–6. doi: 10.1016/S0022-5347(06)00512-X. discussion 246. [DOI] [PubMed] [Google Scholar]
- 21.Ghani KR, Sammon JD, Bhojani N, et al. Trends in percutaneous nephrolithotomy use and outcomes in the United States. J Urol. 2013;190:558–64. doi: 10.1016/j.juro.2013.02.036. [DOI] [PubMed] [Google Scholar]


