Abstract
The overall survival for patients with advanced papillary renal carcinoma (RCC) is still limited. Although multikinase inhibitors have recently been developed for clear cell carcinoma, response rates in other histology non-clear cell RCC are poor and patients often face dose-limiting toxicities which lead to a reduction in prognosis and treatment success. We present a patient with hereditary leiomyomatosis and RCC (HLRCC), showing a sustained response for more than 12 months to gemcitabine-bevacizumab therapy after failure tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR) therapies.
Introduction
The hereditary leiomyomatosis and renal cell cancer syndrome (HLRCC) are caused by heterozygous germline mutations of the fumarate hydratase (FH) gene on chromosome 1q34.1 The intracellular hypoxia inducible factor (HIF) is stabilized due to an accumulation of FH based on the mutations in this gene region. The final consequence is the release of different tumour promoting factors, such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and transforming growth factor alpha (TGF-α).2
Molecularly targeted agents have not been studied as extensively in patients with non-clear cell carcinoma as in those with clear cell renal tumours. However, multiple reports suggest that the vascular endothelial growth factor (VEGFR)-tyrosine kinase inhibitors (TKI) and mammalian target of rapamycin (mTOR) inhibitors may be clinically useful in these patients.3
The case describes the extraordinary success in treating a patient diagnosed HLRCC with metastatic papillary renal carcinoma by a sequential therapy with multikinase inhibitors, external irradiation, and gemcitabine-bevacizumab (BVZ).
Case report
A 30-year-old man presented with cutaneous leiomyoma in his chest and was subsequently found to have a renal mass. In February 2008 the patient underwent a right laparoscopic nephrectomy. At this time no metastases were observed on computed tomography (CT). Pathological studies revealed a type II papillary RCC, TNM staging was pT1NxM0. The genetic study conducted identified a pathogenic change C698G, a missense mutation in FH gene.
About 1 year after the nephrectomy, a routine CT scan showed multiple liver metastases (Fig. 1). An exploratory laparotomy was performed. Pathological review of the metastatic lesions confirmed relapsed renal papillary carcinoma. A magnetic resonance (MRI) scan of the spine was performed for back pain and confirmed a metastatic deposit in the sacrum at S2 (Fig. 1).
Fig. 1.
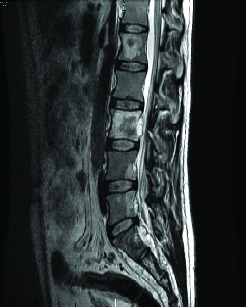
Magnetic resonance imaging showing bone metastases.
Following surgical treatment, the patient received sunitinib 50 mg/daily in 6-week cycles (4 weeks on, 2 weeks off). The CT scan was used for response evaluation according to the Response Evaluation Criteria in Solid Tumors (RECIST). Examination after 6 cycles of treatment showed stable disease. Further 6 cycles of sunitinib were administered to the patient with no dose reduction. Treatment was stopped in September 2010 as the CT evaluation showed liver progression disease.
Between February and October 2011, the patient received sequential everolimus 10 mg/daily (3 months) and pazopanib 800 mg/daily (2 months) with no response. In October 2011, the patient had progressive disease of the liver metastases, peritoneal carcinomatosis, and bone lesions (Fig. 2). Eastern Cooperative Oncology Group (ECOG) performance status (PS) score was 2 secondary to severe abdominal pain and massive ascites. After assessing the benefits and possible risks of new targeted therapy, we administered BVZ 10 mg/kg/ every 14 days intravenously. After the second cycle of BVZ, the ascites resolved without drainage and the patient symptomatically improved.
Fig. 2.

A computed tomography scan showing peritoneal and liver metastases.
In December 2011, vertebral column MRI study showed an epidural soft-tissue mass causing spinal cord compression from L3-S2. Prior to radiotherapy of the spinal (10 × 3.0 Gy/week, total dose 30 Gy), the patient received 3 cycles of BVZ with gemcitabine (GEM) (cycle 1: 1500 mg/m2 GEM, every 14 days intravenously). Two weeks after the completion of radiotherapy, the patient received 8 additional cycles of GEM-BVZ. During this treatment, the patient was clinically well and his ECOG PS score was 0. However, 12 months after starting BVZ, the patient underwent spinal MRI and abdominal CT which revealed meningeal and peritoneal tumour progression (Fig. 3). The patient was transferred to the palliative care team for supportive care.
Discussion
We report the success in treating metastatic papillary renal carcinoma in a single patient, with a survival time of more than 48 months since the initial time of metastatic diagnosis and more than 43 months since beginning sunitinib therapy.
Personalized medicine is expanding field in oncology. We are continuing to learn more about the molecular biology (the specific role of HIF, VEGF and mTOR pathways in RCC).4 We also know that the cause of tumoural development in HLRCC is the mutation in FH.5
The syndrome
HLRCC is a relatively rare autosomal dominant condition which causes cutaneous and uterine leiomyomas and early-onset RCC, typically papillary carcinoma type II.6–8 This syndrome is also known as the multiple cutaneous and uterine leiomyomatosis syndrome (MCUL1) or Reed’s syndrome.
The genetic basis for HLRCC syndrome is germ-line inactivating mutation in the gene for the Krebs/tricarboxylic acid cycle enzyme, fumarate hydratase (FH), located in the enzyme that converts fumarate to malate.7 Affected individuals are predisposed to development of leiomyomas of the skin and uterus, as well as highly aggressive RCC. Inhibition of FH should result in significant decrease in oxidative phosphorylation necessitating that glycolysis followed by fermentation of pyruvate to lactate will be required to provide adequate adenosine triphosphate (ATP), as well as to regenerate NAD+.8 Moreover, FH deficiency is known to up-regulate expression of hypoxia-inducible factor (HIF)-1alpha by enhancing the stability of HIF transcript. This leads to the activation of various HIF-regulated genes, including VEGFR and glucose transporter GLUT1 and increased expression of several glycolytic enzymes.8–10
HLRCC has an autosomal dominant inheritance, and the FH gene is thought to act as a tumour suppressor gene. Germline alterations that have been identified include missense, nonsense, insertion, deletion, and splice-site mutations. In our patient it was identified a pathogenic change C698G, a missense mutation in FH gene, one of the 125 identified mutation.9
Renal tumours occur in 20% to 30% of patients. These renal carcinomas tend to be aggressive, with rapid nodal and distant dissemination even if the primary tumour is relatively small and contained. Patients with suspected HLRCC should undergo a thorough imaging evaluation and early intervention of any suspected lesions.1 Complete wide excision, including lymph node dissection, has been recommended in patients with localized or locally advanced disease. Close follow-up is required after initial treatment. Systemic therapy has shown a wide range of outcomes for patients with metastatic disease. A multidisciplinary approach, including gynecologists, dermatologists, and genetic counselors, is required for optimal patient management.
Treatment
Choueiri and colleagues11 showed that patients with papillary RCC (PRCC) and chromophobe RCC (ChRCC) may have prolonged progression-free survival (PFS) with sunitinib and sorafenib, although clinical responses remain overall low in PRCC. Molina and colleagues did not find any objective response in 8 patients with PRCC treated with Sunitinib (Table 1).12 Additional prospective trials with these agents in non-clear cell RCC will further clarify their use in the future.
Table 1.
Studies with tyrosine kinase inhibitors
| Choueiri TK et al.11 | Phase II | PRCC/ChRCC | SU 41 patients; SO 12 patients | PFS SU 11.9 PFS SO 5.1 |
| Molina AM et al.12 | Phase II | Non clear (8 patients PRCC) | SU 23 patients | PFS 5.6 (PRCC) |
| Tannir NM et al.16 | Phase II | Non clear (27 patients RCC) | SU 57 patients | PFS 2.7 PFS (PRCC =1.5) |
PRCC: papillary renal cell carcinoma; PFS: progression-free survival; SU: sunitinib; SO: sorafenib; RCC: renal cell carcinoma.
The use of mTOR inhibitors and TKIs are still limited. Current clinical practice guidelines, such as the European Society of Medical Oncology (ESMO),13 support the use of mTOR inhibitors in patients with non-clear cell RCC; these recommendations are based on low levels of evidence (subgroups analysis and retrospective data) (Table 2). Further results from randomized, controlled clinical trials are needed to determine the optimal choice of therapy for patients with non-clear cell RCC.
Table 2.
Studies with mTOR
| Dutcher JP et al.17 | Exploratory from Phase III | Papillary subtype (25 patients) | Temsirolimus | PFS 5.9 |
| Other (10 patients) | Temsirolimus | PFS 7.9 |
mTOR: mammalian target of rapamycin; PFS: progression-free survival.
Information on the use of anti-VEGF (BVZ) is still limited in patients with nccRCC.14 Lactate dehydrogenase-A (LDH-A) is an enzyme involved in fermentative glycolysis. This molecular pathway is overexpressed in patients with FH deficient and stabilized by HIF-1a. LDH-A inhibition results in increased apoptosis via ROS production in an A549 surrogate FH knockdown cell line.15
Conclusion
There is a clear lack of evidence about how to treat PRCC tumours. The experience in a single patient with different targeted therapies showed that the GEM-BVZ might provide a benefit in this setting. Therapeutic agents target a variety of signaling pathways, and provide better molecular profiling to help us understand and select treatment for each patient. Non-clear cell RCC tumours are biologically heterogeneous and may not uniformly respond to treatment. Clinical trials are required to clarify the role of these agents, and patients should be encouraged to enroll in appropriately designed studies.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Chung EK, Posadas EM, Kasza K, et al. A phase II trial of gemcitabine, capecitabine, and bevacizumab in metastatic renal carcinoma. Am J Clin Oncol. 2011;34:150–4. doi: 10.1097/COC.0b013e3181d6b2fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Hariry I, Powles T, Lau MR, et al. Amplification of epidermal growth factor receptor gene in renal cell carcinoma. Eur J Cancer. 2010;46:859–62. doi: 10.1016/j.ejca.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Furge KA, Chen J, Koeman J, et al. Detection of DNA copy number changes and oncogenic signaling abnormalities from gene expression data reveals MYC activation in high-grade papillary renal cell carcinoma. Cancer Res. 2007;67:3171–6. doi: 10.1158/0008-5472.CAN-06-4571. [DOI] [PubMed] [Google Scholar]
- 4.Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–42. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiuru M, Lehtonen R, Arola J, et al. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62:4554–7. [PubMed] [Google Scholar]
- 6.Osanto S, Qin Y, Buermans HP, et al. Genome-wide microRNA expression analysis of clear cell renal cell carcinoma by next generation deep sequencing. PLoS ONE. 2012;7:e38298. doi: 10.1371/journal.pone.0038298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson IPM, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–31. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 12.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30:335–40. doi: 10.1007/s10637-010-9491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudier B, Eisen T, Porta C, et al. ESMO Guidelines Working Group Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii65–71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 14.Chung EK, Posadas EM, Kasza K, et al. A phase II trial of gemcitabine, capecitabine, and bevacizumab in metastatic renal carcinoma. Am J Clin Oncol. 2011;34:150–4. doi: 10.1097/COC.0b013e3181d6b2fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie H, Valera VA, Merino MJ, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–35. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–9. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–9. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]


