Abstract
In complex environments, behavioural plasticity depends on the ability of an animal to integrate numerous sensory stimuli. The multidimensionality of factors interacting to shape plastic behaviour means it is difficult for both organisms and researchers to predict what constitutes an adaptive response to a given set of conditions. Although researchers may be able to map the fitness pay-offs of different behavioural strategies in changing environments, there is no guarantee that the study species will be able to perceive these pay-offs. We thus risk a disconnect between our own predictions about adaptive behaviour and what is behaviourally achievable given the umwelt of the animal being studied. This may lead to erroneous conclusions about maladaptive behaviour in circumstances when the behaviour exhibited is the most adaptive possible given sensory limitations. With advances in the computational resources available to behavioural ecologists, we can now measure vast numbers of interactions among behaviours and environments to create adaptive behavioural surfaces. These surfaces have massive heuristic, predictive and analytical potential in understanding adaptive animal behaviour, but researchers using them are destined to fail if they ignore the sensory ecology of the species they study. Here, we advocate the continued use of these approaches while directly linking them to perceptual space to ensure that the topology of the generated adaptive landscape matches the perceptual reality of the animal it intends to study. Doing so will allow predictive models of animal behaviour to reflect the reality faced by the agents on adaptive surfaces, vastly improving our ability to determine what constitutes an adaptive response for the animal in question.
Keywords: adaptive landscape, fitness surface, behavioural plasticity, perception, sensory ecology
1. A complex problem
Understanding how plasticity in behaviour is generated through the interaction between genetic, epigenetic and environmental factors is a major research area in behavioural ecology [1–3]. Despite decades of research, characterizing the ways animals respond to their environments remains a challenge for behavioural ecologists. The incredible complexity of interactions among phenotypes, their development and the environment in which they reside means that accurately predicting how an animal will respond under a given set of conditions is only rarely possible and never straightforward. The limitations occur on two fronts: first, as researchers, we may be unable to measure or observe the internal and external aspects of the animal's environment that influence its behaviour [4]. Second, even if we are able to identify factors of the environment that are known to influence behaviour, then we need also to understand the umwelt of the animal—how it perceives the world around it as a function of its sensory abilities. The multidimensionality of potential interactions among factors that influence behaviour means that making predictions about the optimality, adaptive value and evolutionary trajectories of behavioural phenotypes is a formidable challenge, to say nothing of the difficulty in measuring how animals perceive these factors. Nevertheless, it is a challenge that must be met if we are ultimately to understand the evolution of behaviour. In this review, we discuss how modern analytical approaches may combine studies of optimality, sensory ecology and behavioural plasticity to meet this challenge.
2. Considering the umwelt of our study species
The field of sensory ecology has made great inroads into understanding how variation in the sensory abilities among individuals and species influences animal behaviour [5,6]. Although traditionally focused on the mechanistic bases of sensory biology [7–9], the field of sensory ecology increasingly incorporates evolutionary theory into analyses of sensory biology, examining the phylogenetic, developmental and allocation trade-offs inherent in sensory systems [4]. These considerations are especially pertinent when considering behavioural plasticity in response to variable environments. To maximize fitness across all contexts, the optimal behavioural strategy would of course be to perceive and respond to information about the environment with perfect accuracy. Yet, the perceptual space or umwelt of an animal—the world that it can access through its own sensory systems—may place bounds on its ability to adaptively respond to environmental stimuli. Similarly, in cases where an individual is able to perceive differences in relevant ecological factors, it may lack the behavioural plasticity required to effectively respond to these factors. Turning a blind eye (or deaf ear, or anosmic nose) to the ability of the individual to perceive and respond to its environment will lead to a disconnect between our predictions of adaptive animal behaviour and the sensory reality defined by the umwelt of our study species. Underlying sensory limitations may lead us to conclude that animals are making maladaptive transitions among behavioural states when they are actually acting optimally given the information they are able to collect from their environments. Alternatively, in some cases, organisms have access to perceptual space that is inaccessible to humans; birds and bees [4,5] can see UV light [6], and bats can hear ultrasound [7], potentially clouding our ability to make reasonable predictions about adaptive animal behaviour based on our own experience.
A good example of the limitations sensory biology may place on behaviour is seen in the relationship between temperature, sex determination and adaptive sex allocation. For some fish [10] and many reptiles [11], sex is determined by the ambient temperature during early development (temperature-dependent sex determination, TSD). This can facilitate adaptively plastic allocation of offspring sex if brood sex ratio can be manipulated to favour the rarer sex. A recent study on painted turtles (Chrysemys picta) demonstrated that females choose nest sites with temperatures that will give rise to an adaptive offspring sex ratio [12]. This result may lead to predications about optimal sex allocation in other related taxa. Yet, most studies have failed to convincingly demonstrate that females choose nests that result in optimal sex ratios [12]. Indeed, the variation in environment that potentiates adaptive sex allocation may render populations susceptible to maladaptive sex ratios if these cannot be accurately perceived or predicted [13]. Although there is a clear benefit of sensing both the population sex ratio and the likely nest temperatures to optimally adjust sex ratios, there is a paucity of evidence that this actually occurs. Arguments about adaptive nest site choice based on temperature must therefore be tempered by the fact that it is far from clear that animals with TSD can always sense temperature or population sex ratio.
Sexual selection by mate choice is another, and an especially intriguing, domain where perceptual biology plays a significant role [14]. A controversy in sexual selection is the degree to which females base their mate choice on traits that are correlated with heritable variation for offspring survivorship [15]. Despite well-developed theory, the evidence for a ‘good genes' effect is sparse, and when it does occur, the effect is small [16]. The reasons for the paucity of evidence and effect, despite decades of research on good-genes mate choice, are varied. For example, there could be lack of heritable variation for survivorship, and direct benefits could be far more important than indirect benefits in driving the evolution of preferences [17]. Another possibility is that females might not always have perceptual access to underlying genetic variation, either because there is no phenotype–genotype correlation, or the correlation is so subtle that it cannot be perceived. This might be why the two most successful demonstrations of genetically based mate choice are associated with perceptual pathways known to be both accessible to choosers and indicative of genotype: choice for conspecifics versus heterospecifics [14], and choice for MHC compatibility [18]. In the former case, we know that there is strong linkage between the species-specific courtship traits and the genetic identity of the species; even humans are able to identify species of many birds, frogs, crickets and fireflies by their courtship signals. In the latter, choice for compatible MHC genes appears to be based exclusively on odour cues [18,19]. There is no reason to think that all traits in all modalities will show the same statistical linkage with underlying genetic variation, and that this meaningful variation (i.e. the phenotype–genotype correlation) will be equally accessible in all modalities. Thus, arguments about optimal mate choice, even when based on theoretical ground as well traversed as good genes hypotheses, may break down when sensory ecology is ignored.
More generally, across modalities and taxa, we find examples of sensory limitations or errors leading to suboptimal or maladaptive behaviour [4]. In many bird species, photoperiod cues influence laying date, which may be adaptive if they align with later life-history events such as nesting time, but which can become maladaptive under the novel environmental conditions generated, for instance, by anthropogenic disturbance [20]. Similarly, for many invertebrates, anthropogenic sources of light can lead to navigation errors. Moths fly to the black-lights of entomologists as well as to urban lights [21], and mayflies lay their eggs on dry asphalt roads because they perceive the reflected polarized light as a water surface [22]. Perceptual limitations may also be directly exploited to manipulate the behaviour of con- and heterospecifics [14]. Male goodeid fishes have yellow bands that mimic worms, attracting females in search of prey, and male characin swordtails have a lure on the pectoral spine that resembles an ant; water mites drum the water's surface to mimic the vibrations of their copepod prey, and moths mimic the echolocation calls of predatory bats to freeze their females in fright in order to gain sexual access (reviewed in [14]). Even in systems as seemingly robust as kin recognition in eusocial insects, perceptual limitations can lead to maladaptive responses. Workers of the killer bee Apis mellifera scutellata mistake the pheromonal bouquet of the Cape honeybee A. mellifera capensis for that of their own queen, rearing the larvae of these unrelated individuals and reducing their inclusive fitness to zero. In these cases, a cue has the potential to lead to an adaptive response in a plastic behavioural trait, but incorrect perception of the cue leads to maladaptive behaviour [23].
3. Adaptive behaviour on ‘powerfully seductive’ landscapes
How then can we incorporate the nuance generated by variation among individuals and species in their ability to even detect the parameters of our models? Although the complexity of relationships among factors influencing behaviour means that simple linear predictions become impossible, more sophisticated approaches to understanding adaptive behaviour in a complex world may be applied. Faced with comparable complexity in examinations of gene frequencies or phenotypes across generations, population geneticists and evolutionary biologists map fitness outcomes in multidimensional space using approaches based on genotype (e.g. adaptive landscapes [24]) or phenotype space (e.g. Pareto fronts [25]). As visual and heuristic models of evolutionary processes, fitness landscapes can be ‘powerfully seductive’ [26] owing to the appeal and intuitive understanding of terms such as peaks, ridges and valleys to describe fitness consequences of different allele frequencies or gene-by-environment interactions. These visual models provide us insights into the qualitative directions in which selection may push populations or phenotypes. Applying equivalent approaches to the study of animal behaviour holds great potential to predict or assess ‘adaptive’ behaviour, as they allow us to make predictions across a range of contexts, extrapolating into areas of unmeasured space, for example in the form of nutrient spaces [27] or landscapes of fear [28,29]. Moreover, the topology of the behavioural pay-off surfaces can be manipulated to reflect the sensory ecology of the animal being studied, potentially by directly combining perceptual space with adaptive space.
A major point to consider is how the original implementation of fitness surfaces and adaptive landscapes differs from any application of surfaces in behavioural studies. The fundamental difference comes in the perceptual capacity of the agents (alleles versus behaving organisms) on these surfaces. On Wrightian adaptive landscapes, evolutionary processes such as natural selection, drift and migration may change allele frequencies and cause populations to move through fitness peaks and troughs on the landscape [30], yet the process is blind because populations at any position on the surface cannot shift to maxima through any volitional process. Movement on these landscapes therefore represents the ultimate case of perceptual limitation—alleles are more or less frequent in the next generation, but themselves are inviolate ‘billiard balls' knocked about by selection [31]. Contrast this with behaving animals in fluctuating environmental conditions, which have the ability to perceive, to a greater or lesser extent [28], the pay-offs of different behavioural strategies. In this model, landscapes can be considered visual matrices where peaks and troughs represent direct/immediate (as opposed to indirect/generational) pay-offs as fitness proxies for reproductive success or energetic gains. The individual agents on these surfaces therefore have the potential to move about in real time, shifting directly to pay-off optima without moving through troughs of low fitness. Male spiders choosing among female mates, for example, respond to changing competitive contexts by rapidly switching their choices to increase the reproductive pay-offs under the new social conditions [32]. This capacity hinges on the individual's ability to perceive and assess the pay-offs of a particular behaviour in a given context, which can only be assessed with a detailed understanding of the perceptual and cognitive abilities of the animal being studied. Studies into behavioural optimality must therefore also assess the capacity of the behaving agent to distinguish among local and global optima. While this is a mathematically straightforward exercise, it may be beyond the abilities of study organisms in complex environments. Any study into the optimality of behaviour across environmental contexts therefore naturally dovetails with an assessment of perceptual and cognitive biology [33], but novel approaches may be required to successfully join these fields.
4. Successful use of surfaces in behavioural ecology
Pay-off surfaces have already been used successfully in behavioural ecology, for example, in the implementation of nutrient spaces to predict adaptive foraging behaviour [27]. On these surfaces, the axes represent the amount of protein and carbohydrate, respectively, in sources of food. The animals used in these studies have ‘protein targets', amounts of protein that they must reach in order to be satiated, and by plotting the ratio of protein to carbohydrate in nutrient space, testable predictions can be made about the foraging strategy animals will use. The high coherence between predictions and observed behaviour is a strong indication that the foraging animals are able to perceive the differing amounts of protein and carbohydrate in their food sources, i.e. they are able to perceive the surface on which their behaviour is being plotted. This may not always be a reasonable assumption, as we now outline using a very similar foraging problem based on food colour.
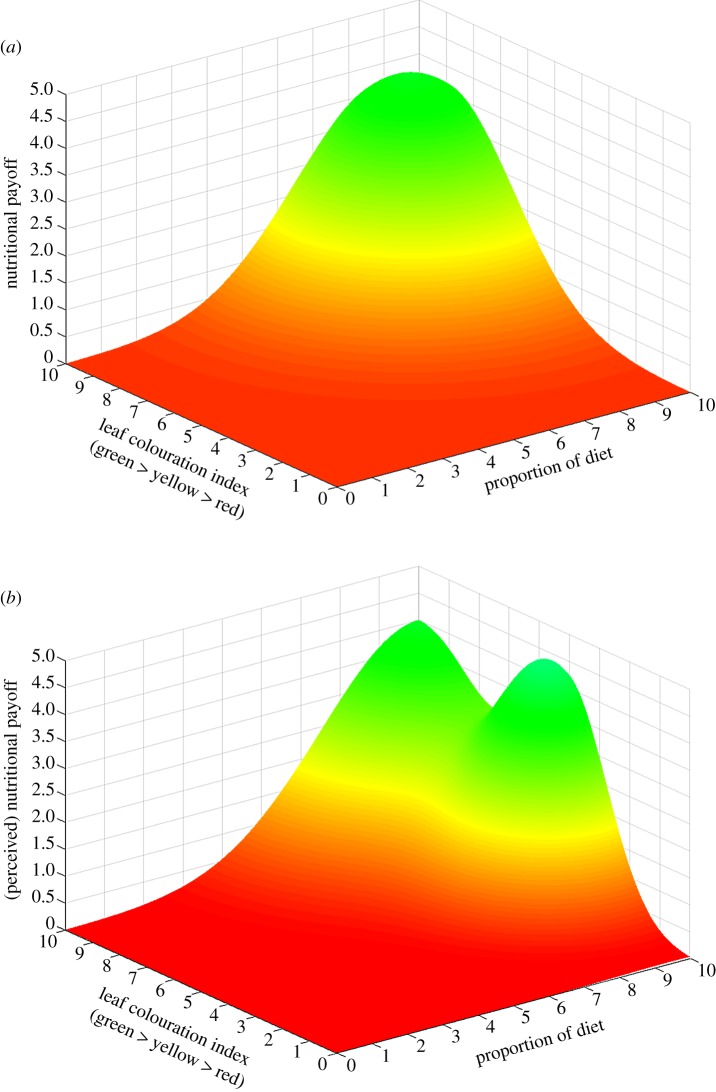
Many animals feed on food sources that differ in colour, and the colour of food sources represents some aspect of their nutritional value (e.g. Sylvia warblers and fruit colour [34]). When foraging animals need to balance their intake of different nutrients, it is intuitive and informative to use landscapes to visualize the fitness outcomes of differing foraging strategies [27]. As autumnal leaves senesce, they lose up to 70% of their nitrogen [35,36], which then decreases their nutritional value to herbivores. A visually spectacular correlate of this senescence (for the human umwelt at least) is a colour change from green to yellow to red, allowing us to create a hypothetical landscape with axes representing the nutritional pay-offs of probabilistic foraging on leaves of various colours. From this landscape, we might predict the adaptive optima for foraging on leaves of differing colours (figure 1a). But this exercise would ignore a caution long ago raised by Lord Rayleigh: the assumption that the world we can see and measure as scientists is in any way similar to that perceived by the animals we study ‘is a good deal to take for granted’ [37]. In fact, the evolutionary basis for this colour transition and the consequences for herbivores have been hotly debated since Hamilton & Brown [38] suggested that autumnal coloration is a case of aposematic coloration, deterring aphids from feeding on leaves in autumn (see also [39,40]). This claim led to the essential question posed by Chittka & Doring [35]: can aphids perceive these allegedly aposematic ‘signals', keeping in mind that no herbivorous insect studied to date has red colour receptors [36]? These researchers demonstrated that the yellow coloration of leaves actually acts as a super-normal stimulus because, to the visual receptors of aphids, yellow appears ‘very’ green and is more attractive to most species of aphids than green, whereas red colours appear dull to the aphid [35]. If we construct a landscape based on increasing toxicity or reduced nutrient value, and hence lower fitness of feeding on yellow or red leaves, we might generate figure 1a in which fitness peaks occur when a higher proportion of green leaves are eaten. On observing that aphids feed more readily on yellow leaves, and therefore sit lower on the fitness landscape, we may assume that the aphids currently reside on a suboptimal point on the landscape, and that selection would drive behaviour to feed primarily on green leaves. But, of course, to an aphid, a yellow leaf looks greener than a green one! As such, the fitness ‘optima’ perceived by the organism (figure 1b) is different from that perceived by the researcher. An adaptive behavioural response is therefore out of reach, because there is a fundamental disconnect between what we can visualize on a foraging fitness landscape and the biological reality of the surface that can be perceived by the organism.
Figure 1.
A visual nutrient space of autumn leaves. The colour of autumn leaves has been suggested to signal either their toxicity (aposematism) or nutrient value to herbivores (e.g. green leaves are higher in nitrogen than yellow or red leaves). (a) Feeding on greener leaves with a higher probability (y-axis) than yellow or red leaves (x-axis) therefore leads to higher fitness pay-offs. (b) To the visual system of aphids, however, yellow leaves appear as a super-normal green stimulus, and will potentially be fed on with greater probability than other leaves. The fitness surface according to the aphid (b) is therefore very different from the actual fitness surface (a). (Online version in colour.)
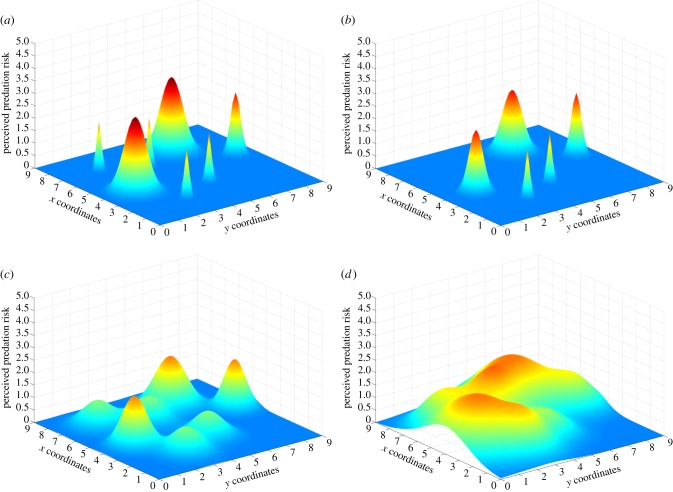
Signals or cues that are employed to intentionally deceive the receiver highlight a more widespread phenomenon in which one individual's adaptive landscape is masked to benefit another individual. While the previously discussed limitation may be classified simply as an error, this category is better described as forced errors. In the context of heterospecific interactions, most predator–prey interactions are based on imperfect perception of the landscape in the sense that prey that could perfectly assess predation risk may never be eaten. This relationship between foraging and predation risk has been usefully explored in landscapes representing the ‘ecology of fear’ [28,29], again an adaptation of the Wrightian landscape. In these landscapes, resource matching interacts with predation risk to modify the spatial distribution and quitting times of foragers. When habitats vary in both their productivity and risk of predation, individuals are predicted to distribute themselves in a manner that maximizes the ratio of reward to risk [28,29]. Yet, for the predators, any behaviour that causes foragers to underestimate the risk of a particular patch will increase the chances of a successful predation event. Thus, there are a number of sensory pathways that prey may use to detect predation risk, and we illustrate how an animal's perceptual abilities influence its perception of such a landscape in figure 2. Studies have shown that the presence of predators can influence the behaviour of potential prey within the predators' range, such as restricting foraging activities [28,29]. Figure 2a illustrates the distribution of a predator species across space. In this example, there are three prey species that perceive the presence of the predator, each through one of three different sensory modalities, vision (figure 2b), sound (figure 2c) and smell (figure 2d). These three modalities give very different impressions of where predators are in terms of accuracy of present location, area over which prey are detected and time over which prey are detected. These perceptual differences could result in very different adjustments that each prey makes to activities that carry high predation risk, such as foraging and sexual displays. In particular, using any one of these surfaces to predict or assess adaptive responses to changes in predator distribution needs be done with respect to the relevant sensory pathways with which these predators are perceived.
Figure 2.
(a) The distribution of a hypothetical predator across space. The presence of predators can create a ‘landscape of fear’ [8], altering the behaviour of prey. The landscape of fear, however, will vary with the ability of prey to perceive the presence of predators, but not all prey and not all sensory modalities sense predators in the same way. (b) The perceived presence of predators by prey that detect predators through the visual modality. Vision gives accurate information about the presence of a predator when it is seen. It relies on direct line of sight, so detection rate can be lower than in other modalities (the prey might not see all the predators in its visual field) and the size of the detection field is typically smaller than in other modalities, but the information about spatial location is more accurate. Note that compared with (a) the peaks in (b) are lower (fewer predators are detected than are actually present), the area of the base of the peaks is smaller (the visual field does not encompass the entire landscape), but the gradient is still steep (i.e. greater accuracy of information). (c) The perceived presence of predators by prey that detect predators through the auditory modality. Sound gives less accurate information about location than visual cues, but because a direct line of sight is not required predators can be detected by acoustic cues over a larger distance than visual cues. Sound is only emitted by the predator when it moves and when it voluntarily makes sound, such as in acoustic communication. Thus, compared with (b) the height of the peaks (predators detected) could be lower if predators tend to be silent, the areas around the peaks are larger (the acoustic detection field is larger than the visual detection field), and the peaks are less steep (the accuracy of localization information is lower). (d) The perceived presence of predators by prey that detect predators through the olfactory modality. Here, we assume that odours are deposited by a predator to mark its territory or home range and thus are non-volatile. Odours provide accurate information about where an individual was but almost no information as to where it is, and this information cannot be detected at any substantial distance from the odour source when odours are used for marking (unlike long-distance olfactory communication in moths and some other animals). Thus, compared with (c) the peaks are high (many prey are detected), the area over which prey can be detected is small and restricted to be within the actual range of the predator (unlike with visual and especially auditory cues when a predator can be detected from a prey outside of its range), and the peaks are not at all steep since the information about the where a predator is at the time the odour cue is sensed in not very accurate. (Online version in colour.)
5. Summary
As our knowledge of the complex interactions among phenotypes, environments and sensory ecology increases, behavioural ecologists increasingly require sophisticated tools to assess what constitutes an adaptive response. We have focused here on the clear heuristic and predictive power that behavioural fitness surfaces may provide, but also emphasize the caution that must be applied to ensure a meaningful link between the predictions we may generate from such models and the biological reality, or umwelt, of the taxon in question. With the advent of increased competition for readership from online and open access journals, many editors lean towards papers with increased visual impact, and computer generated multicoloured surfaces emphatically provide this. Nevertheless, without a proper grounding in the biological reality of the organisms being studied, these surfaces risk placing the cart-before-the-horse, providing predictions into space that cannot be accessed or distinguished by the organism being studied. A pressing question we must always ask in the application of fitness landscapes is whether the topology of the generated landscape matches the perceptual world of the animal it supposes to study. Here, we advocate overlaying traditional adaptive landscapes with the sensory reality of an animal's perceptual space; such an approach could open new research avenues and provide powerful predictive tools of adaptive behaviour.
Let us close by saying that even when an organism has the sensory ability to assess the environment, the cognitive capacity to process this information and the behavioural plasticity to move to places of higher fitness pay-off, this may not occur. In any number of realms, humans are able to accurately predict the pay-offs of behaviours yet we fail to approach behavioural optima. From fisheries management to tobacco smoking, humans provide a wonderful illustration that even when an organism has near perfect information of the pay-off landscape (often by virtue of having created it), it cannot be assumed that adaptive peaks will be reached, or the ascent even attempted.
Acknowledgements
We thank Michael Wade, May Dixon, members of the Ryan Laboratory and two anonymous reviewers for their helpful comments on the manuscript.
Competing interests
We report no competing interests.
Funding
We received no funding for this study.
References
- 1.DeWitt TJ, Scheiner SM. 2004. Phenotypic plasticity: functional and conceptual approaches. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Ghalambor CK, Angeloni LM, Carroll SP. 2010. Behavior as phenotypic plasticity. In Evolutionary behavioral ecology (eds Westneat DF, Fox CW.), pp. 90–107. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Dangles O, Irschick D, Chittka L, Casas J. 2009. Variability in sensory ecology: expanding the bridge between physiology and evolutionary biology. Q. Rev. Biol. 84, 51–74. ( 10.1086/596463) [DOI] [PubMed] [Google Scholar]
- 5.Dusenbery DB. 1992. Sensory ecology: how organisms acquire and respond to information. New York, NY: WH Freeman. [Google Scholar]
- 6.Chittka L, Thomson JD. (eds). 2001. Cognitive ecology of pollination: animal behaviour and floral evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Barlow HB, Mollon JD. 1982. The senses. Cambridge Texts in the Physiological Sciences, vol. 3. Cambridge, UK: CUP Archive. [Google Scholar]
- 8.Greenfield MD. 2002. Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Barth FG, Schmid A. 2001. Ecology of sensing. Berlin, Germany: Springer. [Google Scholar]
- 10.Conover DO. 1984. Adaptive significance of temperature-dependent sex determination in a fish. Am. Nat. 123, 297–313. ( 10.1086/284205) [DOI] [Google Scholar]
- 11.Warner D, Shine R. 2008. The adaptive significance of temperature-dependent sex determination in a reptile. Nature 451, 566–568. ( 10.1038/nature06519) [DOI] [PubMed] [Google Scholar]
- 12.Mitchell TS, Maciel JA, Janzen FJ. 2013. Does sex-ratio selection influence nest-site choice in a reptile with temperature-dependent sex determination? Proc. R. Soc. B 280, 20132460 ( 10.1098/rspb.2013.2460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allsop DJ, Warner DA, Langkilde T, Du W, Shine R. 2006. Do operational sex ratios influence sex allocation in viviparous lizards with temperature-dependent sex determination? J. Evol. Biol. 19, 1175–1182. ( 10.1111/j.1420-9101.2006.01086.x) [DOI] [PubMed] [Google Scholar]
- 14.Ryan MJ, Cummings ME. 2013. Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 44, 437–459. ( 10.1146/annurev-ecolsys-110512-135901) [DOI] [Google Scholar]
- 15.Kirkpatrick M, Ryan MJ. 1991. The paradox of the lek and the evolution of mating preferences. Nature 350, 33–38. ( 10.1038/350033a0) [DOI] [Google Scholar]
- 16.Møller AP, Alatalo RV. 1999. Good-genes effects in sexual selection. Proc. R. Soc. Lond. B 266, 85–91. ( 10.1098/rspb.1999.0607) [DOI] [Google Scholar]
- 17.Kirkpatrick M, Barton NH. 1997. The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA 94, 1282–1286. ( 10.1073/pnas.94.4.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamazaki K, et al. 1976. Control of mating preferences in mice by genes in the major histocompatibility complex. J. Exp. Med. 144, 1324–1335. ( 10.1084/jem.144.5.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overath P, Sturm T, Rammensee HG. 2014. Of volatiles and peptides: in search for MHC-dependent olfactory signals in social communication. Cell. Mol. Life Sci. 71, 1–14. ( 10.1007/s00018-014-1559-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry H, Lil A, Wong B. 2013. Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. ( 10.1111/brv.12012) [DOI] [PubMed] [Google Scholar]
- 21.Baker RR, Sadovy Y. 1978. The distance and nature of the light-trap response of moths. Nature 276, 818–821. ( 10.1038/276818a0) [DOI] [Google Scholar]
- 22.Kriska G, Horváth G, Andrikovics S. 1998. Why do mayflies lay their eggs en masse on dry asphalt roads? Water-imitating polarized light reflected from asphalt attracts Ephemeroptera. J. Exp. Biol. 201, 2273–2286. [DOI] [PubMed] [Google Scholar]
- 23.Jordan LA, Allsopp MH, Oldroyd BP, Wossler TC, Beekman M. 2008. Cheating honeybee workers produce royal offspring. Proc. R. Soc. B 275, 345–351. ( 10.1098/rspb.2007.1422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright S. 1931. Statistical theory of evolution. J. Am. Stat. Assoc. 26, 201–208. ( 10.2307/2277618) [DOI] [Google Scholar]
- 25.Shoval O, Sheftel H, Shinar G, Hart Y, Ramote O, Mayo A, Dekel E, Kavanagh K, Alon U. 2012. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science 336, 1157–1160. ( 10.1126/science.1217405) [DOI] [PubMed] [Google Scholar]
- 26.Jones T.1995. One operator, one landscape. Santa Fe Inst. Tech. Rep. 1995, 95–02. SFI working paper: 1995-02-025.
- 27.Simpson SJ, Sibly RM, Lee KP, Behmer ST, Raubenheimer D. 2004. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68, 1299–1311. ( 10.1016/j.anbehav.2004.03.003) [DOI] [Google Scholar]
- 28.van der Merwe M, Brown JS. 2008. Mapping the landscape of fear of the cape ground squirrel (Xerus inauris). J. Mammal. 89, 1162–1169. ( 10.1644/08-MAMM-A-035.1) [DOI] [Google Scholar]
- 29.Laundre JW, et al. 2014. The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology 95, 1141–1152. ( 10.1890/13-1083.1) [DOI] [PubMed] [Google Scholar]
- 30.Richter H, Engelbrecht A. 2014. Recent advances in the theory and application of fitness landscapes. Berlin, Germany: Springer. [Google Scholar]
- 31.Bateson G. 1979. Mind and nature: a necessary unity. New York, NY: Dutton. [Google Scholar]
- 32.Jordan LA, Kokko H, Kasumovic MM. 2014. Reproductive foragers: spider males choose mates by selecting among available competitive environments. Am. Nat. 183, 638–649. ( 10.1086/675755) [DOI] [PubMed] [Google Scholar]
- 33.Mappes J, Stevens M. 2010. Sensory ecology and information use. In Evolutionary behavioral ecology (eds Westneat DF, Fox CW.), pp. 148–151. Oxford, UK: Oxford University Press. [Google Scholar]
- 34.Schaefer HM, Valido A, Jordano P. 2014. Birds see the true colours of fruits to live off the fat of the land. Proc. R. Soc. B 281, 20132516 ( 10.1098/rspb.2013.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chittka L, Döring TF. 2007. Are autumn foliage colors red signals to aphids? PLoS Biol. 5, 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Döring TF, Chittka L. 2007. Visual ecology of aphids—a critical review on the role of colours in host finding. Arthropod-Plant Int. 1, 3–16. ( 10.1007/s11829-006-9000-1) [DOI] [Google Scholar]
- 37.Lord Rayleigh JWS. 1874. Insects and the colours of flowers. Nature 11, 6. [Google Scholar]
- 38.Hamilton WD, Brown SP. 2001. Autumn tree colours as a handicap signal. Proc. R. Soc. Lond. B 268, 1489–1493. ( 10.1098/rspb.2001.1672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archetti M, Brown SP. 2004. The coevolution theory of autumn colours. Proc. R. Soc. Lond. B 271, 1219–1223. ( 10.1098/rspb.2004.2728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archetti M. 2000. The origin of autumn colours by coevolution. J. Theor. Biol. 205, 625–630. ( 10.1006/jtbi.2000.2089) [DOI] [PubMed] [Google Scholar]