Abstract
Animals use a range of sensory cues for finding food, avoiding predators and choosing mates. In this regard, the aquatic environment is particularly suitable for the use of olfactory and other chemical cues. Nevertheless, mate choice research, even on aquatic organisms, has focused on visual signals, while chemical cues relevant in sexual selection have been assumed to be ‘intrinsic’ excretions of mate candidates. Here, using the sand goby Pomatoschistus minutus, a small fish with paternal egg care, we investigated the possibility that ‘extrinsic’ chemical cues in the males’ nests could also have a significant contribution to mating success. We found that females strongly avoided laying eggs into nests subject to the odour of Saprolegnia water moulds (an egg infection) and that this effect was independent of the females’ initial, visually based preference for males. To the best of our knowledge, this is the first study to show that chemical cues related to parental failure can play a large role in sexual selection.
Keywords: chemical signal, mate choice, odour, olfactory cue, parental care, sexual selection
1. Introduction
Animals across a range of taxa can use multiple sensory mechanisms when choosing mates [1]. For instance, a combination of visual, acoustic and chemical cues can be assessed simultaneously or sequentially [1]. In this respect, owing to the solubility and dispersal properties of chemical cues in water, such cues are used in aquatic environments over various spatial scales, not only in mate choice, but also in homing, foraging and predation avoidance [2,3]. For example, olfactory cues can be used for avoiding habitats of high predation risk, as seen in the aquatic Oklahoma salamander, Eurycea tynerensis [4].
Among aquatic organisms, teleost fishes use chemical cues for a particularly broad range of purposes [2,3], especially in the context of reproduction, as demonstrated by the remarkable homing runs to breeding grounds by many salmonid species [2]. At smaller spatial scales, olfactory cues are used in species recognition, in synchronizing reproductive behaviours and for choosing compatible mates among conspecific suitors [2,3,5]. For example in Pseudotropheus cichlids, females prefer conspecific over congeneric males only when both olfactory and visual cues are available [6]. More generally, studies investigating the effects of chemical cues on reproduction have predominantly focused on orientation over long distances and assessment of ‘intrinsic’ cues excreted by potential mates at short distances [2,7].
When the opposite sex provides parental care, direct benefits of mating with an apt carer can be substantial. Therefore, we hypothesize that ‘extrinsic’ chemical cues that signal parental failure can be more reliable than any intrinsic signals of mate quality. For example in species with exclusive male egg care, females should benefit if they can assess the fate of the male suitors’ previously tended eggs, instead of needing to rely (solely) on potentially unreliable sexual signals. An excellent model for testing the importance of such extrinsic chemical cues in sexual selection is the sand goby, Pomatoschistus minutus, a sexually dimorphic fish with paternal egg care. During the extended breeding season, males try to attract females to spawn in their nests. Females typically assess multiple male candidates [8] based on visual cues and potentially also acoustic signals [9,10]. In this study, we set out to assess the significance of extrinsic chemical cues in sexual selection by manipulating the ‘smell’ of male candidates’ nests with Saprolegnia water moulds. These moulds are widespread pathogens that grow on eggs of aquatic organisms, compromising egg survival of gobies [11] and globally causing major losses to aquaculture [12]. In particular, Saprolegnia is much more likely to attack poorly- than well-attended eggs ([11] and references therein).
2. Material and methods
The study was conducted in 2014 at the Tvärminne Zoological Station on the coast of the Baltic Sea (59°50.7′ N; 23°15.0′ E). Sand gobies, collected in the proximity of the field station, were separated by sex and maintained in holding aquaria (50–100 l). All holding and experimental tanks had flow-through seawater and were subjected to natural light rhythm, water temperature and salinity.
Before the experiment, the body size of each fish was measured to the nearest millimetre and 0.01 g. Two size-matched males (n = 23 pairs; size difference (mean ± s.e.): 0.4 ± 0.1 mm; 0.03 ± 0.01 g) were then placed in each test tank, divided into three compartments by two removable, transparent Perspex dividers with holes for water exchange. The male compartments, one at each end of the tank (19 × 25 cm), were separated from each other by a central female compartment (30 × 25 cm; for an illustration, see [10]). A 4 cm layer of fine sand was provided as substrate. Both male compartments also had a halved clay flowerpot (diameter: 6.5 cm), which served as a nesting resource [10,13]. Seawater was pumped into the male compartments and flowed out via the female compartment. The tanks were shielded against disturbance with plastic blinds. We proceeded with the experiment only when both nests had been built. Males that did not build a nest within 60 h were replaced.
The female mate choice trials were conducted in two phases. The aim of phase 1 was to give the female the opportunity to establish her baseline mate preference. After placing a gravid female in the central compartment, behaviour of the female and both males was video-recorded for 3 h. The video footage was later spot-sampled every 5 min to count the number of times the female had been within 3 cm from each male's compartment, while oriented towards him. In sand gobies, such association preferences are reliable and repeatable indicators of female mating intentions [13].
Before the start of a replicate, we placed two containers, each filled with 3.5 l of water, next to the test tank. In one container, we had one or two pieces of thin acetate film covered with adhesive sand goby eggs (from one to three females) that had been incubated in contact with Saprolegnia moulds until threads of Saprolegnia were seen growing on them. The other container had either one or two similar pieces of film without eggs, from here on called ‘control A’ (n = 15 replicates), or with sand goby eggs (again from one to three females) that had no visible traces of Saprolegnia infection, from here on ‘control B’ (n = 14 replicates). Phase 2 was started immediately after phase 1 (i.e. at 3 h) by removing the two transparent dividers, so that the female had access to the entire tank. At the same time, a peristaltic pump started to pump water through the glass capillary (diameter 3 mm) with which each nesting resource was equipped. Whether a particular male/nest received Saprolegnia or non-Saprolegnia (i.e. ‘control’) water (flow rate: approx. 0.01 l min−1) was assigned at random and blind to the results of phase 1. The pump was left to operate until both containers ran out of water (approx. 6 h later). In total, phase 2 was continued for a maximum of 11 h, the first 1.5 h of which were video-recorded. We later counted, blind to the treatment, the number of times the focal female visited the two nest entrances (including visits inside the nests) and the number of minutes each male was actively courting the female. If no spawning occurred, the female was removed (control A: n = 5; control B: n = 2). The same pair of males was later re-used with a new female (nA = 4; nB = 2). If the second female also failed to spawn (nA = 1; nB = 1), the two males were also replaced. Hence, each female (n = 29; total length: 49.0 ± 0.6 mm) was used only once, and each male (n = 2 × 23 = 46; total length: 51.0 ± 0.5 mm) was only used in one replicate that resulted in a spawning (nA = 9; nB = 11). One female laid eggs in both nests and was deemed to prefer the male that received over two-thirds of the eggs. Video recordings of two replicates captured only the central compartment and were therefore useful for phase 1 only.
3. Results
The focal female spawned in the nest of the control male in nine of nine (binomial test, p = 0.0039) and 10 of 11 (binomial test, p = 0.012) replicates in set-ups A and B, respectively (figure 1). This indicates that there was no significant difference between the two control types (G-test of independence, G1 = 0.8213, p = 0.36). Thus, overall, the female spawned with the control male in 19 of 20 replicates (binomial test, p < 0.0001). Of the 20 male pairs, in 13 cases, the preferred male was, in phase 2, (randomly) allocated to the Saprolegnia treatment and the non-preferred male to the control treatment, and vice versa for the remaining seven replicates (binomial test, p = 0.26). In phase 2, control and Saprolegnia treatments did not significantly differ in terms of nest inspections by the female or male courtship rate (binomial tests for separate and combined data: all p > 0.10; figure 2).
Figure 1.
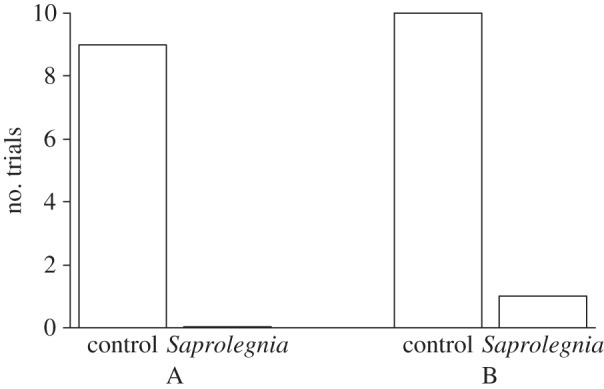
The number of males mating with the focal female (in phase 2) in the control versus Saprolegnia treatments. A and B refer to our two control treatment types.
Figure 2.
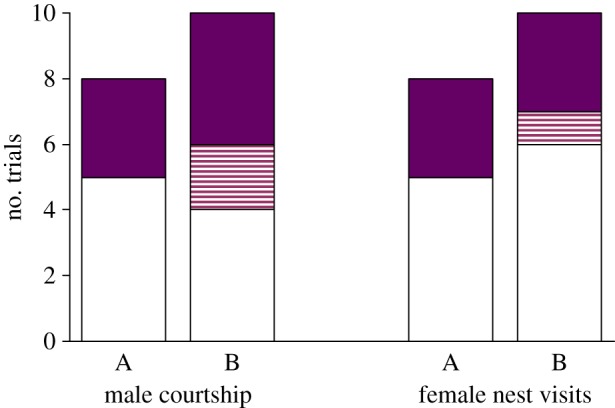
The number of replicates in which the control male (white) versus the Saprolegnia treatment male (filled) was displaying more courtship and was visited more often by the female. Replicates with ties are indicated with a striped pattern. A and B refer to the two control types. (Online version in colour.)
4. Discussion
Our study demonstrated that extrinsic chemical cues, i.e. Saprolegnia-infected eggs, had a drastic impact on the distribution of mating success: independent of their initial preference, almost all females spawned with the male whose nest received non-Saprolegnia control water (figure 1). This is notable, because in many animals (including sand gobies), visual- and acoustic-based mate preferences tend to have more modest effect sizes [1,14]. Similarly, courtship displays in some taxa (including the sand goby) seem to function predominantly to attract the attention of females, rather than to provide reliable cues of male quality [15,16]. In other words, our results suggest that extrinsic chemical cues have at least as important impact on the distribution of mating success as visual or acoustic sexual signals. This is important because, to date, research efforts have mainly focused on visual signals produced by competing males [1], or, less commonly, acoustic or intrinsic chemical cues [7,15]. Indeed, to the best of our knowledge, this is the first study to show that chemical cues that are linked to earlier parental failure are important contributors to mating success.
Earlier results have suggested that females may show a bias against nests from which eggs had recently been removed [17]. However, the mechanisms resulting in this avoidance behaviour were not assessed. In contrast, here we show that extrinsic chemical cues, such as ‘Saprolegnia odour’, can have a strong effect on the distribution of male mating success. Although we cannot completely exclude the possibility that an adjustment of male behaviour might have contributed to the result, we find this possibility unlikely, because the treatments did not differ in the males’ eagerness to court the focal female. Furthermore, similar to females that sample multiple mate candidates in the wild [8], most of our females inspected both males/nests multiple times before making their mating decision.
In conclusion, our study suggests that chemical cues other than those excreted by mate candidates may play a larger role in sexual selection than previously acknowledged. Therefore, future assessments of sexual selection, especially in aquatic animals, should more regularly consider the role of such extrinsic odour cues in mate choice.
Supplementary Material
Acknowledgements
We thank Tvärminne Zoological Station and Kai Lindström for logistic support, and Anniina Saarinen, Karine Gagnon, Santeri Lehtonen, Vojtěch ‘Voltage’ Lanta and Will Sowersby for assistance.
Ethics statement
All animal experimentation of this study adheres to the Finnish and EU guidelines for the use of animals for scientific purposes and was approved by ELLA—the National Animal Experiment Board of Finland.
Data accessibility
Data are provided as the electronic supplementary material.
Funding statement
We thank the Department of Biology, University of Turku (T.K.L.) and the Swedish Research Council (C.K.).
Authors' contributions
C.K. conceived the experiments with T.K.L. providing input on the design. T.K.L. performed the experiments and analysed the data. T.K.L. wrote the manuscript with C.K. providing edits and advice.
Competing interests
We have no competing interests.
References
- 1.Candolin U. 2003. The use of multiple cues in mate choice. Biol. Rev. 78, 575–595. ( 10.1017/S1464793103006158) [DOI] [PubMed] [Google Scholar]
- 2.DeBose JL, Nevitt GA. 2008. The use of odors at different spatial scales: comparing birds with fish. J. Chem. Ecol. 34, 867–881. ( 10.1007/s10886-008-9493-4) [DOI] [PubMed] [Google Scholar]
- 3.Wisenden BD. 2000. Olfactory assessment of predation risk in the aquatic environment. Phil. Trans. R. Soc. Lond. B 355, 1205–1208. ( 10.1098/rstb.2000.0668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathis A, Unger S. 2012. Learning to avoid dangerous habitat types by aquatic salamanders, Eurycea tynerensis. Ethology 118, 57–62. ( 10.1111/j.1439-0310.2011.01987.x) [DOI] [Google Scholar]
- 5.Smadja C, Butlin RK. 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102, 77–97. ( 10.1038/hdy.2008.55) [DOI] [PubMed] [Google Scholar]
- 6.Plenderleith M, van Oosterhout C, Robinson RL, Turner GF. 2005. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 1, 411–414. ( 10.1098/rsbl.2005.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson BG, Jones TM. 2007. The role of chemical communication in mate choice. Biol. Rev. 82, 265–289. ( 10.1111/j.1469-185X.2007.00009.x) [DOI] [PubMed] [Google Scholar]
- 8.Forsgren E. 1997. Mate sampling in a population of sand gobies. Anim. Behav. 53, 267–276. ( 10.1006/anbe.1996.0374) [DOI] [Google Scholar]
- 9.Lindström K, Lugli M. 2000. A quantitative analysis of the courtship acoustic behaviour and sound patterning in male sand goby, Pomatoschistus minutus. Environ. Biol. Fish. 58, 411–424. ( 10.1023/A:1007695526177) [DOI] [Google Scholar]
- 10.Lehtonen TK, Rintakoski S, Lindström K. 2007. Mate preference for multiple cues: interplay between male and nest size in the sand goby, Pomatoschistus minutus. Behav. Ecol. 18, 696–700. ( 10.1093/beheco/arm032) [DOI] [Google Scholar]
- 11.Lehtonen TK, Kvarnemo C. In press. Infections may select for filial cannibalism by impacting egg survival in interactions with water salinity and egg density. Oecologia. ( 10.1007/s00442-015-3246-1) [DOI] [PubMed] [Google Scholar]
- 12.van West P. 2006. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challenges for an old problem. Mycologist 20, 99–104. ( 10.1016/j.mycol.2006.06.004) [DOI] [Google Scholar]
- 13.Lehtonen TK, Lindström K. 2008. Repeatability of mating preferences in the sand goby. Anim. Behav. 75, 55–61. ( 10.1016/j.anbehav.2007.04.011) [DOI] [Google Scholar]
- 14.Lehtonen TK, Wong BBM, Lindström K. 2010. Fluctuating mate preferences in a marine fish. Biol. Lett. 6, 21–23. ( 10.1098/rsbl.2009.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amorim MCP, Pedroso SS, Bolgan M, Jordão JM, Caiano M, Fonseca PJ. 2013. Painted gobies sing their quality out loud: acoustic rather than visual signals advertise male quality and contribute to mating success. Funct. Ecol. 27, 289–298. ( 10.1111/1365-2435.12032) [DOI] [Google Scholar]
- 16.Lehtonen TK. 2012. Signal value of male courtship effort in a fish with paternal care. Anim. Behav. 83, 1153–1161. ( 10.1016/j.anbehav.2012.01.040) [DOI] [Google Scholar]
- 17.Lindström K, Kangas N. 1996. Egg presence, egg loss, and female mate preferences in the sand goby (Pomatoschistus minutus). Behav. Ecol. 7, 213–217. ( 10.1093/beheco/7.2.213) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided as the electronic supplementary material.


