Abstract
Birds' eggshells are renowned for their striking colours and varied patterns. Although often considered exceptionally diverse, we report that avian eggshell coloration, sampled here across the full phylogenetic diversity of birds, occupies only 0.08–0.10% of the avian perceivable colour space. The concentrations of the two known tetrapyrrole eggshell pigments (protoporphyrin and biliverdin) are generally poor predictors of colour, both intra- and interspecifically. Here, we show that the constrained diversity of eggshell coloration can be accurately predicted by colour mixing models based on the relative contribution of both pigments and we demonstrate that the models' predictions can be improved by accounting for the reflectance of the eggshell's calcium carbonate matrix. The establishment of these proximate links between pigmentation and colour will enable future tests of hypotheses on the functions of perceived avian eggshell colours that depend on eggshell chemistry. More generally, colour mixing models are not limited to avian eggshell colours but apply to any natural colour. Our approach illustrates how modelling can aid the understanding of constraints on phenotypic diversity.
Keywords: biliverdin, eggshell colour, protoporphyrin, subtractive colour mixing
1. Introduction
Birds' eggshells display a variety of colours and striking patterns that have captured the attention of philosophers, artists and scientists since the time of Aristotle [1]. The diversity of colour is generally attributed to biliverdin IXα, appearing blue–green, and protoporphyrin IX, appearing rusty-brown [2]. There is strong evidence that eggshell colours and their physical–chemical bases are adaptive in many contexts [3].
Contrary to dietary sources of avian coloration (e.g. carotenoids, as found in birds' feathers), biliverdin and protoporphyrin are synthesized pigments [4,5]. One limitation to understanding the function of eggshell coloration is the unresolved relationship between pigment concentrations and their perceived colours. While some studies have found correlations between pigment concentrations and eggshell coloration within species [6,7], others have not found these patterns within [8] or among species for either ground coloration [2] or maculation patterns [9]. However, such a quantitative link between variation in eggshell pigmentation and avian-perceived variation in eggshell colour is fundamental for testing evolutionary and functional hypotheses.
Here, we integrate empirical and model-based approaches to examine avian-perceived eggshell colours. We generate predicted colours using two subtractive colour mixing models that each combined different components of eggshell colour [10]. First, we mixed the colours of a purely biliverdin-pigmented eggshell and a purely protoporphyrin-pigmented eggshell (hereafter ‘simple model’). Second, we then additionally mixed the colour of an unpigmented eggshell, representing a pure calcium carbonate eggshell matrix (hereafter ‘general model’). Using eggs representing the full phylogenetic diversity of birds (electronic supplementary material, figure S1), we tested whether these ‘model-predicted’ eggshell colours encompassed the entire avian eggshell colour gamut (i.e. the complete range of avian-perceivable eggshell colours).
2. Material and methods
(a). Colour analyses
We used the average reflectance spectra of avian eggshells stored in natural history museums (figure 1a) from 636 species (electronic supplementary material, figure S1) originally collected by Hanley et al. [11] (for further details, see electronic supplementary material). We calculated avian-perceived variation in colour using receptor-noise-limited models [13] accounting for the visual sensitivity of the average ultraviolet-sensitive (UVS) or violet-sensitive (VS) avian receivers [14], the double cone sensitivity of the blue tit, Cyanistes caeruleus, and domestic chicken, Gallus gallus, respectively, and irradiance spectra (scaled by 10 000) representing bright illumination under direct daylight and filtered forest light viewing conditions. These calculations generated values that represented the relative stimulation of birds' four single cones and double cones (electronic supplementary material, table S1). We converted these values into spatial coordinates within the UVS and VS avian tetrahedral colour spaces (hereafter ‘natural eggshell colours’). The avian tetrahedral colour space removes achromatic information; however, chromatic and achromatic variation is thought to be perceived via separate mechanisms in birds [12]. Colour analyses were conducted using the ‘pavo’ software package [15].
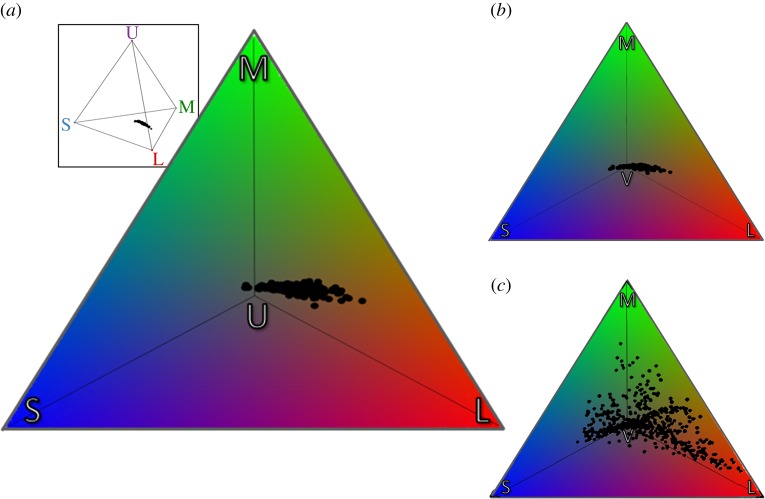
Figure 1.
The distribution of (a) birds' eggshell colours (this study) within the ultraviolet-sensitive (UVS) avian tetrahedral colour space (inset) when viewed under daylight conditions. We compared the perceptual spaces occupied by (b) avian eggshell colours with (c) avian feather colours (sourced and adapted from [12]) in the violet-sensitive (VS) avian colour space as they were originally presented using ‘a standard constant illumination across all visible wavelengths' sensu [12]. The plots illustrate the stimulation of the short (S), medium (M), long (L), and either (a) ultraviolet (U) or (b,c) violet (V) wavelength-sensitive photoreceptors. All plots are shown from above the U or V vertex of the tetrahedral colour space. (Online version in colour.)
(b). Comparing pigment mixing model outputs with the range of natural eggshell colours
Based on the spectra for two eggshells, each containing only a single pigment, 100 intermediate reflectance spectra were generated. These intermediate spectra were derived using a Yule–Nielsen subtractive colour mixing model [10] as follows:
| 2.1 |
where Nc represents the number of colorants, R represents the reflectance at each wavelength (λ) and c represents the relative concentration such that the sum of all relative concentrations equals 1. Here, the American robin (Turdus migratorius: electronic supplementary material, table S2) was used as a purely biliverdin-based eggshell [2] and the peregrine falcon (Falco peregrinus: electronic supplementary material, table S2) as a purely protoporphyrin-based eggshell [2].
Next, we also incorporated the spectral characteristics of the calcium carbonate eggshell matrix into the subtractive model (the ‘general model’: figure 2c), by including the reflectance of an immaculate white Northern fulmar (Fulmarus glacialis: electronic supplementary material, table S2) eggshell, representing an unpigmented eggshell [2]. We again generated 100 intermediate reflectance spectra (figure 2c). The predictive ability of each model was examined with three approaches: we compared the overlap between the actual and model-generated colour spaces, we determined how close the model-generated colours were to the line natural eggshell colours formed through three-dimensional visual space (hereafter ‘absolute residual’), and we calculated how dispersed the x-coordinates of the model-generated colours were relative to the full range of the avian eggshell colour gamut (for further details, see electronic supplementary material). Using different species to represent purely pigmented or unpigmented eggshells did not change our conclusions (electronic supplementary material).
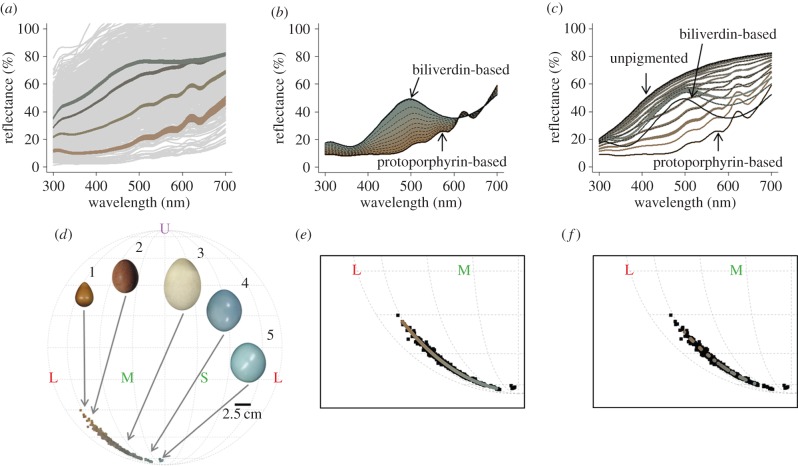
Figure 2.
The reflectance spectra of (a) all avian eggshells from [11] (grey), summarized by four k-means clusters (means ± s.e.; plotted in their actual colours), (b) the simple model's output and (c) the general model's output with reflectance spectra of pure/no pigments (solid black lines), every 10th spectrum (dashed lines), and all intermediate spectra (full colour shading). We illustrate a Mollweide projection of the hue distribution of (d) natural eggshell colour in UVS avian colour space, plotted in the actual colours that maintained their relative brightness, with five representative eggs: (1) Hydrophasianus chirurgus (FMNH 15312), (2) Falco peregrinus (UMMZ 231817), (3) Fulmarus glacialis (FMNH 4913), (4) Tinamus major (UMMZ 191600) and (5) Tinamus osgoodi (FMNH 2856). The letters represent the ultraviolet (U), short (S), medium (M) and long (L) wavelength-sensitive photoreceptors. We depict the (e) simple and (f) general model outputs' hue distributions above natural eggshell colours (black). (Online version in colour.)
3. Results
Avian eggshell colours occupied very little (less than 1%) of the UVS avian-perceivable colour space: 0.09% in daylight (figure 1a), and 0.08% in forest light conditions. Similarly, eggshell colours occupied only 0.10% (figure 1b) of the VS avian-perceivable colour space in daylight conditions, and 0.08% of the colour space in forest light conditions.
Both the simple and general models generated colours that fell completely (100%) within the natural eggshell colour gamut. However, the simple model output did not match natural eggshell colours as accurately as randomly sampled natural eggshell colours matched themselves (hereafter ‘null model’; t = 21.26, d.f. = 150.53, p < 0.0001; electronic supplementary material, figure S2). By contrast, the general model output matched natural egg colours better than randomly selected natural egg colours matched themselves (t = −16.36, d.f. = 197.07, p < 0.0001; electronic supplementary material, figure S2), which was a substantial improvement over the output of the simple model (t = −30.11, d.f. = 136.50, p < 0.0001). All colours from the simple, general and null models had significantly smaller (all p < 0.0001) absolute residuals than points randomly drawn from the UVS avian colour space (electronic supplementary material, figure S2).
The dispersion of the x-coordinates of the colours generated by the simple model represented 76% of the dispersion of natural eggshell colours (figure 2e). The general model produced colours that were 54% as dispersed as natural eggshell colours (figure 2f).
4. Discussion
Given the continued and widespread scientific and aesthetic interest in colourful avian eggshells, and the traditional awe over their diversity, the avian eggshell colour gamut is surprisingly small. In fact, to a bird's eyes, their eggs are 200- to 400-times less diverse in colour than their feathers (this study versus [12]; figure 1b,c). Additionally, we document that variation in avian eggshell colour is directly associated with the relative contribution of biliverdin and protoporphyrin, particularly when accounting for their integration within a calcium carbonate matrix of the eggshell.
Both sets of model-generated colours were within the avian eggshell colour gamut and varied along the same axis of variation as real eggshells. We found that the simple model-generated colours more thoroughly covered the entire range of natural eggshell colours (i.e. dispersion: figure 2e,f), but the general model-generated colours more accurately matched the spectral reflectance of natural eggshell colours (figure 2c; electronic supplementary material, figure S2). Nonetheless, these models cannot yet predict the limits of eggshell colour diversity because the colours of some natural eggshells, with unknown pigment concentrations, fall outside the model-predicted ranges (figure 2e,f). Currently, our models also assume an even mixing of the pigments throughout the eggshell, but in some species pigment concentrations vary across the eggshell layers [16]; therefore, further analyses are required for such species. Future research explicitly interested in eggshell appearance should consider ground coloration (as we did), luminance and eggshell patterning.
Just as with birds' feathers [12], avian eggshell colours should be limited within the proximate limits set by colour production mechanisms and the ultimate limits set by selective pressures. Variation in the colours of birds' feathers is mostly attributable to structural colour, with pigments contributing little to the colour diversity (approx. 7% of the total 26% of the VS colour space occupied by feather colours) [12]; in feathers, individual pigment classes occupy very little of avian perceptual colour space indeed, from 0.1% for porphyrins to 3.5% for carotenoids [12]. Just as with tetrapyrrole feather pigments (turacin and turacoverdin) [12], our models predict that tetrapyrrole eggshell pigments (protoporphyrin and biliverdin) occupy very little of avian colour space (approx. 0.10%).
Our evidence supports chemical analyses [17] that found just two pigments responsible for birds' eggshell colours and implies that structural or other factors are only minor contributors to avian eggshell coloration [18,19]. The constraint in perceivable chromatic variation may suggest the relative importance of the achromatic component of eggshell colour or suggest alternative non-visual functions for eggshell pigments [3]. These colour mixing models can be applied to any natural colour, and, more generally, they demonstrate a novel approach to understanding trait diversity. This study enables future exploration of the expression and constraint of avian eggshell coloration by establishing a direct link between pigmentation and avian-perceived eggshell colours.
Supplementary Material
Acknowledgements
We thank J. L. Cuthbert for editorial assistance and MetaCentrum reg. no. CZ.1.05/3.2.00/08.0144 for computational resources. We also thank Oxford University Press for permission to adapt Fig. 4c from reference [12] for figure 1c in this article.
Ethics
No live animals were studied.
Data Accessibility
Reflectances: http://dx.doi.org/10.5061/dryad.2q3r2.
Authors' Contribution
D.H. and M.E.H. conceived the study; D.H. and P.C. collected the data; D.H., M.E.H. and T.G. planned the analyses; D.H. generated the models and ran the analyses; and D.H., P.C., T.G. and M.E.H. wrote the manuscript. All authors approved publication.
Competing Interests
We declare we have no competing interests.
Funding
We thank the European Social Fund, and the state budget of the Czech Republic, project no. CZ.1.07/2.3.00/30.0041 (T.G. and D.H.), and the Human Frontier Science Program (T.G., P.C. and M.E.H.).
References
- 1.Aristotle. 350 BC The history of animals. Boston, MA: Internet Classics Archive, MIT. [Google Scholar]
- 2.Cassey P, Thomas GH, Portugal SJ, Maurer G, Hauber ME, Grim T, Lovell PG, Mikšík I. 2012. Why are birds’ eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biol. J. Linn. Soc. 106, 657–672. ( 10.1111/j.1095-8312.2012.01877.x) [DOI] [Google Scholar]
- 3.Cassey P, Maurer G, Lovell PG, Hanley D. 2011. Conspicuous eggs and colourful hypotheses: testing the role of multiple influences on avian eggshell appearance. Avian Biol. Res. 4, 185–195. ( 10.3184/175815511X13207699868421) [DOI] [Google Scholar]
- 4.Baird T, Solomon SE, Tedstone DR. 1975. Localisation and characterisation of egg shell porphyrins in several avian species. Br. Poult. Sci. 16, 201–208. ( 10.1080/00071667508416177) [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xu GY, Liu ZZ, Li JY, Yang N. 2006. A study on eggshell pigmentation: biliverdin in blue-shelled chickens. Poult. Sci. 85, 546–549. ( 10.1093/ps/85.3.546) [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Rull I, Miksik I, Gil D. 2008. Egg pigmentation reflects female and egg quality in the spotless starling Sturnus unicolor. Behav. Ecol. Sociobiol. 62, 1877–1884. ( 10.1007/s00265-008-0617-1) [DOI] [Google Scholar]
- 7.Moreno J, Lobato E, Morales J, Merino S, Tomas G, Martinez-de la Puente J, Sanz JJ, Mateo R, Soler JJ. 2006. Experimental evidence that egg color indicates female condition at laying in a songbird. Behav. Ecol. 17, 651–655. ( 10.1093/beheco/ark014) [DOI] [Google Scholar]
- 8.Cassey P, et al. 2012. Avian eggshell pigments are not consistently correlated with colour measurements or egg constituents in two Turdus thrushes. J. Avian Biol. 43, 503–512. ( 10.1111/j.1600-048X.2012.05576.x) [DOI] [Google Scholar]
- 9.Brulez K, Cassey P, Meeson A, Mikšík I, Webber SL, Gosler AG, Reynolds SJ. 2014. Eggshell spot scoring methods cannot be used as a reliable proxy to determine pigment quantity. J. Avian Biol. 45, 94–102. ( 10.1111/j.1600-048X.2013.00236.x) [DOI] [Google Scholar]
- 10.Simonot L, Hébert M. 2014. Between additive and subtractive color mixings: intermediate mixing models. J. Opt. Soc. Am. A 31, 58–66. ( 10.1364/JOSAA.31.000058) [DOI] [PubMed] [Google Scholar]
- 11.Hanley D, Cassey P, Doucet SM. 2013. Parents, predators, parasites, and the evolution of eggshell colour in open nesting birds. Evol. Ecol. 27, 593–617. ( 10.1007/s10682-012-9619-6) [DOI] [Google Scholar]
- 12.Stoddard M, Prum R. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052. ( 10.1093/beheco/arr088) [DOI] [Google Scholar]
- 13.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431. ( 10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 15.Maia R, Eliason CM, Bitton P, Doucet SM, Shawkey MD. 2013. pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol. Evol. 4, 906–913. ( 10.1111/2041-210X.12069) [DOI] [Google Scholar]
- 16.Liu HC, Hsiao MC, Hu YH, Lee SR, Cheng WTK. 2010. Eggshell pigmentation study in blue-shelled and white-shelled ducks. Asian Australas. J. Anim. Sci. 23, 162–168. ( 10.5713/ajas.2010.90256) [DOI] [Google Scholar]
- 17.Gorchein A, Lim CK, Cassey P. 2009. Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 23, 602–606. ( 10.1002/bmc.1158) [DOI] [PubMed] [Google Scholar]
- 18.Igic B, et al. 2015. A nanostructural basis for gloss of avian eggshells. J. R. Soc. Interface 12, 20141210 ( 10.1098/rsif.2014.1210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fecheyr-Lippens DC, Igic B, D'Alba L, Hanley D, Verdes A, Holford M, Waterhouse GIN, Grim T, Hauber ME, Shawkey MD. 2015. The cuticle modulates ultraviolet reflectance of avian eggshells. Biology Open ( 10.1242/bio.012211) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reflectances: http://dx.doi.org/10.5061/dryad.2q3r2.