Abstract
Arthropod sex ratios can be manipulated by a diverse range of selfish genetic elements, including maternally inherited Wolbachia bacteria. Feminization by Wolbachia is rare but has been described for Eurema mandarina butterflies. In this species, some phenotypic and functional females, thought to be ZZ genetic males, are infected with a feminizing Wolbachia strain, wFem. Meanwhile, heterogametic WZ females are not infected with wFem. Here, we establish a quantitative PCR assay allowing reliable sexing in three Eurema species. Against expectation, all E. mandarina females, including wFem females, had only one Z chromosome that was paternally inherited. Observation of somatic interphase nuclei confirmed that W chromatin was absent in wFem females, but present in females without wFem. We conclude that the sex bias in wFem lines is due to meiotic drive (MD) that excludes the maternal Z and thus prevents formation of ZZ males. Furthermore, wFem lines may have lost the W chromosome or harbour a dysfunctional version, yet rely on wFem for female development; removal of wFem results in all-male offspring. This is the first study that demonstrates an interaction between MD and Wolbachia feminization, and it highlights endosymbionts as potentially confounding factors in MD of sex chromosomes.
Keywords: meiotic drive, Wolbachia, W chromatin body, gene dosage, sex chromosome, sex determination
1. Introduction
Selfish genetic elements can highjack sex determination systems and distort sex ratios in order to enhance their own transmission in host populations. Examples are meiotic drive (MD) genes of sex chromosomes [1] and endosymbiotic microorganisms [2] such as Wolbachia, a maternally inherited bacterium of arthropods that can induce cytoplasmic incompatibility (CI), thelytokous parthenogenesis, male-killing (MK) and feminization. Feminization is least common and results in female development of individuals with an assumed male chromosome composition [3].
Wolbachia-induced feminization has been reported for several terrestrial crustaceans and is best described for the isopod Armadillidium vulgare with presumed heterogametic females (WZ). In this species, Wolbachia causes individuals to develop into functional females via manipulation of the androgenic gland [4]. Consequently, the frequency of the W chromosome in infected populations is expected to decline until its eventual elimination, such that female sex is controlled by the presence of Wolbachia rather than W, while males may develop when Wolbachia transmission is leaky [4].
Wolbachia-induced feminization has also been recorded for three insect species, including two Eurema butterfly species [5–7]. Eurema mandarina butterfly populations are nearly fixed for wCI infections. In some populations, females are co-infected with wFem, a strain thought to cause feminization [6,8]. For example, on Tanegashima Island in Japan, most females harbour both strains and produce only daughters with similar offspring numbers to the mixed sex broods produced by wCI females [8,9].
Lepidoptera are diplodiploid insects with female heterogamety—females are WZ or 0Z, males are ZZ. As in most Lepidoptera [10], the W chromosome of uninfected and wCI E. mandarina females forms a heterochromatic body during interphase of somatic cells [6]. However, in wFem females this W chromatin is missing, which has led to the assumption that they have a male ZZ chromosome composition [6,9]. Both MK and MD have previously been excluded as mechanisms for the sex ratio bias, because antibiotic treatment of wFem females did not change offspring numbers and did not restore even sex ratios, but yielded all-male broods [6]. Further antibiotic experiments provided evidence that wFem has a continuous feminizing action on individuals during larval development [11]. Here, we scrutinized the genetic basis of the sex ratio bias in E. mandarina, and directly tested the hypothesized ZZ composition of wFem females. We also compared the inheritance of Z in all-female and mixed-sex families to probe them for any segregation distortions.
2. Material and methods
(a). Sampling and Wolbachia screening
We tested 57 E. mandarina from Tanegashima produced by five and three field-collected mothers that produced all-female and mixed-sex broods, respectively. This number also included six tetracycline-treated individuals from one mixed-sex family. Controls were six E. mandarina from Hachijō-jima Island, Japan, as well as 10 individuals each of Australian Eurema hecabe and Australian Eurema smilax. Wolbachia infections were confirmed and sequenced by using strain-specific PCR primers [9] (electronic supplementary material, S1).
(b). W chromatin body assays
After oviposition, the eight field-collected E. mandarina females were analysed for presence of the W chromatin body [10,12] using previously established methods for Eurema [10,12].
(c). Real-time quantitative PCR
The gene dose ratio (GDR) of Z-linked genes Tpi and kettin with the autosomal gene EF-1α was inferred by quantitative PCR (qPCR) [13]. We tested the offspring of the eight field-collected E. mandarina females, six control individuals from Hachijō-jima and 10 individuals each of E. hecabe and E. smilax (electronic supplementary material, S1).
(d). Tpi sequence analysis
Inheritance of the Z chromosome was revealed through Tpi sequence analysis of mothers and their offspring. Paternal alleles remained unknown as females were caught after mating.
3. Results
(a). Wolbachia infection status
Offspring of all-female families were infected with both wCI and wFem. By contrast, offspring of mixed-sex families were only infected with wCI. The six tetracycline-treated offspring individuals of a wCI female were uninfected. All wild-caught E. mandarina from Hachijō-jima and Australian E. hecabe were positive for wCI, and Australian E. smilax were uninfected (electronic supplementary material, S2).
(b). W chromatin body assays
The W chromatin body was detected in the three mothers of wCI-infected and wCI-cured individuals but not in the five mothers of wFem females (electronic supplementary material, S2). This confirmed previously published absence of W in wFem E. mandarina and wFem E. hecabe [7,8].
(c). Gene dose ratio of Z-linked genes in males and females
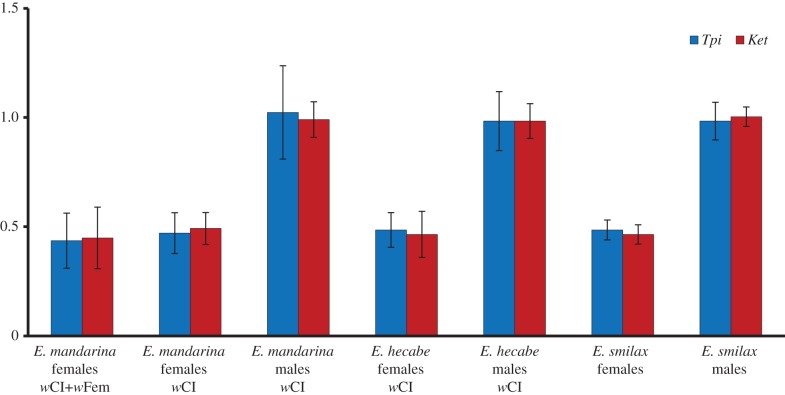
Our qPCR approach correctly determined sex in Eurema butterflies, independent of their infection status. Both genes had a GDR close to 1 for all males in all species (figure 1). Females of wCI E. hecabe, uninfected E. smilax, wCI and uninfected E. mandarina had a GDR close to 0.5. Contrary to expectation, GDR of wFem E. mandarina females was also 0.5.
Figure 1.
Gene dose ratio of Z-linked Tpi and kettin normalized to autosomal Ef-1α in Eurema individuals. Error bars represent s.e. (Online version in colour.)
(d). Inheritance of the Z chromosome
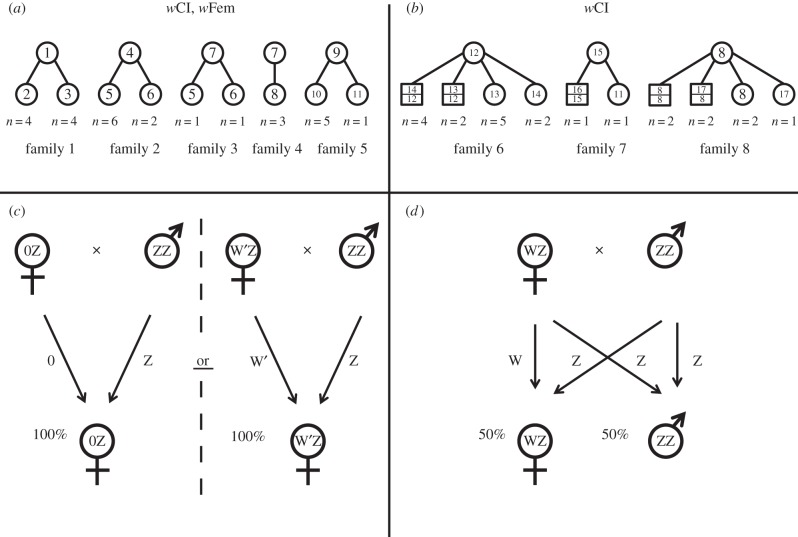
Sequence analysis of the Z-linked Tpi gene provided further evidence that all females had a single Z (figure 2a,b), while most males were heterozygous with two different alleles (figure 2b). In wFem families, the mother's allele was not observed in daughters (n = 27 over five families), implying MD against the maternal Z. In families without wFem, normal Mendelian segregation was seen, with maternal Z alleles appearing in sons and not in daughters (figure 2; electronic supplementary material, S3).
Figure 2.
Family pedigrees of wFem co-infected (a) and wCI-infected (b) Eurema mandarina. Each number represents a different allele; circles represent females with one Z, squares males with two Z alleles. wFem leads to either a loss of W or a modified W’ chromosome; wFem individuals carry the paternal Z (c). In wCI-infected lineages with equal sex ratios, sex chromosomes experience Mendelian inheritance (d).
4. Discussion
By using qPCR, we accurately identified sex in three Eurema species; the GDR of two Z-linked genes in males was twice that in females. This matched the detection of W chromatin in wCI-infected E. mandarina females but was not in line with the absence of W chromatin in wFem females. Thus, contrary to previous hypotheses, wFem females did not have male ZZ genotypes. We then investigated inheritance of the Z chromosome. Alleles of Z-linked Tpi in wFem females always differed from their maternal genotype, revealing paternal inheritance of Z, and more specifically, the exclusion of maternal Z from progeny by a yet unknown MD mechanism.
Based on our findings, we conclude that wFem lineages do not possess a W chromosome, or carry a modified W’ that is dysfunctional and cannot be visualized in W chromatin assays (figure 2c). A previous study detected W in just one wFem female [9]; perhaps wFem-infected lineages have a modified W’ that can only occasionally be visualized as W chromatin. Irrespective of whether W is lost or modified, wFem still compensates for it and triggers female development of individuals with a single Z chromosome. This is shown by previous experiments demonstrating that Wolbachia must be present in larvae for female development [11].
In addition, MD prevents inheritance of the maternal Z chromosome. MD can polarize the meiotic spindle, leading to a non-random segregation of sex chromosomes [14] where no sex chromosome or W’ may be preferentially inherited while Z may be pulled towards the polar body. It is not yet known whether Wolbachia is directly involved in MD of E. mandarina or whether MD and Wolbachia feminization are two independent mechanisms. The answer depends on the currently unknown Z chromosome composition of the all-male offspring of females cured of wFem; the re-establishment of Mendelian Z inheritance would provide evidence that Wolbachia causes the observed MD.
Here, we propose a new conceptual framework in which MD is responsible for the uniform sex chromosome composition within sex-biased lines. wFem does not feminize ZZ males but feminizes individuals with a single Z (0Z or W'Z). wFem compensates for the loss of the female differentiation pathway. Thus, the combined action of MD and feminization may have led to the evolution of 0Z female genotypes, analogous to the loss of the Y chromosome in male heterogametic systems that can result in the evolution of X0 systems [15].
The production of all-male broods after wFem curing could follow the mechanism seen in Bombyx mori, where embryos with only one Z chromosome become males when the sex determination signal of the W chromosome, a female-specific piRNA, is silenced [16]. Furthermore, in the moth Ostrinia scapulalis, a MK Wolbachia strain was found to carry a feminizing factor, while the moth's W chromosome was dysfunctional [17]. How Wolbachia induces femaleness in Z individuals remains hidden. One possibility is mimicry of the primary sex determination signal itself. Wolbachia has recently been reported to manipulate the host's piRNA machinery in Aedes aegypti [18].
While the capacity to induce MD has not yet been demonstrated for endosymbionts, possible interactions of endosymbionts with other selfish genetic elements have previously been discussed [19]. Our study is the first to suggest the combined action of different reproductive manipulations, MD and feminization. It highlights that reproductive manipulations in Eurema butterflies are more complex than previously anticipated, and this may apply to current models of Wolbachia feminization in general. In addition, our study raises the possibility that endosymbionts might cause MD in their hosts.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Jennifer Morrow and three reviewers for comments on earlier manuscript versions.
Data accessibility
Tpi and kettin sequences were submitted to GenBank (electronic supplementary material, S1).
Funding statement
This work was part of a PhD research project funded by the Hawkesbury Institute for the Environment.
Authors' contributions
P.K., M.R., D.K. and J.M.C. designed the study. P.K. and D.K. collected and analysed the data. P.K. and M.R. wrote the manuscript with input from D.K. and J.M.C. All authors agreed on the final version of the manuscript.
Conflict of interests
We have no conflict of interests.
References
- 1.Werren JH. 2011. Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc. Natl Acad. Sci. USA 108, 10 863–10 870. ( 10.1073/pnas.1102343108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kageyama D, Narita S, Watanabe M. 2012. Insect sex determination manipulated by their endosymbionts: incidences, mechanisms and implications. Insects 3, 161–199. ( 10.3390/insects3010161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 4.Cordaux R, Bouchon D, Grève P. 2011. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 27, 332–341. ( 10.1016/j.tig.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 5.Negri I, Pellecchia M, Mazzoglio PJ, Patetta A, Alma A. 2006. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc. R. Soc. B 273, 2409–2416. ( 10.1098/rspb.2006.3592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiroki M, Kato Y, Kamito T, Miura K. 2002. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 89, 167–170. ( 10.1007/s00114-002-0303-5) [DOI] [PubMed] [Google Scholar]
- 7.Narita S, Kageyama D, Hiroki M, Sanpei T, Hashimoto S, Kamitoh T, Kato Y. 2011. Wolbachia-induced feminisation newly found in Eurema hecabe, a sibling species of Eurema mandarina (Lepidoptera: Pieridae). Ecol. Entomol. 36, 309–317. ( 10.1111/j.1365-2311.2011.01274.x) [DOI] [Google Scholar]
- 8.Hiroki M, Tagami Y, Miura K, Kato Y. 2004. Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe. Proc. R. Soc. Lond. B 271, 1751–1755. ( 10.1098/rspb.2004.2769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narita S, Nomura M, Kageyama D. 2007. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol. Ecol. 61, 235–245. ( 10.1111/j.1574-6941.2007.00333.x) [DOI] [PubMed] [Google Scholar]
- 10.Traut W, Marec F. 1996. Sex chromatin in Lepidoptera. Q. Rev. Biol. 71, 239–256. ( 10.2307/3035648) [DOI] [PubMed] [Google Scholar]
- 11.Narita S, Kageyama D, Nomura M, Fukatsu T. 2007. Unexpected mechanism of symbiont-induced reversal of insect sex: feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl. Environ. Microbiol. 73, 4332–4341. ( 10.1128/aem.00145-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kageyama D, Traut W. 2004. Opposite sex–specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc. R. Soc. Lond. B 271, 251–258. ( 10.1098/rspb.2003.2604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen P, Sýkorová M, Šíchová J, Kůta V, Dalíková M, Čapková Frydrychová R, Neven LG, Sahara K, Marec F. 2013. Neo-sex chromosomes and adaptive potential in tortricid pests. Proc. Natl Acad. Sci. USA 110, 6931–6936. ( 10.1073/pnas.1220372110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardo-Manuel de Villena F, Sapienza C. 2001. Nonrandom segregation during meiosis: the unfairness of females. Mamm. Genome 12, 331–339. ( 10.1007/s003350040003) [DOI] [PubMed] [Google Scholar]
- 15.Charlesworth B. 1996. The evolution of chromosomal sex determination and dosage compensation. Curr. Biol. 6, 149–162. ( 10.1016/S0960-9822(02)00448-7) [DOI] [PubMed] [Google Scholar]
- 16.Kiuchi T, et al. 2014. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 509, 633–636. ( 10.1038/nature13315) [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto TN, Ishikawa Y. 2012. A male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sex-determining system of its host. Biol. Lett. 8, 412–415. ( 10.1098/rsbl.2011.1114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayoral JG, Etebari K, Hussain M, Khromykh AA, Asgari S. 2014. Wolbachia infection modifies the profile, shuttling and structure of microRNAs in a mosquito cell line. PLoS ONE 9, e96107 ( 10.1371/journal.pone.0096107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riegler M, Sidhu M, Miller WJ, O'Neill SL. 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr. Biol. 15, 1428–1433. ( 10.1016/j.cub.2005.06.069) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Tpi and kettin sequences were submitted to GenBank (electronic supplementary material, S1).