Abstract
In many host populations, one of the most striking differences among hosts is their age. While parasite prevalence differences in relation to host age are well known, little is known on how host age impacts ecological and evolutionary dynamics of diseases. Using two clones of the water flea Daphnia magna and two clones of its bacterial parasite Pasteuria ramosa, we examined how host age at exposure influences within-host parasite competition and virulence. We found that multiply-exposed hosts were more susceptible to infection and suffered higher mortality than singly-exposed hosts. Hosts oldest at exposure were least often infected and vice versa. Furthermore, we found that in young multiply-exposed hosts competition was weak, allowing coexistence and transmission of both parasite clones, whereas in older multiply-exposed hosts competitive exclusion was observed. Thus, age-dependent parasite exposure and host demography (age structure) could together play an important role in mediating parasite evolution. At the individual level, our results demonstrate a previously unnoticed interaction of the host's immune system with host age, suggesting that the specificity of immune function changes as hosts mature. Therefore, evolutionary models of parasite virulence might benefit from incorporating age-dependent epidemiological parameters.
Keywords: age-structured interactions, Daphnia–Pasteuria system, epidemiology, optimal virulence, stage-structured theory, trade-off hypothesis
1. Introduction
The SARS epidemic in 2003 and the H1N1 influenza pandemic in 2009 have highlighted the need for a joint epidemiological and evolutionary investigation of rapidly evolving virulent parasites [1]. Particularly in microparasites where ecological dynamics such as within-host competition can strongly influence evolutionary change, they must be studied conjointly to better understand disease evolution [2]. Understanding the interplay between the epidemiology and ecology of host and parasite populations, and identifying the conditions that lead to the coexistence of different strains of a given disease agent, are thus fundamental goals of infectious disease epidemiology and evolutionary ecology [3].
Here we examine how host age at exposure, a crucial epidemiological variable, influences the outcome of multiple infections by parasites, a naturally occurring and widespread ecological phenomenon [4]. This combined approach, which only recently received theoretical attention [5–7], has not been applied experimentally. Using a tractable host–parasite model system, the freshwater planktonic crustacean Daphnia magna and its obligate-killing bacterial parasite Pasteuria ramosa, we analyse the expression of parasite virulence and lifetime transmission potential as well as the competitive outcome of multiple infections in hosts of different age. Previous studies of this system found that younger hosts were more susceptible to infection [8,9], as well as became castrated faster and produced more parasite transmission stages than late-infected ones [9]. We therefore hypothesized that the immune system of younger hosts would be less equipped to deal with multiple infections in comparison with older hosts. The results of this study, which are based on a molecular analysis of the reproductive success of competing parasite genotypes within hosts, are in accordance with our hypothesis. Moreover, our results are aligned with theoretical predictions [5–7], and suggest that the age structure of the host population and the epidemiology (age at first exposure) of the parasite, both of which depend on ecological and demographic conditions, could influence parasite evolution and might even promote parasite strain coexistence and diversity.
2. Material and methods
The experiment presented here is part of a larger experiment on age-dependent effects of parasitism. The main part has been published before [9]. Here we focus on unpublished parts of this experiment, conducted under the same conditions and with the same material as the earlier part (see [9] for detailed methods). In short, we individually exposed 5-, 15- and 30-day-old females from each D. magna clone (HO2 and M10) for a week either to 20 000 parasite spores of one of the two P. ramosa clones C19 and C24 (single genotype infections, [10]), or to a mixed suspension of 10 000 spores from each P. ramosa clone (multiple infections). Pasteuria ramosa clone C19 originated from isolate P1, whereas P. ramosa clone C24 originated from isolate P4 [10]. In single infections, isolate P1 is more virulent than P4 but is less infective and produces fewer spores than P4 [11,12]. We recorded the release of host offspring and dead Daphnia on a daily basis. Infection is evident by brownish-red coloration about 12 days post-exposure. Dead Daphnia were frozen in 0.1 ml medium at −20°C for spore counting using a haemocytometer (Thoma ruling) under a phase contrast microscope (400×). The effects of host age at exposure on parasite infectivity, virulence and reproduction during single infections were analysed in [9]. Here we focus on single- versus multiple-infection treatments and examine the competitive outcome of multiple infections in the different age classes (see the electronic supplementary material for details of the genetic and statistical analyses).
3. Results
(a). Parasite infectivity and spore production (parasite transmission)
Younger hosts were more susceptible to multiple infections than older hosts, regardless of host clone (table 1 and figure 1a). Individuals in the multiple-infection treatments had a higher probability of becoming infected than those in the single-infection treatments (table 1). Total spore production in the multiple-infection treatments was highest in younger hosts regardless of host clone (ANOVA F2,218 = 38.0, p < 0.0001; figure 1b), and it was similar to that of single infections (ANOVA F1,219 = 0.03, p = 0.86).
Table 1.
Logistic regression analysis of the effects of host clone, infection treatment (single versus multiple infections) and host age at exposure on infection status. All interactions are non-significant. d.f. = degrees of freedom. Bold typeface indicates significant effects.
| independent variable | d.f. | χ2 | p-value |
|---|---|---|---|
| host clone | 1 | 1.0 | 0.315 |
| infection treatment | 1 | 9.2 | 0.002 |
| exposure age | 2 | 112.6 | <0.001 |
Figure 1.
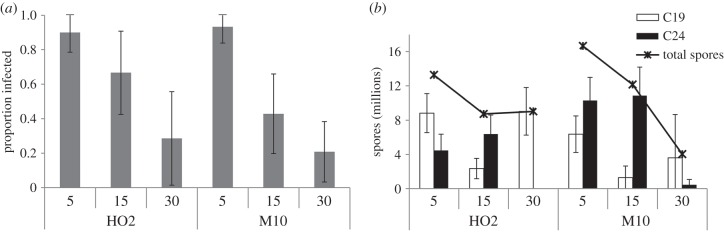
(a) Proportion (±Wilson 95% CI) of D. magna multiply-exposed to two P. ramosa clones for every combination of host clone and host age at exposure (in days). (b) Mean (±s.e.) parasite spore production among the multiple-infection treatments for every combination of host clone and host age at exposure.
(b). Parasite-induced host mortality (virulence) and parasitic castration
Individuals in the multiple-infection treatments suffered higher mortality than those in single-infection treatments (table 2). Younger hosts became castrated faster than older hosts, though time from exposure to castration did not differ between multiple- and single-infection treatments (table 2). Host offspring production was lower in hosts treated with multiple infections than in hosts treated with single infections (electronic supplementary material, figure S1; ANOVA F1,219 = 12.44, p = 0.0005), but it was also affected by host clone (ANOVA F1,219 = 11.8, p = 0.0007) and host age at exposure (ANOVA F2,218 = 179.0, p < 0.0001).
Table 2.
Cox regression analysis for the effects of host clone, infection treatment (single versus multiple infections) and host age at exposure on time-from-exposure-to-castration and time-from-exposure-to-host-death (virulence). All interactions are non-significant. d.f. = degrees of freedom. Bold typeface indicates significant effects.
| time from exposure to castration |
time from exposure to host death (virulence) |
||||
|---|---|---|---|---|---|
| independent variable | d.f. | χ2 | p-value | χ2 | p-value |
| host clone | 1 | 0.4 | 0.525 | 9.2 | 0.002 |
| infection treatment | 1 | 2.3 | 0.127 | 7.0 | 0.008 |
| exposure age | 2 | 26.0 | <0.001 | 0.4 | 0.829 |
(c). Within-host parasite competition
The relative success of each parasite clone in producing spores differed among host-age-at-exposure treatments, but the pattern was similar in both host clones. When infecting 5-day-old Daphnia, both parasite clones produced large numbers of spores; when infecting 15-day-old Daphnia, parasite clone C24 produced considerably more spores than parasite clone C19; but when infecting 30-day-old Daphnia, parasite clone C19 almost completely excluded parasite clone C24 (figures 1b and 2). In other words, younger hosts allowed coexistence of both parasite clones (co-infection), while older hosts promoted competitive exclusion (superinfection).
Figure 2.

Proportion of D. magna co-infected by both P. ramosa clones for every combination of host clone and host age at exposure (in days).
4. Discussion
We examined how the age at exposure of multiply-exposed hosts influences within-host dynamics. We found that multiply-exposed hosts were more susceptible to infection and suffered higher mortality than singly-exposed hosts. More importantly, young multiply-exposed hosts facilitated transmission of both parasite clones (co-infection), whereas older multiply-exposed hosts promoted competitive exclusion (superinfection).
There is considerable evidence that host heterogeneity, particularly in the form of host species richness or genetic diversity within a single host species, is closely linked to parasite diversity [13,14]. Our study suggests that host age at exposure is another form of heterogeneity that can affect parasite evolution and diversity. Accurate forecasting of parasite diversity will likely require intimate knowledge of the heterogeneities to disease effects present in host populations [15]. The influence of host age at exposure on parasite strain coexistence has only recently been modelled [5–7], being driven by field studies that examined age-dependent strain diversity of helminth parasites [16], and by surveys that investigated age-dependent changes in the frequencies of Streptococcus pneumoniae and Escherichia coli strains infecting humans [17,18]. Our study is, we believe, the first experimental demonstration of the link between host age at exposure and parasite strain coexistence.
Mechanistic factors such as moulting frequency, increased host size or higher food intake cannot explain the observed differences among age groups [9]. Although younger animals generally have a weaker immune system than older ones, we can only speculate on the underlying mechanisms that lead to strong changes in parasite success across ages of infection. For example, younger hosts might only be able to mount a weak immune response to infection, which is likely to permit coexistence via resource partitioning, while eliciting a strong immune response by older hosts would result in exclusion [6]. Alternatively, if host age classes exhibit variation in resource supply and immune responses, then Pasteuria clones co-infecting younger hosts may be subject to exploitation competition, whereas Pasteuria clones co-infecting older hosts may be subject to apparent (immune-mediated) competition [4,19]. Our finding that multiply-exposed hosts were more susceptible to infection than singly-exposed hosts further suggests that the parasite clones may facilitate each other. Since the hosts were naive, we can rule out the possibility of immune priming. Differences in the type of competition that governs within-host dynamics could, in principle, lead to differences in the competitive outcome. Either way, this does not explain why Pasteuria clone C24 dominated the competition in 15-day-old hosts, while clone C19 dominated the competition in 30-day-old hosts (figure 1b). Nevertheless, the changes in the relative abundances of the parasite clones strongly indicate that the immune response of the host interacts in a specific way with each parasite clone (as opposed to treating them equally). This increased discriminating power of the immune system seems to go hand in hand with its maturation.
Our study shows that the age at infection can crucially influence the success of different parasite strains. Moving from the individual to the population level, this implies that different age structures of the host population can result in different trajectories for parasite evolution. Age-structured interactions within a host population, coupled with consumer–resource interactions such as predator–prey or host–parasite, can lead to population cycles [20]. In Daphnia–algal systems, these cycles arise owing to competitive interactions between juveniles and adults, and their amplitude is determined by the ratio of the cycle period to juvenile stage duration [21]. Our results indicate that these competitive interactions, through their influence on the host age structure, may feedback on patterns of strain coexistence/exclusion and parasite diversity. As a result, host resource and disease dynamics could be shaped quite strongly.
Our results also have implications for our understanding of virulence evolution. Epidemiological models that study virulence evolution in response to multiple infections typically assume that only one of the co-infecting parasite strains can be transmitted at the same time (i.e. superinfection scenario), which is often at odds with the observed biology in nature [22]. Even when co-infections are allowed, which brings biological realism at the cost of more complex and less tractable models, they usually preclude competitive exclusion by one of the parasite strains [22]. Our results show that both scenarios are possible within the same system. The relationship between virulence and parasite transmission is believed to be a primary driver of the evolution of virulence, and it can be used to predict the optimal level of virulence [23]. Izhar & Ben-Ami [9] showed that this relationship is age-specific, because younger hosts produced more transmission stages than older ones even though parasite-induced host mortality (virulence) did not vary with host age. Our study further shows that the genetic composition of parasite strains in young multiply-exposed hosts is different from that in older hosts. Such variation in parasite transmission, while maintaining similar rates of parasite-induced host mortality (virulence) across host age, could result in different levels of optimal virulence for each host age. Similarly, while epidemiological models of disease dynamics have long recognized the importance of incorporating host age structure into standard susceptible–infected–recovered compartment models [24], models of virulence evolution do not take the dependence of virulence and parasite transmission with regard to host age at exposure into account. Furthermore, varying host susceptibility and its ability to mount an effective immune response in different age classes may also influence the classic virulence–transmission trade-off.
In summary, our results emphasize that the outcome of within-host competition is influenced by the complex interplay between host age at infection and the changing specificity of the host's immune system. This finding suggests that the host age structure may play a previously undetected role in the evolution of host–parasite interactions. Elucidating the underlying dynamics will improve our understanding of disease ecology and virulence evolution.
Supplementary Material
Acknowledgements
We thank R. Honeycutt and two anonymous reviewers for valuable comments on the manuscript.
Data Accessibility
Data used in this manuscript will be archived on Dryad. We will provide an Excel file with the host clone, parasite clone, infection treatment (single versus multiple infections), exposure age, infection status, host offspring counts, time from exposure to castration, time from exposure to host death and parasite spore production (per parasite clone) for each Daphnia that was used in the analyses.
Authors' Contributions
R.I. participated in the design of the study, carried out the infection assays, performed data and statistical analyses, and drafted the manuscript; J.R. carried out the molecular laboratory work, and read and critically approved the manuscript; F.B.A. conceived the study, participated in the design of the study, coordinated the study, and helped draft and critically revised the manuscript. All authors gave final approval for publication.
Competing Interests
We have no competing interests.
Funding
We received no funding for this study.
References
- 1.Pybus OG, Fraser C, Rambaut A. 2013. Evolutionary epidemiology: preparing for an age of genomic plenty. Phil. Trans. R. Soc. B 368, 20120193 ( 10.1098/rstb.2012.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvani AP. 2003. Epidemiology meets evolutionary ecology. Trends Ecol. Evol. 18, 132–139. ( 10.1016/S0169-5347(02)00050-2) [DOI] [Google Scholar]
- 3.Holt RD, Dobson AP. 2006. Extending the principles of community ecology to address the epidemiology of host-pathogen systems. In Disease ecology: community structure and pathogen dynamics (eds Collinge SK, Ray C.), pp. 6–27. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Read AF, Taylor LH. 2001. The ecology of genetically diverse infections. Science 292, 1099–1102. ( 10.1126/science.1059410) [DOI] [PubMed] [Google Scholar]
- 5.Li X-Z, Liu J-X, Martcheva M. 2010. An age-structured two-strain epidemic model with super-infection. Math. Biosci. Eng. 7, 123–147. ( 10.3934/mbe.2010.7.123) [DOI] [PubMed] [Google Scholar]
- 6.Martcheva M, Pilyugin SS, Holt RD. 2007. Subthreshold and superthreshold coexistence of pathogen variants: the impact of host age-structure. Math. Biosci. 207, 58–77. ( 10.1016/j.mbs.2006.09.010) [DOI] [PubMed] [Google Scholar]
- 7.Qiu Z, Li X, Martcheva M. 2012. Multi-strain persistence induced by host age structure. J. Math. Anal. Appl. 391, 595–612. ( 10.1016/j.jmaa.2012.02.052) [DOI] [Google Scholar]
- 8.Garbutt JS, O'Donoghue AJP, McTaggart SJ, Wilson PJ, Little TJ. 2014. The development of pathogen resistance in Daphnia magna: implications for disease spread in age-structured populations. J. Exp. Biol. 217, 3929–3934. ( 10.1242/jeb.111260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izhar R, Ben-Ami F. 2015. Host age modulates parasite infectivity, virulence and reproduction. J. Anim. Ecol. 84 ( 10.1111/1365-2656.12352) [DOI] [PubMed] [Google Scholar]
- 10.Luijckx P, Ben-Ami F, Mouton L, Du Pasquier L, Ebert D. 2011. Cloning of the unculturable parasite Pasteuria ramosa and its Daphnia host reveals extreme genotype–genotype interactions. Ecol. Lett. 14, 125–131. ( 10.1111/j.1461-0248.2010.01561.x) [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ami F, Mouton L, Ebert D. 2008. The effects of multiple infections on the expression and evolution of virulence in a Daphnia-endoparasite system. Evolution 62, 1700–1711. ( 10.1111/j.1558-5646.2008.00391.x) [DOI] [PubMed] [Google Scholar]
- 12.Ben-Ami F, Routtu J. 2013. The expression and evolution of virulence in multiple infections: the role of specificity, relative virulence and relative dose. BMC Evol. Biol. 13, 97 ( 10.1186/1471-2148-13-97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. 2013. Host and parasite diversity jointly control disease risk in complex communities. Proc. Natl Acad. Sci. USA 110, 16 916–16 921. ( 10.1073/pnas.1310557110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hechinger RF, Lafferty KD. 2005. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc. R. Soc. B 272, 1059–1066. ( 10.1098/rspb.2005.3070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PD, Day T. 2008. Epidemiological and evolutionary consequences of targeted vaccination. Mol. Ecol. 17, 485–499. ( 10.1111/j.1365-294X.2007.03418.x) [DOI] [PubMed] [Google Scholar]
- 16.Galvani AP. 2005. Age-dependent epidemiological patterns and strain diversity in helminth parasites. J. Parasitol. 91, 24–30. ( 10.1645/GE-191R1) [DOI] [PubMed] [Google Scholar]
- 17.Gordon DM, Stern SE, Collingnon PJ. 2004. Influence of age and sex of human hosts on the distribution of Escherichia coli ECOR groups and virulence traits. Microbiology 151, 15–23. ( 10.1099/mic.0.27425-0) [DOI] [PubMed] [Google Scholar]
- 18.Inostroza J, Vinet AM, Retamal G, Lorca P, Ossa G, Facklam RR, Sorensen RU. 2001. Influence of patient age on Streptococcus pneumoniae serotypes causing invasive disease. Clin. Diagn. Lab. Immunol. 8, 556–559. ( 10.1128/CDLI.8.3.556-559.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Cáceres CE. 2009. Resource ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am. Nat. 174, 149–162. ( 10.1086/600086) [DOI] [PubMed] [Google Scholar]
- 20.de Roos AM, Persson L. 2013. Population and community ecology of ontogenetic development. Princeton, NJ: Princeton University Press. [Google Scholar]
- 21.McCauley E, Nelson WA, Nisbet RM. 2008. Small-amplitude cycles emerge from stage-structured interactions in Daphnia–algal systems. Nature 455, 1240–1243. ( 10.1038/nature07220) [DOI] [PubMed] [Google Scholar]
- 22.Alizon S. 2013. Co-infection and super-infection models in evolutionary epidemiology. Interface Focus 3, 20130031 ( 10.1098/rsfs.2013.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alizon S, Hurford A, Mideo N, Van Baalen M. 2009. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245–259. ( 10.1111/j.1420-9101.2008.01658.x) [DOI] [PubMed] [Google Scholar]
- 24.Castillo-Chavez C, Hethcote HW, Andreasen V, Levin SA, Liu WM. 1989. Epidemiological models with age structure, proportionate mixing, and cross-immunity. J. Math. Biol. 27, 233–258. ( 10.1007/BF00275810) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this manuscript will be archived on Dryad. We will provide an Excel file with the host clone, parasite clone, infection treatment (single versus multiple infections), exposure age, infection status, host offspring counts, time from exposure to castration, time from exposure to host death and parasite spore production (per parasite clone) for each Daphnia that was used in the analyses.


