Abstract
The naked mole-rat (NMR) Heterocephalus glaber is a unique and fascinating mammal exhibiting many unusual adaptations to a subterranean lifestyle. The recent discovery of their resistance to cancer and exceptional longevity has opened up new and important avenues of research. Part of this resistance to cancer has been attributed to the fact that NMRs produce a modified form of hyaluronan—a key constituent of the extracellular matrix—that is thought to confer increased elasticity of the skin as an adaptation for living in narrow tunnels. This so-called high molecular mass hyaluronan (HMM-HA) stems from two apparently unique substitutions in the hyaluronan synthase 2 enzyme (HAS2). To test whether other subterranean mammals with similar selection pressures also show molecular adaptation in their HAS2 gene, we sequenced the HAS2 gene for 11 subterranean mammals and closely related species, and combined these with data from 57 other mammals. Comparative screening revealed that one of the two putatively important HAS2 substitutions in the NMR predicted to have a significant effect on hyaluronan synthase function was uniquely shared by all African mole-rats. Interestingly, we also identified multiple other amino acid substitutions in key domains of the HAS2 molecule, although the biological consequences of these for hyaluronan synthesis remain to be determined. Despite these results, we found evidence of strong purifying selection acting on the HAS2 gene across all mammals, and the NMR remains unique in its particular HAS2 sequence. Our results indicate that more work is needed to determine whether the apparent cancer resistance seen in NMR is shared by other members of the African mole-rat clade.
Keywords: naked mole-rat, Heterocephalus, hyaluronan, hyaluronan synthase, Bathyergidae, subterranean mammal
1. Introduction
The naked mole-rat (NMR) Heterocephaus glaber is emerging as an important ‘non-model’ organism for the study of longevity and healthy ageing. A range of physiological and molecular/biochemical adaptations underpin the lack of senescence observed in this small Hystricomorph rodent that can live for 32 years—10 times longer than a mouse and more than five times longer than predicted for its body size [1].
There has been considerable interest in the ability of NMRs to resist cancer [1,2], and recently, a mechanism involving the production of a high molecular mass hyaluronan (HMM-HA) has been proposed [3]. Hyaluronan (HA) is a glycosaminoglycan and its presence in a variety of tissues and the extracellular matrix has been linked to many cellular processes, such as cell division, motility and morphogenesis [4], and also implicated in the development of some cancers [5]. HMM-HA is up to six times the mass of the largest human HA. The novel anti-cancer mechanism identified in the NMR has been termed early contact inhibition (ECI). This is a process whereby cell growth occurring when cells come into contact with each other, or with the extracellular matrix, is arrested at much lower densities than in the mouse. Contact inhibition is lost in cancer cells, and the loss of ECI makes cells more susceptible to malignant transformation [6]. ECI is controlled by the interaction of HA with the CD44–NF2 pathway, which mediates contact inhibition [3]. In addition, NMRs also produce a novel tumour suppressor protein (an isoform of INK4) in response to HMM-HA stimulation. Induction of INK4 is associated with contact inhibition and leads to cell-cycle arrest, contributing to tumour resistance [7]. In the NMR, it has been postulated that selection for its characteristic loose, elastic skin is an adaptation to living underground in tight tunnels and confined spaces, and that the elasticity of the skin is facilitated by HMM-HA [3]. Thus, the cancer resistance imparted by HMM-HA may be a secondary and fortuitous consequence of the primary function of HMM-HA under selection.
Hyaluronan synthase 2 (HAS2) is one of three characterized membrane-embedded HA synthases responsible for the synthesis of HA from intracellular precursors, and deposition into the extracellular matrix [8]. Tian et al. [3] showed that HMM-HA is produced in NMRs by a uniquely modified version of the HAS2 gene, and accumulates owing to extremely low hyaluronidase activity. Specifically, two serine substitutions at highly conserved sites in the cytoplasmic domains of exons 2 and 4 appear to confer upon the protein molecule the ability to produce HMM-HA: when NMR HAS2 was overexpressed in a human HEK293 cell culture, secretion of HMM-HA was observed [3].
Given the link between NMR cancer resistance, HMM-HA and specific mutations in HAS2, together with the advantages of an elastic skin in the subterranean niche, we predict that production of HMM-HA may not be unique to NMRs, and that discovery of other HAS2 mutations may be of potential interest to cancer research. This study, therefore, aims to test for possible parallel signatures of adaptive evolution in the HAS2 gene in other mammals with shared selection pressures; in particular, other members of the African mole-rat family (Bathyergidae), as well as divergent subterranean insectivorous mammals within the superorders Afrotheria and Laurasiatheria.
2. Material and methods
We generated new HAS2 sequence data from 11 subterranean mammal species representing five divergent families (table 1; electronic supplementary material, Methods). These were combined with new sequences from two close outgroups of the Bathyergidae (cane rat and Cape porcupine), and a further 57 representative mammalian HAS2 sequences from all species available at the time of the study on GenBank (table 1). Sequences were aligned manually for analysis using Mesquite [9] and genetic distances calculated using MEGA v. 6 [10]. To test for signatures of selection acting along 519 codons of HAS2 (exons 2 and 4) across all 70 mammal species included in our study, we implemented site, branch-site and clade models with the codeml package in PAML v. 4.4 [11], using a mammal species tree topology based on published studies [12–14]. Amino acid polymorphisms were analysed using MAPP [15], which implements a predictive statistical framework to score the physico-chemical impact of substitutions in multiple alignments of orthologues. Novel sequences have been deposited in GenBank.
Table 1.
Sample list, taxonomy and accession numbers/EMSEMBL Transcript ID for the HAS2 sequences included in our analysis.
| scientific name | common name | order; family | accession number |
|---|---|---|---|
| Homo sapiens | human | Primates; Hominidae | U54804.1 |
| Pan troglodytes | chimpanzee | Primates; Hominidae | XM528222.4 |
| Pan paniscus | bonobo | Primates; Hominidae | XM3820492.1 |
| Pongo abelii | orangutan | Primates; Hominidae | XM3777303.1 |
| Nomascus leucogenys | northern white-cheeked gibbon | Primates; Cercopithecidae | XM3256164.2 |
| Macaca fascicularis | crab-eating macaque | Primates; Cercopithecidae | XM5564005.1 |
| Macaca mulatta | rhesus macaque | Primates; Cercopithecidae | XM1098841.2 |
| Chlorocebus sabaeus | green monkey | Primates; Cercopithecidae | XM8001453.1 |
| Saimiri boliviensis boliviensis | squirrel monkey | Primates; Cebidae | XM3933098.1 |
| Callithrix jacchus | common marmoset | Primates; Callitrichidae | XM2759268.2 |
| Otolemur garnettii | Garnett's greater galago | Primates; Galagidae | XM_3782374.1 |
| Tarsius syrichta | Philippine tarsier | Primates; Tarsiidae | XM8056607.1 |
| Tupaia chinensis | Chinese tree shrew | Scandentia; Tupaiidae | XM_6157522.1 |
| Lipotes vexillifer | Yangtze River dolphin | Cetacea; Lipotidae | XM_7445586.1 |
| Physeter catodon | sperm whale | Cetacea; Physeteridae | XM_7106932.1 |
| Odobenus rosmarus divergens | walrus | Carnivora; Odobenidae | XM_4416640.1 |
| Leptonychotes weddellii | Weddell seal | Carnivora; Phocidae | XM_6739332.1 |
| Canis lupus familiaris | dog | Carnivora; Canidae | XM_539153.4 |
| Panthera tigris altaica | tiger | Carnivora; Felidae | XM_7075592.1 |
| Felis catus | cat | Carnivora; Felidae | XM_4000089.2 |
| Ailuropoda melanoleuca | panda | Carnivora; Ursidae | XM_2927908.1 |
| Mustela putoriua furo | ferret | Carnivora; Mustelidae | XM_4804975.1 |
| Myotis davidii | David's mouse-eared bat | Chiroptera; Vespertilionidae | XM_6769087.1 |
| Myotis lucifugus | little brown bat | Chiroptera; Vespertilionidae | XM_6085251.1 |
| Myotis brandtii | Brandt's bat | Chiroptera; Vespertilionidae | XM_5885867.1 |
| Eptesicus fuscus | big brown bat | Chiroptera; Vespertilionidae | XM_8143356.1 |
| Pteropus alecto | black flying fox | Chiroptera; Pteropodidae | XM6916584.1 |
| Elephantulus edwardii | Cape elephant shrew | Macroscelidea; Macroscelididae | XM_6879317.1 |
| Orycteropus afer afer | aardvark | Tubulidentata; Orycteropodidae | XM_7943422.1 |
| Trichechus manatus latirostris | Florida manatee | Sirenia; Trichechidae | XM_4372979.1 |
| Procavia capensis | rock hyrax | Hyracoidea; Procaviidae | ENSPCAG00000005792 |
| Loxodonta africana | African elephant | Proboscidea; Elephantidae | XM_003408169 |
| Echinops telfairi | lesser hedgehog tenrec | Afrosoricida; Tenrecidae | XM_004697409 |
| Amblysomus hottentotusa | golden mole | Afrosoricida; Chrysochloridae | KR057419 |
| Oryctolagus cuniculus | European rabbit | Lagomorpha; Leporidae | AB055978.1 |
| Ochotona princeps | American pika | Lagomorpha; Ochotonidae | XM_6982381.1 |
| Spermophilus tridecemlineatus | thirteen-lined ground squirrel | Rodentia; Sciuridae | XM_5316195.1 |
| Mus musculus | mouse | Rodentia; Muridae | U52524.2 |
| Rattus norvegicus | rat | Rodentia; Muridae | AF008201.1 |
| Peromyscus maniculatus bairdii | prairie deer mouse | Rodentia; Cricetidae | XM_6982381.1 |
| Cricetulus griseus | Chinese hamster | Rodentia; Cricetidae | XM_7638417.1 |
| Mesocricetus auratus | golden hamster | Rodentia; Cricetidae | XM_5082729.1 |
| Tachyorychtes splendensa | East African root rat | Rodentia; Spalacidae | KR057420 |
| Nannospalax galili | blind mole-rat | Rodentia; Spalacidae | XM_008837986.1 |
| Fukomys zechia | Ghana mole-rat | Rodentia; Bathyergidae | KR057425 |
| Fukomys damarensisa | Damaraland mole-rat | Rodentia; Bathyergidae | KR057427 |
| Georychus capensisa | Cape dune mole-rat | Rodentia; Bathyergidae | KR057424 |
| Cryptomys hottentotusa | common mole-rat | Rodentia; Bathyergidae | KR057422 |
| Bathyergus janettaa | Namaqua dune mole-rat | Rodentia; Bathyergidae | KR057423 |
| Bathyergus suillusa | dune mole-rat | Rodentia; Bathyergidae | KR057421 |
| Heliophobius kapitia | silvery mole-rat | Rodentia; Bathyergidae | KR057426 |
| Heterocephalus glaber | naked mole-rat | Rodentia; Bathyergidae | XM_004883123 |
| Thryonomys swinderianusa | cane rat | Rodentia; Thryonomyidae | KR057428 |
| Hystrix africaeaustralisa | Cape porcupine | Rodentia; Hystricidae | KR057429 |
| Ctenomys perrensia | tuco-tuco | Rodentia; Ctenomyidae | KR057430 |
| Cavia porcellus | guinea pig | Rodentia; Caviidae | XM_003463665 |
| Chinchilla lanigera | chinchilla | Rodentia; Chinchillidae | XM5410908.1 |
| Sorex araneus | common shrew | Eulipotyphla; Soricidae | XM4607644.1 |
| Talpa europaeaa | European mole | Eulipotyphla; Talpidae | KR057431 |
| Condylura cristata | star-nosed mole | Eulipotyphla; Talpidae | XM_4679612.1 |
| Erinaceus europaeus | European hedgehog | Eulipotyphla; Erinaceidae | XM7520808.1 |
| Vicugna pacos | alpaca | Artiodactyla; Camelidae | XM_6211418.1 |
| Bos taurus | cow | Artiodactyla; Bovidae | XM_174079.2 |
| Capra hircus | goat | Artiodactyla; Bovidae | XM_5688873.1 |
| Sus scrofa | pig | Artiodactyla; Suidae | XM_214053.1 |
| Equus caballus | horse | Perissodactyla; Equidae | XM_1081801.1 |
| Ceratotherium simum simum | white rhinoceros | Perissodactyla; Rhinocerotidae | XM_4431107.1 |
| Dasypus novemcinctus | nine-banded armadillo | Cingulata; Dasypodidae | XM_4480750.1 |
| Monodelphis domestica | opossum | Didelphimorphia; Didelphidae | XM_1370252.2 |
| Ornithorhynchus anatinus | duck-billed platypus | Monotremata; Ornithorhynchidae | XM_1505190.2 |
aSpecies sequenced for this study.
3. Results and discussion
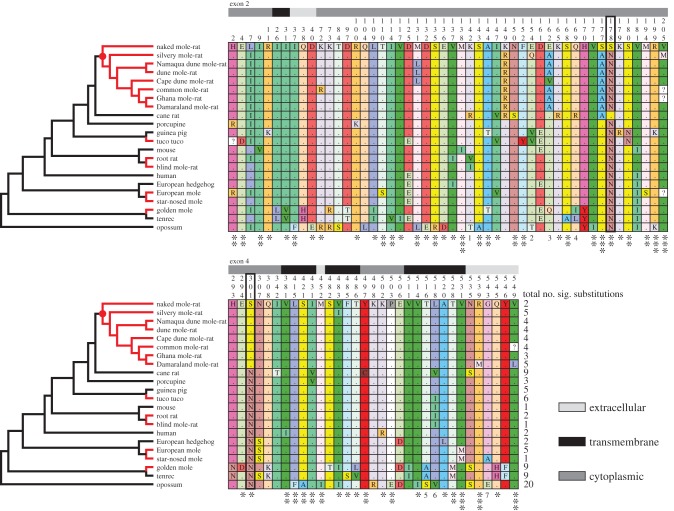
Overall measures of variation among 70 taxa revealed p-distances (nucleotide) ranging from 0.08 (chimp versus bonobo) to 19.53% (Philippine tarsier versus Opossum) and from zero (e.g. human versus chimp) to 15.26% (Cape elephant shrew versus opossum) for amino acid substitutions (see electronic supplementary material, table S2 for distances and table S3 for a complete amino acid alignment for all taxa). Site, branch-site and clade models implemented in PAML did not find evidence for positive selection; instead, all models suggest that HAS2 is under purifying (negative) selection with ω values of less than 1 across all mammals (p < 0.001; see electronic supplementary material, Results). Despite this, we identified multiple amino acid substitutions in key domains of the HAS2 molecule, including those previously described for the NMR (see figure 1; electronic supplementary material, table S3), although we observed no obvious substitutions shared across subterranean mammals. Of particular interest are the residues at sites 178 and 301 that facilitate production of HMM-HA in NMRs. Our results show that the serine substitution at site 178 in NMRs is only present in one other mammal, the cane rat, a close outgroup to the Bathyergidae, and thus perhaps arose convergently. Interestingly, however, the neighbouring site 177 has a serine residue substituted with an alanine in the cane rat and all bathyergids except the NMR. Replacement of a serine (which is readily phosphorylated and often important in the active site of enzymes) with an alanine is likely to have functional significance (p < 0.001 in MAPP analysis). The serine substitution at site 301 of the NMR is present in all bathyergid genera, but no other mammals, and is a shared derived character (synapomorphy) for the group (electronic supplementary material, figures S1–S3). There are also a number of other unique substitutions in particular species of the Bathyergidae/cane rat (e.g. sites 149 and 162; figure 1). Analysis of these polymorphisms using MAPP revealed multiple significant mutations (figure 1). Among the bathyergids, the Damaraland and silvery mole-rats ranked highest with five significant substitutions, followed by four in common, Cape dune, dune and Namaqua dune mole-rats, and three in the Ghana mole-rat. The NMR had just two—the aforementioned substitutions at sites 178 and 301 (figure 1; electronic supplementary material, figure S4 and table S3). The blind mole-rat HAS2 sequence is unremarkable and similar to the mouse and root rat, despite the fact that this species has also been reported to produce HMM-HA [3]. Thus, the mechanism of HMM-HA production in blind mole-rats may differ from that of NMRs, and cancer resistance also reported in this species appears to be mediated by a different mechanism [16,17]. It is noteworthy that these substitutions were relatively low within the context of the entire mammal dataset examined, where a maximum value of 21 substitutions predicted to have a significant effect was observed in the Cape elephant shrew (electronic supplementary material, table S3 and Supplementary results).
Figure 1.
Phylogenetic relationships and corresponding HAS2 sequences of five clades containing subterranean mammals (red branches), including non-subterranean ingroup comparisons, the human and a marsupial (opossum) outgroup (black branches). The Bathyergidae are the monophyletic clade denoted by the red circle. Adjacent panels show the respective variable amino acids for exons 2 and 4 (site numbers indicated above columns). Shaded bars indicate the relative locations of sites in the molecule (extracellular, transmembrane or cytoplasmic). The key amino acid residues at sites 178 and 301—that facilitate production of HMM-HA in NMRs—are indicated by the bold border. Asterisks below sites denote significance of substitutions estimated by MAPP analysis: *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant; 1: R = ns, V = **, I = *, T = **; 2: T = **, V = *, Q = ns; 3: V = ***, A = **, Q = ns; 4: R = **, I = ns, L = ns; 5:A = *, S = ns; 6: V = *, I = ns; 7: E = *, A = ns. Numbers at the end of the respective sequence alignments denote the number of substitutions per taxon that are predicted to have a significant impact on protein function.
These results raise interesting questions regarding the functional significance of the observed changes in the HAS2 amino acid sequence, and whether there is any correlation with longevity and cancer resistance (where known). Within the Bathyergidae, so far no other taxa are known to live as long as NMRs. Species such as the dune mole-rats (genus Bathyergus) and Georychus generally have short lifespans in the order of 4–6 years ([18], N. C. Bennett 2014, unpublished data), although two Georychus are known to have lived for 10 and 11 years, respectively, in captivity [19]. The Silvery mole-rat (Heliophobius) and some Fukomys (e.g. Damaraland and Zambian mole-rats) may commonly live more than 7 and 10 years, respectively [19,20], and sometimes up to 15+ years [20]. The absence of cancer has only been noted in NMRs, but there is a paucity of studies on other species in this context. Thus, the role of HAS2 and HA in other species remains unclear, but it is likely that multiple factors contribute to longevity. Nevertheless, our results and analysis provide the basis for further studies to establish the functional significance of HAS2 variants, using in vitro methods to characterize the different versions of HA produced, and the role they may play in cancer resistance.
Supplementary Material
Acknowledgements
We thank Ruth Rose for cloning, and the following for sample collection: Walter Verheyen (Tachyoryctes and Heliophobius), Patricia Mirol (Ctenomys), Kweku Dakwa (F. zechi) and Clair and Mark Rylands (Talpa). N.C.B. acknowledges funding from the DST-NRF SARChI Chair for Behavioural Ecology and Physiology; S.J.R. and K.T.J.D. were supported by the European Research Council.
Ethical statement
All procedures involving live animals and sample collection described in this manuscript were conducted in accordance with appropriate national and provincial guidelines, permits and regulations.
Data accessibility
New DNA sequence data have been deposited in GenBank accession numbers: KR057419–KR057431 (table 1).
Authors' contributions
C.G.F. conceived of, designed and coordinated the study, carried out the molecular laboratory work and sequence alignments, participated in data analysis and drafted the manuscript; K.T.J.D. designed primers, participated in data analysis and bioinformatics; S.J.R. participated in data analysis; N.C.B. provided samples and funding. All authors contributed to critical assessment of the results, manuscript revisions and gave final approval for publication.
Conflict of interest
We have no competing interests.
References
- 1.Buffenstein R. 2008. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B 178, 439–445. ( 10.1007/s00360-007-0237-5) [DOI] [PubMed] [Google Scholar]
- 2.Delaney MA, Nagy L, Kinsel MJ, Treuting PM. 2013. Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Vet. Pathol. 50, 607–621. ( 10.1177/0300985812471543) [DOI] [PubMed] [Google Scholar]
- 3.Tian X, et al. 2013. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349. ( 10.1038/nature12234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser JRE, Laurent TC, Laurent UBG. 1997. Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 242, 27–33. ( 10.1046/j.1365-2796.1997.00170.x) [DOI] [PubMed] [Google Scholar]
- 5.Stern R. 2009. Association between cancer and ‘acid mucopolysaccharides’: an old concept comes of age, finally. In Hyaluronan in cancer biology (ed. Stern R.), pp. 3–16. San Diego, CA: Academic Press/Elsevier. [DOI] [PubMed] [Google Scholar]
- 6.Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC, Gorbunova V. 2009. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc. Natl Acad. Sci. USA 106, 19 352–19 357. ( 10.1073/pnas.0905252106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian X, Azpurua J, Ke Z, Augereau A, Zhang ZD, Vijg J, Gladyshev VN, Gorbunova V, Seluanov A. 2015. INK4 locus of the tumor-resistant rodent, the naked mole rat, expresses a functional p15/p16 hybrid isoform. Proc. Natl Acad. Sci. USA 112, 1053–1058. ( 10.1073/pnas.1418203112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe K, Yamaguchi Y. 1996. Molecular identification of a putative human hyaluronan synthase. J. Biol. Chem. 271, 22 945–22 948. ( 10.1074/jbc.271.38.22945) [DOI] [PubMed] [Google Scholar]
- 9.Maddison WP, Maddison DR. 2014. Mesquite: a modular system for evolutionary analysis. Version 3.01. See http://mesquiteproject.org.
- 10.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. ( 10.1093/molbev/mst197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang ZH. 2007. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591. ( 10.1093/molbev/msm088) [DOI] [PubMed] [Google Scholar]
- 12.Faulkes CG, Verheyen E, Verheyen W, Jarvis JUM, Bennett NC. 2004. Phylogeographic patterns of speciation and genetic divergence in African mole-rats (Family Bathyergidae). Mol. Ecol. 13, 613–629. ( 10.1046/j.1365-294X.2004.02099.x) [DOI] [PubMed] [Google Scholar]
- 13.Blanga-Kanfi S, Miranda H, Penn O, Pupko T, DeBry R, Huchon D. 2009. Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 9, 71 ( 10.1186/1471-2148-9-71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meredith RW, et al. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524. ( 10.1126/science.1211028) [DOI] [PubMed] [Google Scholar]
- 15.Stone EA, Siddow A. 2005. Physicochemical constraint violation by missense substitutions mediates impairment of protein function and disease severity. Genome Res. 15, 978–986. ( 10.1101/gr.3804205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorbunova V, Hinea C, Tian X, Ablaeva J, Gudkov AV, Nevo E, Seluanov A. 2012. Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. Proc. Natl Acad. Sci. USA 109, 19 392–19 396. ( 10.1073/pnas.1217211109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. 2014. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat. Rev. Genet. 15, 531–540. ( 10.1038/nrg3728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett NC, Maree S, Faulkes CG. 2006. Georychus capensis. Mamm. Species 799, 1–4. ( 10.1644/799.1) [DOI] [Google Scholar]
- 19.Weigl R. 2005. Longevity of mammals in captivity; from the living collections of the world. Stuttgart, Germany: Kleine Senckenberg-Reihe 48. [Google Scholar]
- 20.Tacutu R, Craig T, Budovsky T, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhães JP. 2013. Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 41, D102–D1033. ( 10.1093/nar/gks1155) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
New DNA sequence data have been deposited in GenBank accession numbers: KR057419–KR057431 (table 1).