Abstract
Telomeres are sensitive to damage induced by oxidative stress, and thus it is expected that dietary antioxidants may support the maintenance of telomere length in animals, particularly those with a fast rate of life (e.g. fast metabolism, activity and growth). We tested experimentally the effect of antioxidant supplements on telomere length during early development in wild gull chicks with natural individual variations in behaviour pattern and growth rate. Proactive chicks had shorter telomeres than reactive chicks, but the penalty for the bold behaviour pattern was reduced by antioxidant supplementation. Chicks growing faster had longer telomeres during early growth, suggesting that inherited quality supports a fast life history.
Keywords: antioxidants, growth, oxidative stress, telomere, tonic immobility
1. Introduction
Conditions during early life can have profound fitness consequences via several physiological and genetic mechanisms, one of which involves telomere dynamics [1]. Telomeres are DNA and protein complexes that protect eukaryote chromosome ends from fusion and degradation, and they are subject to shortening at each cycle of cell division. Recent studies have shown links between stress exposure in early life and telomere length [2,3]. However, behavioural strategies to cope with environmental challenges often vary between individuals [4], and this variation is also associated with metabolic and life-history traits [5]. Therefore, the level of early life stress may differ among individuals according to their behaviour and life-history trajectories, resulting in links between lifestyle and telomere length [6].
Telomeres are particularly sensitive to damage induced by oxidative stress [7,8], and thus it is expected that antioxidants may support the maintenance of telomere length during development by alleviating the oxidative costs of growth [9]. Some correlational studies have shown that consumption of dietary antioxidants is associated with longer telomere length in humans [10,11]. However, whether exogenous antioxidants can help maintain telomere length in wild animals in relation to their behavioural pattern or life history is an unexplored question.
Our aims in this study were to examine the relationships of telomere length to behavioural pattern and growth rate and to test experimentally the effect of antioxidant supplementation on telomere length during early development in wild gull chicks. We evaluated the tonic immobility (TI) response of chicks as a proxy for the bold–fearful behavioural axis [12]. TI is an innate paralysis response, and a long duration of TI is considered an indication of fearfulness and reactive behaviour in birds [12]. Boldness/fearfulness is a principal component of animal personality that has coevolved with a suite of physiology and life-history particularities related to the rate of life [5]. We predict that supplementation of dietary antioxidants (vitamins C and E) will influence the maintenance of telomere length particularly in chicks with bold behaviour or fast growth, because these phenotypes are linked with accelerated metabolism [13].
2. Material and methods
We performed the field experiment from April to June 2010 at a colony of yellow-legged gulls (Larus michahellis) in Sálvora Island, Spain (42°28′ N, 09°00′ W). We used 108 nests containing a clutch of three eggs with known laying date, order and size. Nests were randomly assigned to either a non-supplement (n = 52 nests) or a vitamin supplement group (n = 56 nests). We cross-fostered all three eggs 1 day after clutch completion within a group of four nests in which the second and third eggs were laid on the same days. In this way, all three eggs from the same original nest were incubated (then the hatchlings were raised) in three different foster nests other than the original nest, but conserving the expected chick order (for details, see [14]). By cross-fostering, any possible natural covariation between parental effects and telomere length were disrupted. All hatchlings were individually identified by using a leg flag.
Chicks assigned to the experimental group received daily a suspension of vitamins C and E (Lohmann Animal Health GmbH & Co. KG, Germany), beginning on the day of hatching (day 0) until age 7 days. From a suspension of 10 mg vitamin E and 6 mg vitamin C mixed in 100 ml water, each chick daily received 0.6 ml on days 0–3 and 1 ml on days 4–7 via oral administration with a syringe (i.e. approx. 20% the estimated natural daily intake [14]). The control group received the same amount of water without vitamins C and E.
Chicks were weighed on days 0 and 7 and growth rate was calculated as [ln(weight on day 7) − ln(weight on day 0)]/7 days. Chick response to manual restraint (TI, [12]) was tested on day 2 as a measure of boldness [5]. TI was tested by following a previously published protocol adjusted to gull chicks [15]. Each chick was placed in a concave container, covered with a textile and restrained for approximately 15 s. After removing the textile, the time until the chick righted itself was measured up to 180 s. A blood sample was collected from the brachial vein of each chick on days 0 and 7, using a sterile needle and heparinized capillary tubes. Centrifuged red blood cells from hatchlings were mixed with alcohol and used for molecular sexing [16], and those from day 7 were stored at −80°C to determine telomere length.
Telomere length was measured in DNA from red blood cells using quantitative PCR [17] (electronic supplementary material). We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene, with primer sequences designed from the GAPDH gene intron (electronic supplementary material, figure S1). Samples were analysed in duplicate, and T/S ratios (telomere repeat copy number/single gene copy number) were calculated, controlling for plate efficiency (electronic supplementary material).
The effects of vitamin supplementation were analysed using linear mixed-effect models (LMM) and generalized linear mixed models (GLMM). As TI followed a bimodal distribution, we categorized this variable in two values by using the median (12.38 s) as cut-off point. Thus, we analysed TI using a GLMM with a binomial distribution and logit link. Growth rate and telomere length in the first week of life were analysed using LMMs. Identities of cross-foster group [14] and original and foster nests (nested within the cross-foster group) were included as random effects. Experimental treatment, chick sex, hatching date, egg volume and laying order were included as fixed effects. Growth rate was also included as an additional fixed effect in the model of telomere length. Two-way interactions of interest were also included in the models. Non-significant fixed effects were dropped sequentially from the full model by using deletion tests. Then, the final model was fitted to obtain estimates and significance levels of the remaining fixed effects. The p-values of non-significant terms were determined based on model comparisons with the final model.
3. Results
Among 280 hatchlings, 221 survived until age 7 days, but all parameters used in this study (i.e. TI, growth rate and telomere length) were measured only in 212 chicks (electronic supplementary material, table S1). TI of 2-day-old chicks was not affected by vitamin supplement, sex, hatching date, hatching order or egg volume (p > 0.09), and all two-way interactions between experimental treatment and other fixed effects were not significant (p > 0.12). Chicks hatching earlier in the season grew faster than late chicks during the first week of life (F1,202 = 20.91, p < 0.001), but chick growth rate was not affected by other factors, covariates and interactions (p > 0.09). Vitamin supplementation had no effect on telomere length by itself (p = 0.763), but there was a significant interacting effect of vitamin supplement and TI level on telomere length of chicks at age 7 days (F1,206 = 4.41, p = 0.037, figure 1). In the non-supplement group, bold chicks had shorter telomeres than fearful chicks (p = 0.004), but this difference did not appear in vitamin-supplemented chicks (p = 0.941). Telomere length was positively correlated with chick growth irrespective of vitamin supplement treatment (r = 0.16, F1,206 = 5.33, p = 0.022). In addition, males had longer telomeres than females (male T/S ratio: 1.83 ± 0.07, female T/S ratio: 1.64 ± 0.07; F1,206 = 3.94, p = 0.048), but other effects were not significant (p > 0.28).
Figure 1.
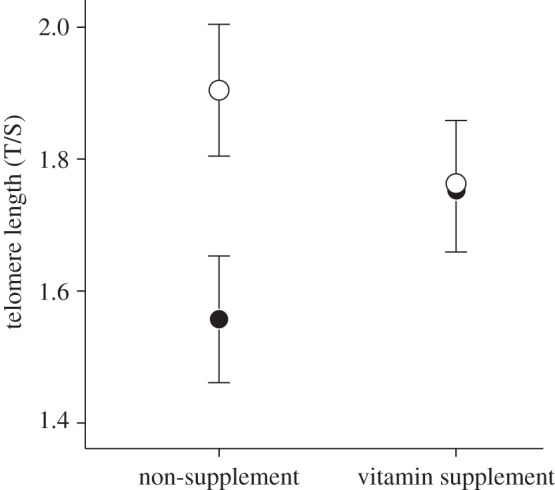
Telomere length (mean ± s.e.) of gull chicks (n = 212) at age 7 days according to the experimental treatment and TI level. Closed circles represent proactive, bold chicks and open circles reactive, fearful chicks.
4. Discussion
Our results indicate that proactive chicks had shorter telomeres than reactive chicks, but this penalty for bold temperament was reduced by a dietary supplement of antioxidants. Between-individual differences in personality-related behavioural traits covary with life-history and physiological differences at the within-population level [5]. In general, bold personality is correlated with a fast lifestyle that involves fast metabolism [5,13], and thus antioxidants may be a limiting resource particularly for proactive individuals. Probably for this reason, vitamin supplementation benefited proactive chicks by reducing oxidative damage to telomeres, whereas antioxidants obtained from food were sufficient to maintain homeostasis in reactive chicks. The overall effect of vitamin supplementation on telomere length was not significant, suggesting that the protective effects of antioxidants against telomere loss cannot be generalized.
Our finding that there is a positive relationship between telomere length and growth rate was opposite to our prediction that fast growth will lead to reduced telomere length [1]. In agreement with this prediction, a recent study of captive zebra finches demonstrated that telomere length was negatively correlated with their body mass at the end of the growth period [18]. Life-history trajectories often have delayed effects on physiological condition [19], whereas the effects of behaviour patterns can appear more immediately. We measured telomere length in gull chicks only during the early stage of growth, but the direct effect of fast growth on telomere length through increased cell division rate or the indirect effect through accelerated metabolism may appear beyond this period. On the other hand, individuals with strong capacity to cope with oxidative stress may support a fast lifestyle, giving rise to a positive relationship between telomere length and growth rate. It is also possible that chicks' developmental conditions independently influenced their telomere length and growth. Indeed, a recent study on a wild bird showed that chicks under stressful conditions grew more slowly and lost more telomere repeats [2]. Interestingly, our study also indicates that telomere length differed between sexes, suggesting that sex-specific mechanisms may govern life-history trajectories in a very early stage of life (see also [18]).
In conclusion, our results show that dietary antioxidants can help the maintenance of telomere length during early life in individuals that probably suffer greater telomere damage owing to consistent early lifestyle associated with bold behaviours. Exogenous antioxidants may also be involved in the expression of developmental phenotypes through the regulation of endocrine signals and endogenous antioxidant systems [14]. Telomere length at the beginning of life is an important predictor of fitness-related traits, but telomere attrition is also greatest during this period when growth is still occurring [20]. Therefore, the availability of dietary antioxidants during early life should have long-term consequences by protecting telomeres in fast-living individuals.
Supplementary Material
Acknowledgements
We thank two anonymous reviewers for helpful comments and A. Tato, J. C. Noguera, A. Lucas, C. Pérez, J. Díaz and the National Park staff for help during the study.
Ethics statement
This study was conducted under permission from the Xunta de Galicia (177/2010), and all the procedures complied with the current laws of Spain.
Data accessibility
The data supporting this article will be deposited in Dryad.
Funding statement
Funding was provided by the Spanish Ministerio de Economía y Competitividad (CGL2012-40229-C02-02) and the Xunta de Galicia (2012/305).
Authors' contributions
S.-Y.K. and A.V. conceived the study, performed the experiment and statistical analyses and wrote the article. Both authors gave final approval for publication.
Conflict of interests
We have no competing interests.
References
- 1.Monaghan P. 2014. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 2.Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. ( 10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 5.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savolainen K, Eriksson JG, Kananen L, Kajantie E, Pesonen AK, Heinonen K, Räikkönen K. 2014. Associations between early life stress, self-reported traumatic experiences across the lifespan and leukocyte telomere length in elderly adults. Biol. Psychol. 97, 35–42. ( 10.1016/j.biopsycho.2014.02.002) [DOI] [PubMed] [Google Scholar]
- 7.Kawanishi S, Oikawa S. 2004. Mechanism of telomere shortening by oxidative stress. Ann. NY Acad. Sci. 1019, 278–284. ( 10.1196/annals.1297.047) [DOI] [PubMed] [Google Scholar]
- 8.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. 2008. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic. Biol. Med. 44, 235–246. ( 10.1016/j.freeradbiomed.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 9.Kim S-Y, Noguera JC, Morales J, Velando A. 2011. Quantitative genetic evidence for trade-off between growth and resistance to oxidative stress in a wild bird. Evol. Ecol. 25, 461–472. ( 10.1007/s10682-010-9426-x) [DOI] [Google Scholar]
- 10.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. 2009. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 89, 1857–1863. ( 10.3945/ajcn.2008.26986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Calzón S, Moleres A, Martínez-González MA, Martínez JA, Zalba G, Marti A. In press. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin. Nutr. ( 10.1016/j.clnu.2014.07.015) [DOI] [PubMed] [Google Scholar]
- 12.Jones RB. 1986. The tonic immobility reaction of the domestic fowl: a review. World’s Poult. Sci. J. 42, 82–96. ( 10.1079/WPS19860008) [DOI] [Google Scholar]
- 13.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. ( 10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 14.Kim S-Y, Noguera JC, Tato A, Velando A. 2013. Vitamins, stress and growth: the availability of antioxidants in early life influences the expression of cryptic genetic variation. J. Evol. Biol. 26, 1341–1352. ( 10.1111/jeb.12136) [DOI] [PubMed] [Google Scholar]
- 15.Rubolini D, Romano M, Boncoraglio G, Ferrari RP, Martinelli R, Galeotti P, Fasola M, Saino N. 2005. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm. Behav. 47, 592–605. ( 10.1016/j.yhbeh.2005.01.006) [DOI] [PubMed] [Google Scholar]
- 16.Fridolfsson AK, Ellegren H. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121. ( 10.2307/3677252) [DOI] [Google Scholar]
- 17.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 18.Noguera JC, Metcalfe NB, Boner W, Monaghan P. 2015. Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taenioyigia guttata). Biol. Lett. 11, 20140938 ( 10.1098/rsbl.2014.0938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yearsley JM, Kyriazakis I, Gordon IJ. 2004. Delayed costs of growth and compensatory growth rates. Funct. Ecol. 18, 563–570. ( 10.1111/j.0269-8463.2004.00879.x) [DOI] [Google Scholar]
- 20.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article will be deposited in Dryad.


