Abstract
Although parasite-mediated selection is assumed to be the main driver of immune gene evolution, empirical evidence that parasites induce allele frequency changes at host immune genes in time and/or space remains scarce. Here, I show that the frequency of a protective gene variant of the innate immune receptor Toll-like receptor 2 in natural bank vole (Myodes glareolus) populations is positively associated with the strength of Borrelia burgdorferi sensu lato infection risk across the European continent. Thereby, this study provides rare evidence for the role of spatially variable infection pressures in moulding the vertebrate immune system.
Keywords: host–parasite interactions, Lyme disease, wildlife disease, genetic variation, resistance evolution, rodents
1. Introduction
Parasites negatively affect host fitness and thereby select for host responses that prevent or reduce infection. The immune system has a key role in host defence, and parasites are therefore assumed to be main drivers of immune gene evolution [1]. Associations between immune gene variants and parasite resistance are regularly observed in natural populations [2–4]. However, in most cases, it is difficult to track changes in allele frequencies over time to directly demonstrate parasite-driven evolution of the hosts' immune system in the wild [5].
As an alternative, spatial variation in parasite abundance might provide insights into the role of infection pressures in shaping immune system evolution. In humans, for example, spatial variation in Plasmodium sp. prevalence is a strong predictor of the frequency of malaria resistance alleles across countries [6–8]. Evidence for an association between parasite pressure and the frequency of resistance alleles in populations of non-human vertebrates, however, remains scant because of a lack of information on spatial variation in the prevalence of wildlife disease, but also, or mainly, the scarcity of candidate immune genes [9].
Borrelia burgdorferi sensu lato is a common tick-transmitted pathogen in rodents [10], and the causative agent of human Lyme borreliosis (LB), the most common vector-borne disease in Europe and North America [11,12]. The innate immune receptor Toll-like receptor 2 (TLR2) plays a key role in the recognition of bacterial lipoproteins [13] and has been identified as a candidate gene for Borrelia resistance in laboratory mice [14,15]. Furthermore, bank voles (Myodes glareolus) carrying certain TLR2 variants (TLR2 c2 [16]) have a substantially reduced probability of becoming Borrelia infected (i.e. TLR2 c2 confers partial Borrelia resistance), highlighting that TLR2 mediates host–Borrelia interactions also in the wild [16]. Based on this knowledge, I hypothesized that Borrelia-mediated selection shapes the evolution of TLR2 in rodents. Indeed, substantial variation in the frequency of the protective TLR2 variant has been observed in bank vole populations across Europe [17]. Although data on Borrelia prevalence in these populations are not available, rates of human LB cases are reported for most European countries [18], and can be used as a proxy for Borrelia infection risk.
2. Material and methods
Information on LB incidence in a country, measured as the average annual number of LB cases per 100 000 inhabitants, was obtained from [18,19] (table 1). Data on the frequency of the protective TLR2 c2 variant in the local bank vole population were obtained from [17] (table 1).
Table 1.
Frequency of the protective TLR2 c2 variant (TLR2 c2) in bank vole populations across Europe and average number of annual LB cases per 100 000 inhabitants (LB incidence) in the same country. LB incidence data were obtained from [18,19]. N: number of sequenced bank voles; lineage: mitochondrial lineage of the local bank vole population. The exact bank vole sampling locations and TLR2 and cytb genotyping procedures are described in [17].
| country | N | TLR2 c2 | lineage | LB incidence |
|---|---|---|---|---|
| Austria | 17 | 0.27 | Western | 130 |
| Belgium | 19 | 0.05 | Western | 12.58 |
| Czech Republic | 19 | 0.00 | Western | 31.73 |
| Denmark | 19 | 0.05 | Eastern | 1.68 |
| England | 17 | 0.00 | Western | 1.72 |
| Finland | 20 | 0.03 | Eastern | 18.46 |
| Germany 1 | 21 | 0.10 | Western | 25 |
| Germany 2 | 18 | 0.08 | Eastern | 25 |
| Italy 1 | 21 | 0.00 | Italian | 0.02 |
| Italy 2 | 19 | 0.00 | Italian | 0.02 |
| Lithuania | 20 | 0.18 | Carpathian | 42.93 |
| Netherlands | 10 | 0.00 | Western | 2.01 |
| Norway | 21 | 0.00 | Carpathian | 4.5 |
| Poland | 12 | 0.29 | Eastern | 9.29 |
| Russia | 21 | 0.07 | Eastern | 9.24 |
| Scotland | 19 | 0.00 | Carpathian | 1.72 |
| Slovenia | 20 | 0.30 | Western | 136.86 |
| Spain | 13 | 0.00 | Spanish | 9.8 |
| Switzerland | 19 | 0.27 | Western | 25.09 |
| Sweden | 20 | 0.30 | Carpathian | 69 |
| Ukraine | 20 | 0.00 | Eastern | 0.98 |
Eight well-defined mitochondrial bank vole lineages have been described in Europe [20–22], which reflect the colonization history of the species after the last glaciation. Phylogeographic analyses based on the mitochondrial cytochrome b gene were used to identify to which lineage the different study populations belong [17] (table 1). For Germany and Italy, two populations were sampled (table 1). However, the results did not change qualitatively when randomly excluding one of the two populations per country from the analysis.
The frequency of TLR2 c2 in the sampled bank vole populations was analysed using a quasi-binomial generalized linear model in R [23]. LB incidence in a country was included in the model. In addition, I included the mitochondrial lineage to which a population belongs to account for potential differences in TLR2 c2 frequency among populations owing to the colonization history of bank voles in Europe [17,20–22]. The significance of factors was determined by comparing two nested models, with and without the factor of interest, using likelihood-ratio tests.
3. Results and discussion
As predicted, if Borrelia-mediated selection affects the large-scale geographical distribution of the protective TLR2 variant, there was a positive association between the frequency of TLR2 c2 in bank vole populations and human LB incidence across 19 European countries (χ2 = 35.636, d.f. = 1, p = 0.003; figure 1). No indication was found that TLR2 c2 frequency differed among mitochondrial bank vole lineages (χ2 = 13.958, d.f. = 4, p = 0.499), showing that the observed pattern was not influenced by the colonization history of the species in Europe [17,20–22]. Rather, the strong association between human LB incidence and the frequency of the protective TLR2 c2 variant in bank vole populations indicates that Borrelia-mediated selection has shaped the evolution of this innate immune receptor in wild rodents.
Figure 1.
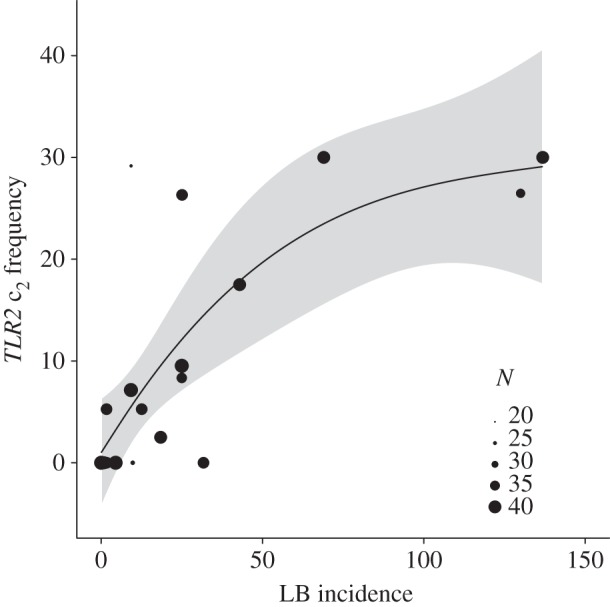
Relationship between human LB incidence (LB cases/100 000 inhabitants) and the frequency of the protective TLR2 c2 variant in the local bank vole population across 19 European countries. The circle size indicates the number of sampled TLR2 alleles (N) in a population. Locally fitted smoothed polynomial surface (solid line) and 95% confidence interval (grey area) are presented.
Clearly, the average annual rate of human LB cases in a country is only a crude estimate of the actual Borrelia exposure bank voles experience at a local scale. LB incidence rates, for example, show regional differences within countries [18]. Also, there might be differences among countries in how accurately LB cases are diagnosed and/or reported [18]. Furthermore, lifestyle differences across countries might change the relationship between Borrelia abundance and human LB incidence [18]. Finally, human LB cases are partly caused by Borrelia genospecies that do not infect rodents (e.g. the bird specialist Borrelia garinii [24]). However, all these factors will add noise to the data and therefore reduce the statistical power to detect a relationship between human LB incidence and the frequency of the protective TLR2 variant in the local bank vole population, rather than generate a spurious correlation. The observed relationship thus likely represents an underestimation of the impact of Borrelia pressure on TLR2 evolution in bank voles.
The finding that the protective TLR2 c2 variant occurred at low frequencies in regions where Borrelia is rare suggests that resistance may be associated with costs in the absence of the pathogen [25–27]. Indeed, it has been shown that a human Toll-like receptor 4 variant reduces mortality during malaria infection, but is disadvantageous in the absence of Plasmodium because it increases the susceptibility to severe bacterial infections [7]. Similar trade-offs may maintain the pronounced balanced polymorphism observed at the bank vole TLR2 [17]. Assessing the costs of carrying resistance alleles in the absence of infection will thus be a fruitful next step to understand the maintenance of immunogenetic variation in wild vertebrates.
In conclusion, this study provides empirical evidence for an association between Borrelia infection risk and the frequency of a protective TLR2 variant in bank vole populations across the European continent. Thereby, it is one of the first to reveal an association between large-scale spatial variation in pathogen pressure and the immunogenetic composition of populations of a wild vertebrate.
Acknowledgements
I thank Erik Postma and two anonymous reviewers for comments on the manuscript. S. Karger AG approved the use of published LB incidence data for this study.
Data accessibility
All data supporting this article are presented in table 1.
Competing interests
I delcare I have no competing interests.
Funding
The study was financially supported by the University of Zurich Research Priority Program ‘Evolution in action: from genomes to ecosystems' and the Swiss National Science Foundation (PP00P3_128386 and PP00P3_157455).
References
- 1.Murphy K, Travers P, Walport M. 2008. Janeway‘s immunobiology, 7th edn New York, NY: Garland Science. [Google Scholar]
- 2.Hill AVS, et al. 1991. Common West African HLA antigens are associated with protection from severe malaria. Nature 352, 595–600. ( 10.1038/352595a0) [DOI] [PubMed] [Google Scholar]
- 3.Bellamy R. 2004. Susceptibility to infectious diseases: the importance of host genetics. New York, NY: Cambridge University Press. [Google Scholar]
- 4.Piertney SB, Oliver MK. 2006. The evolutionary ecology of the major histocompatibility complex. Heredity 96, 7–21. ( 10.1038/sj.hdy.6800724) [DOI] [PubMed] [Google Scholar]
- 5.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 6.Garamszegi LZ. 2014. Global distribution of malaria-resistant MHC-HLA alleles: the number and frequencies of alleles and malaria risk. Malar. J. 13, 349 ( 10.1186/1475-2875-13-349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferwerda B, et al. 2007. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl Acad. Sci. USA 104, 16 645–16 650. ( 10.1073/pnas.0704828104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiatkowski DP. 2005. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77, 171–192. ( 10.1086/432519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurgin LG, Richardson DS. 2010. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc. R. Soc. B 277, 979–988. ( 10.1098/rspb.2009.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, Ogden NH. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 4, 660–669. ( 10.1038/nrmicro1475) [DOI] [PubMed] [Google Scholar]
- 11.Dennis DT, Hayes EB. 2002. Epidemiology of Lyme borreliosis. In Lyme borreliosis: biology, epidemiology and control (eds Kahl O, Gray JS, Lane RS, Stanek G.), pp. 251–280. Oxford, UK: CABI Publications. [Google Scholar]
- 12.Ostfeld RS. 2011. Lyme disease: the ecology of a complex system. New York, NY: Oxford University Press. [Google Scholar]
- 13.Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145. ( 10.1038/35100529) [DOI] [PubMed] [Google Scholar]
- 14.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168, 348–355. ( 10.4049/jimmunol.168.1.348) [DOI] [PubMed] [Google Scholar]
- 15.Roper RJ, et al. 2001. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immunol. 2, 388–397. ( 10.1038/sj.gene.6363801) [DOI] [PubMed] [Google Scholar]
- 16.Tschirren B, Andersson A, Scherman K, Westerdahl H, Mittl P, Råberg L. 2013. Polymorphisms at the innate immune receptor TLR2 are associated with Borrelia infection in a wild rodent population. Proc. R. Soc. B 280, 20130364 ( 10.1098/rspb.2013.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morger J, et al. In press Distinct haplotype structure at the innate immune receptor Toll-like receptor 2 (TLR2) across bank vole populations and lineages in Europe. Biol. J. Linn. Soc. ( 10.1111/bij.12593) [DOI] [Google Scholar]
- 18.Hubálek Z. 2009. Epidemiology of Lyme borreliosis. In Lyme borreliosis: biological and clinical aspects (eds Lipsker D, Jaulhac B.), pp. 31–50. Basel, Switzerland: Karger. [Google Scholar]
- 19.Biletska H, Podavalenko L, Semenyshyn O, Lozynskyj I, Tarasyuk O. 2008. Study of Lyme borreliosis in Ukraine. Int. J. Med. Microbiol. 298, 154–160. ( 10.1016/j.ijmm.2008.04.004) [DOI] [Google Scholar]
- 20.Deffontaine V, Libois R, Kotlík P, Sommer R, Nieberding C, Paradis E, Searle JB, Michaux J. 2005. Beyond the Mediterranean peninsulas: evidence of central European glacial refugia for a temperate forest mammal species, the bank vole (Clethrionomys glareolus). Mol. Ecol. 14, 1727–1739. ( 10.1111/j.1365-294X.2005.02506.x) [DOI] [PubMed] [Google Scholar]
- 21.Kotlík P, Deffontaine V, Mascheretti S, Zima J, Michaux JR, Searle JB. 2006. A northern glacial refugium for bank voles (Clethrionomys glareolus). Proc. Natl Acad. Sci. USA 103, 14 860–14 864. ( 10.1073/pnas.0603237103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deffontaine V, Ledevin R, Fontaine MC, Quere JP, Renaud S, Libois R, Michaux JR. 2009. A relict bank vole lineage highlights the biogeographic history of the Pyrenean region in Europe. Mol. Ecol. 18, 2489–2502. ( 10.1111/j.1365-294X.2009.04162.x) [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team. 2011. R: a language and environment for statistical computing See http://www.R-project.org.
- 24.Baranton G, De Martino SJ. 2009. Borrelia burgdorferi sensu lato diversity and its influence on pathogenicity in humans. In Lyme borreliosis: biological and clinical aspects (eds Lipsker D, Jaulhac B.), pp. 1–17. Basel, Switzerland: Karger. [DOI] [PubMed] [Google Scholar]
- 25.Tschirren B, Richner H. 2006. Parasites shape the optimal investment in immunity. Proc. R. Soc. B 273, 1773–1777. ( 10.1098/rspb.2006.3524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netea MG, Wijmenga C, O'Neill LAJ. 2012. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 13, 535–542. ( 10.1038/ni.2284) [DOI] [PubMed] [Google Scholar]
- 27.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting this article are presented in table 1.


