Abstract
Drosophila performs elaborate well-defined rituals of courtship, which involve several types of sensory inputs. Here, we report that Or47b-neurons promote male-mating success. Males with Or47b-neurons silenced/ablated exhibit reduced copulation frequency and increased copulation latency. Copulation latency of Or47b-manipulated flies increased proportionately with size of the assay arena, whereas in controls it remained unchanged. While competing for mates, Or47b-ablated males are outperformed by intact controls. These results suggest the role of Or47b-neurons in promoting male-mating success.
Keywords: Drosophila, courtship, sensory, olfactory, Or47b
1. Introduction
Innate behaviours ranging from highly conserved circadian rhythms to specialized ones such as waggle dance of honeybees and migration of monarch butterflies have an endogenous basis. Such behavioural programmes are governed by dedicated neuronal circuits that respond to specialized sensory cues [1]. Drosophila exhibits ritualized courtship, which comprises a series of easily observable stereotypic events [2]. The genes and neurocircuitry involved in the regulation of courtship behaviour have been extensively studied [2], and it is known that it involves a cascade of events genetically programmed by two zinc-finger transcription factors fruitless (fru) and doublesex (dsx) [3]. Male-specific fruM is expressed in about 2000 neurons in the central and peripheral nervous system, and is deemed necessary and sufficient for the development of almost all aspects of male courtship behaviour [4,5].
Males recognize females using a wide array of sensory modalities that are visual, olfactory, gustatory, tactile, acoustic and mechanosensory in nature [2,6]. Four olfactory receptors (Or47b, Or65a, Or67d and Or88a) that respond to fly odours belong to the same family of olfactory receptors [7]. In addition, there is another family of receptors that respond to chemosensory cues [8], and it has been shown that one of its members, IR84a, is able to detect food odours and is important for male courtship behaviour [9].
GABA-signalling in Or47b-neurons regulates pheromone receptivity in males, improving their ability to detect females [10]. Two Fru-positive glomerular targets of Or67d (DA1) and Or47b (VA1v) neurons show developmental plasticity and are involved in courtship behaviour [11,12]. In a recent study, Wang et al. [13] have shown that the courtship efficiency of two Or47b loss-of-function mutants (Or47b2 and Or47b3) is comparable to controls, suggesting that Or47b receptors may not be involved in courtship behaviour. However, Or47b null males show defects in courting hydrocarbon-free targets, which are otherwise vigorously courted by wild-type males, suggesting the role of Or47b receptors in detecting chemical compounds involved in sexual behaviours. Taken together, these results suggest that Or47b-neurons and VA1v glomeruli to which they are connected are crucial for the regulation of sexual behaviours in the fruit fly Drosophila melanogaster.
2. Material and methods
(a). Fly strains
Fly strains used are Or47bGAL4 (NCBS, Bangalore), iso31 (isogenic strain), UASdti (dti = diphtheria toxin), UASdORKC1 (Todd Holmes, UC-Irvine), Or47b2 and Or47b3 (Bloomington). Expression of diptheria toxin (DTI) and dORKC1 causes ablation and electrical silencing of neurons [14]. Fly line iso31 is the genetic background strain in the DrosoDel project [15], and was used to backcross (for at least five generations) fly strains in our experiments. The driver and effector lines were confirmed [16].
(b). Copulation frequency assay
To estimate copulation frequency, freshly emerged virgin males and females were dispensed into small glass vials (25 × 90 mm; diameter × length), and 10 such vials per sex per assay were maintained for five days under 12 light : 12 dark cycles, after which lights were turned-off to create constant darkness (DD). All of our assays were carried out under DD using far-red light (λ > 650 nm) to eliminate confounding effects of visual cues on courtship and mating. After 9 h, 10 males and 10 females from a pair of vials were introduced into a mating arena (n = 10) comprising long glass vials (25 × 195 mm). Similarly, in a separate set of experiments, single male–female pairs (n = 16) were aspirated into fresh glass tubes (25 × 95 mm). Flies were monitored for the formation of mating pairs, and the number of such pairs formed in 3/5-min bins over a period of 15/30 min was used to estimate copulation frequency. All our results were verified in two independent trials. To assess the roles of males and females in successful mating, either males or females with ablated Or47b-neurons were used along with intact controls. Additionally, the copulation frequency of Or47b loss-of-function mutants (Or47b2/2 and Or47b3/3) and heterozygous controls (Or47b2/+ and Or47b3/+) was assayed either in groups or in pairs. Data were analysed by Kruskal–Wallis analysis of variance (ANOVA) followed by post hoc multiple comparisons using Dunn–Sidak's test.
(c). Copulation latency assay
To estimate copulation latency (time taken to initiate copulation), 9 h after the onset of DD, a single virgin male from either of the two genotypes (Or47bGAL4/UASdti or Or47bGAL4/iso31) was aspirated into a fresh glass vial approximately 10 min prior to the introduction of a virgin female (n = 16 pairs for each genotype and each experimental condition). Copulation latency was assayed in glass arenas of three sizes (5 × 65 mm = small tubes, 7 × 80 mm = large tubes and 25 × 90 mm = vials).
(d). Mating competition assay
Virgin flies were collected and maintained in a manner similar to that described above. One male each from the two genotypes (Or47bGAL4/UASdti, Or47bGAL4/+ and Or47bGAL4/UASdti, UASdti/+) was introduced into a fresh glass tube (7 × 80 mm) approximately 10 min before a Canton S (CS) female was introduced. Mating success was estimated as the proportion of males of either genotype that were able to secure mating with CS females (n = 16 vials per replicate). Eye-colour (Or47b-ablated flies, dark-red compared with faint-red controls) was used to identify genotype of the successful male. Eye-colour of the successful male was observed first under red light, and subsequently confirmed under normal light.
3. Results
(a). Ablation/silencing of Or47b-neurons affects copulation frequency
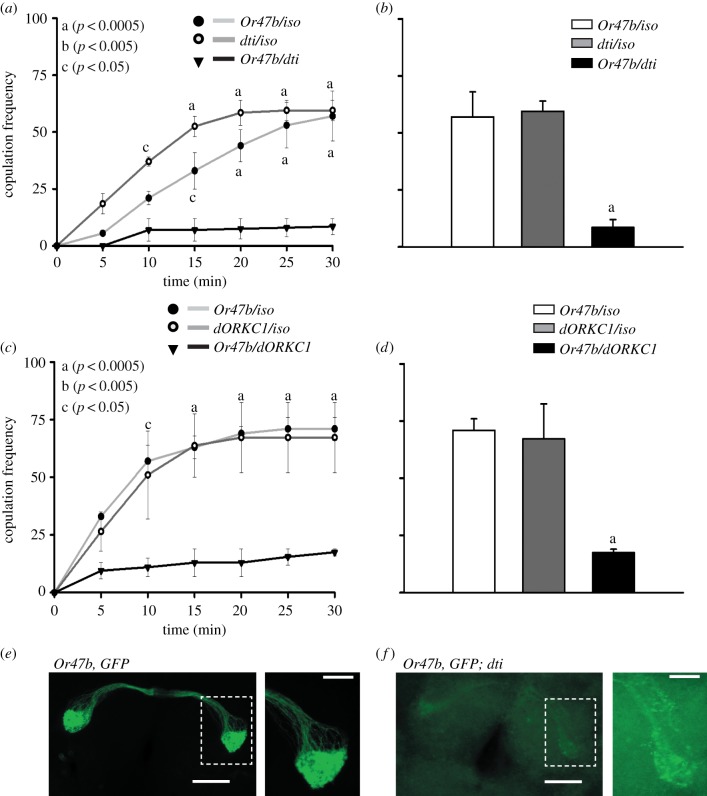
We performed mating assays in groups of Or47b-ablated/silenced flies. ANOVA followed by Dunn–Sidak's multiple comparisons revealed that the copulation frequency of Or47b-ablated/silenced flies was significantly lower than that of intact controls (ablated: 10 min: p < 0.05; 15 min: p < 0.05 for Or47bGAL4, p < 0.0001 for UASdti/+; 20/30 min: p < 0.0001 for both controls, figure 1a,b; silenced: 10 min: p < 0.05, 15 min: p < 0.01, 20/25/30 min: p < 0.0001 for both controls, figure 1c,d). As results on ablated and silenced flies were similar, we chose Or47b-ablated flies for the next set of experiments. Ablation of Or47b-neurons using UASdti was confirmed by immunohistochemistry (electronic supplementary material, S1, n = 20, figure 1e,f). To avoid confounding effects of a large group of individuals on courtship/mating behaviours, we also carried out mating assays with male–female pairs and found that Or47b-ablated males show reduced courtship frequency compared with heterozygous controls (electronic supplementary material, figure S1).
Figure 1.
Ablation/silencing of Or47b-neurons affects copulation frequency. Copulation frequency profiles and copulation frequency at the end of 30 min of (a,b) ablated, (c,d) silenced (inverted triangle) and control flies [UASdti/+, UASdORKC1/+ (open circles), Or47b/+ (solid circles)]. Copulation frequency (percentage of flies copulating) is plotted along y-axis and time (min) is plotted along x-axis. Representative images for (e) Or47b, GFP, and (f) Or47b, GFP; dti brains along with magnified images of the highlighted region. Immunostaining against GFP shows projections of Or47b-neurons in the antennal lobes, which are markedly reduced in Or47b, GFP; dti. In controls, iso refers to iso31, used to backcross fly strains. Scale bars in the main figures (e,f) equal 50 µm and in the magnified images on the right equal 20 µm. Error bars in (a--d) represent standard error of the mean (SEM). (Online version in colour.)
(b). Or47b-neurons in males are necessary for maintaining normal copulation frequency
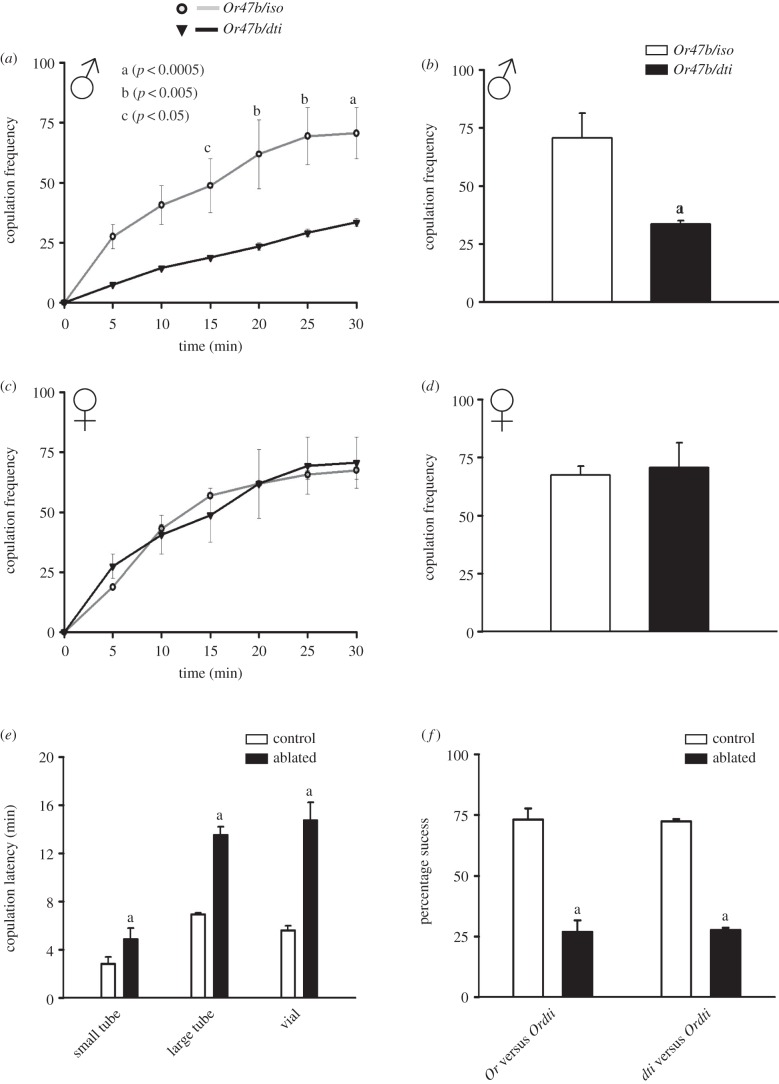
Copulation assay was done on: (i) Or47b-ablated (Ordti) males or intact (Or47b/+) males with CS females, (ii) CS males with ablated (Ordti) females or intact (Or47b/+) females. In (i), copulation frequency of the group with ablated males was significantly lower than the group with intact males (10 min: p < 0.05, 15 min: p < 0.01, 20 min: p < 0.005, 25/30 min: p < 0.0005; figure 2a,b). In (ii), we found no difference in copulation frequencies between the two groups (p > 0.05; figure 2c,d). These results suggest that Or47b-neurons in males rather than females are necessary for ensuring normal copulation frequency.
Figure 2.
Or47b-neurons are necessary in males for normal copulation and male-mating success. Copulation frequency profiles and copulation frequency at the end of 30 min, in assays where (a,b) Or47b-ablated or control males are allowed to mate with CS females, or (c,d) Or47b-ablated or control females (inverted triangle for ablated and circles for intact) are allowed to mate with CS males. (e) Copulation latency of Or47b-ablated (dark bars) and intact control males (light bars) when assayed in three differently sized arenas. The y-axis represents time (min) taken by flies to initiate copulation (copulation latency). (f) Mating success of Or47b-ablated (dark bars) and intact control males (light bars) in competition to secure mating with CS females. Or and dti code for intact controls Or47b/+ and dti/+, whereas Ordti codes for Or47b-ablated flies (Or47bGAL4/UASdti). All other details same as in figure 1.
(c). Or47b-neurons enable males to efficiently secure mating
Regardless of size of the arena, Or47b-ablated flies exhibited significantly higher copulation latency compared with intact controls (p < 0.05; figure 2e). With increasing size of the arena, which would be expected to lower odourant concentrations, Or47b-ablated males took progressively longer to secure matings, while copulation latency of controls remained unchanged.
We also assayed the ability of Or47b-ablated males to secure matings with CS females by allowing them to compete against intact controls. Ablated males secured only approximately 25% of the matings, significantly lower than both the controls (approx. 75%; p < 0.0001; figure 2f), suggesting that Or47b-ablated males are less competitive in securing mates than controls.
(d). Flies with loss-of-function mutation in Or47b receptors do not show defects in mating behaviour
The copulation frequency of flies carrying loss-of-function mutation in the Or47b receptors (Or47b2 and Or47b3) did not differ from that of heterozygous controls (Or47b2/+ and Or47b3/+), when assayed in groups or pairs (p > 0.05; electronic supplementary material, figure S2). This suggests that Or47b receptors may not be involved in the regulation of mating behaviour.
4. Discussion
Or47b-silenced/ablated flies display reduced copulation efficiency, which suggests the role of Or47b-neurons in mating behaviour. Copulation latency of Or47b-ablated flies increases proportionally with size of the assay arena whereas in controls it remains unchanged, which suggests that Or47b-neurons help males to track females in their vicinity efficiently. Or47b-ablated males were less efficient in securing mating when made to compete with intact controls, which further highlights the role of Or47b-neurons in mating behaviour.
Or47b-neurons express fruM, which is activated by both male and female odours [7], and they therefore are believed to play a crucial role in male courtship behaviour [6]. Of the three olfactory neurons, Or67d projects to DA1, Or47b to VA1v and IR84a to VL2a [6]. These three glomeruli are larger in males than females, which could be the reason behind their greater role in males than in females [6]. Furthermore, Or67d and Or47b genes show higher expression in males than females, suggesting their male-specific roles [17]. Previous studies suggest that these two receptors are involved in promoting male reproductive fitness-related behaviours including male–male aggression and male–female courtship [12,13,18]. Furthermore, Or47b along with Or88a receptors promote mating by responding to both male and female odours [7,13]. Although the ligand recognized by Or47b receptors is yet unknown, there is enough evidence suggesting its role in male courtship.
Although ablation of Or47b-neurons reduces male-mating success, males with loss-of-function mutation in Or47b receptors (Or47b2 and Or47b3) do not show such defects [13]. This discrepancy could be owing to basic differences in the way sensory receptors and receptor neurons function and their impact on intra- and inter-glomerular interactions. One possible reason for this discrepancy could be that the function of Or47b-neurons in courtship and mating may be mediated by receptors other than olfactory receptors, such as IRs expressed in these neurons [8]. Another possible reason could be the interactions between glomeruli and the balance between lateral inhibition and lateral excitation [19]. This is probably skewed in such a way that it affects the ability of ablated/silenced males to sense females, which remains unaltered in null males. For example, there exist differences in the synaptic inhibition of GABA-signalling, which is known to modulate olfactory responses [19]. Pheromone-sensing olfactory neurons have high levels of GABA receptors, deemed important for tracking mates [10,20]. Expression of GABABR2-RNAi in Or47b-neurons is found to cause an increase in post-synaptic firing frequency, which suggests pre-synaptic inhibition by GABABR2 receptors. As the above two mutations in the Or47b receptor do not interfere with the development of neuronal projections, they have negligible impact on the GABABR2-signalling in VA1v glomeruli, causing no defect in male-mating efficiency. However, silenced/ablated Or47b-neurons would probably result in the release of pre-synaptic inhibition of GABABR2, causing increased post-synaptic firing and altered inter-glomerular signalling. Interestingly, change in the size of VA1v glomeruli also has a measurable effect on mating behaviour [11], indicating the role of pre-synaptic inhibition. Taken together, our studies suggest that Or47b-neurons promote male-mating success by enhancing mating efficiency.
Supplementary Material
Acknowledgements
We thank Sheeba Vasu and two anonymous reviewers for suggesting improvements to the manuscript, Nisha, Pankaj, Antara, Rajanna and Muniraju for assistance during assays.
Data Accessibility
All data are included in the main figures and electronic supplementary material.
Authors' Contributions
S.R.L. conceived the idea, designed experiments and analysed data. S.R.L. performed experiments along with A.V., M.S. and S.P., V.K.S. supervised the project and provided laboratory space. S.R.L. and V.K.S. drafted the manuscript, and all authors contributed to the writing and approved the final version of the manuscript.
Competing Interests
Authors declare no competing interests.
Funding
We received no funding for this study.
References
- 1.Olsen SR, Wilson RI. 2008. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends NeuroSci. 31, 512–520. ( 10.1016/j.tins.2008.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villella A, Hall JC. 2008. Neurogenetics of courtship and mating in Drosophila. Adv. Gen. 62, 67–184. ( 10.1016/S0065-2660(08)00603-2) [DOI] [PubMed] [Google Scholar]
- 3.Manoli DS, Meissner GW, Baker BS. 2006. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends NeuroSci. 29, 444–451. ( 10.1016/j.tins.2006.06.006) [DOI] [PubMed] [Google Scholar]
- 4.Demir E, Dickson BJ. 2005. fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794. ( 10.1016/j.cell.2005.04.027) [DOI] [PubMed] [Google Scholar]
- 5.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. 2005. Male-specific fruitless specifies the neural substrates of Drosophila courtship behavior. Nature 436, 395–400. ( 10.1038/nature03859) [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AB, Berthelot-Grosjean M, Grosjean Y. 2013. The smell of love in Drosophila. Front. Physiol. 4, 72 ( 10.3389/fphys.2013.00072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Goes van Naters W, Carlson JR. 2007. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612. ( 10.1016/j.cub.2007.02.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162. ( 10.1016/j.cell.2008.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GS, Benton R. 2011. An olfactory receptor for food-derived odors promotes male courtship in Drosophila. Nature 478, 236–240. ( 10.1038/nature10428) [DOI] [PubMed] [Google Scholar]
- 10.Root CM, et al. 2008. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron 59, 311–321. ( 10.1016/j.neuron.2008.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayser MS, Yue Z, Sehgal A. 2014. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 344, 269–274. ( 10.1126/science.1250553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtovic A, Widmer A, Dickson BJ. 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546. ( 10.1038/nature05672) [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. 2011. Hierarchical chemosensory regulation of male–male social interactions in Drosophila. Nat. Neurosci. 14, 757–762. ( 10.1038/nn.2800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venken KJ, Simpson JH, Bellen HJ. 2011. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72, 202–230. ( 10.1016/j.neuron.2011.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryder E, et al. 2011. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167, 797–813. ( 10.1534/genetics.104.026658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lone SR, Sharma VK. 2012. Or47b receptor neurons mediate socio-sexual interactions in the fruit fly Drosophila melanogaster. J. Biol. Rhythms 27, 107–116. ( 10.1177/0748730411434384) [DOI] [PubMed] [Google Scholar]
- 17.Shiao MS, Fan WL, Fang S, Lu MJ, Kondo R, Li WH. 2013. Transcriptional profiling of adult Drosophila antennae by high-throughput sequencing. Zool. Stud. 52, 42 ( 10.1186/1810-522X-52-42) [DOI] [Google Scholar]
- 18.Wang L, Anderson DJ. 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231. ( 10.1038/nature08678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen SR, Wilson RI. 2008. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960. ( 10.1038/nature06864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. 2002. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463–474. ( 10.1016/S0896-6273(02)00975-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the main figures and electronic supplementary material.