Abstract
Two major types of intercellular communication are found in the central nervous system (CNS), namely wiring transmission (point-to-point communication, the prototype being synaptic transmission with axons and terminals) and volume transmission (VT; communication in the extracellular fluid and in the cerebrospinal fluid (CSF)) involving large numbers of cells in the CNS. Volume and synaptic transmission become integrated inter alia through the ability of their chemical signals to activate different types of receptor protomers in heteroreceptor complexes located synaptically or extrasynaptically in the plasma membrane. The demonstration of extracellular dopamine (DA) and serotonin (5-HT) fluorescence around the DA and 5-HT nerve cell bodies with the Falck–Hillarp formaldehyde fluorescence method after treatment with amphetamine and chlorimipramine, respectively, gave the first indications of the existence of VT in the brain, at least at the soma level. There exist different forms of VT. Early studies on VT only involved spread including diffusion and flow of soluble biological signals, especially transmitters and modulators, a communication called extrasynaptic (short distance) and long distance (paraaxonal and paravascular and CSF pathways) VT. Also, the extracellular vesicle type of VT was demonstrated. The exosomes (endosome-derived vesicles) appear to be the major vesicular carriers for VT but the larger microvesicles also participate. Both mainly originate at the soma–dendritic level. They can transfer lipids and proteins, including receptors, Rab GTPases, tetraspanins, cholesterol, sphingolipids and ceramide. Within them there are also subsets of mRNAs and non-coding regulatory microRNAs. At the soma–dendritic membrane, sets of dynamic postsynaptic heteroreceptor complexes (built up of different types of physically interacting receptors and proteins) involving inter alia G protein-coupled receptors including autoreceptors, ion channel receptors and receptor tyrosine kinases are hypothesized to be the molecular basis for learning and memory. At nerve terminals, the presynaptic heteroreceptor complexes are postulated to undergo plastic changes to maintain the pattern of multiple transmitter release reflecting the firing pattern to be learned by the heteroreceptor complexes in the postsynaptic membrane.
Keywords: wiring transmission, volume transmission, G protein-coupled receptors, heteroreceptor complexes, extracellular vesicles, exosomes
1. Introduction
In 1963–1965, the discovery of the brain-stem dopamine (DA), noradrenaline (NA) and serotonin (5-HT) neurons with long ascending and descending projections forming widespread monoamine terminal networks in the central nervous system (CNS) [1–4] and their responses to drugs [5,6] led to the introduction of the theory of volume transmission (VT) in 1986 [7,8]. In the presence of amphetamine, a strong diffuse extracellular greenish DA fluorescence was seen among the DA nerve cell bodies of the substantia nigra pars compacta and the ventral tegmental area, respectively [6]. In the nucleus, raphe dorsalis treatment with the 5-HT reuptake blocker cloimipramine led to the appearance of a strong diffuse yellowish extracellular 5-HT fluorescence in the area of the 5-HT nerve cell bodies [6,9]. The findings in the 5-HT dorsal raphe neurons were validated and beautifully extended by de-Miguel and colleagues in studies on the soma of Retzius neurons, where synaptic and extrasynaptic secretion of 5-HT were demonstrated [10,11]. Extrasynaptic exocytosis from small clear or large granular vesicles appeared to be in dominance (see also [11]). The large granular vesicles probably also released other types of signalling molecules like peptides and proteins.
The essence of VT is as follows: a widespread major intercellular communication that occurs in the extracellular fluid (ECF) and in the cerebrospinal fluid (CSF) of the CNS. VT signals move from source to target cells via energy gradients, mostly thermodynamic (temperature, pressure and concentration gradients), leading to diffusion and flow. The major difference with synaptic transmission is in the transmission channels, which are private in synaptic transmission (axons, terminals) but diffuse in VT represented by the channels of the extracellular space. Of course, the speed is also markedly different occurring within milliseconds for synaptic transmission and seconds-minutes-hours in VT [12–18].
In this review, we will characterize the heterogeneities of VT including the contribution of soluble VT signals and VT signals carried by extracellular vesicles (ECVs) [19], building on the pioneering work of Simons & Raposo [20]. Furthermore, the impact of VT in the trophic units of the grey matter built up of neurons, glial cells (astroglia, microglia), blood vessels with endothelial cells and pericytes, and extracellular matrix of the CNS [21,22] will be discussed. This term was used to indicate the smallest set of cells within the CNS which act in a complementary way to support the trophism of one another. Their VT signalling together with long distance and CSF VT signalling play a major role in modulating the synaptic transmission in the neural network formed by the neural component of the trophic units.
Finally, the theory is introduced that the synaptic and VT signals become integrated in sets of heteroreceptor complexes in the presynaptic and postsynaptic membranes and their signalling cascades [23]. The relevance of this transmission for learning and short- and long-term memory will be discussed [24]. A few examples will be given on how certain 5-HT1A and N-methyl-d-aspartate (NMDA) heteroreceptor complexes can play a role in depression and schizophrenia, respectively, and be targets for novel antidepressant and atypical antipsychotic drugs [25–27].
2. Comparison between soluble and extracellular vesicle-mediated volume transmission signals at the nerve terminal and soma level
(a). Nerve terminal level
(i). Soluble volume transmission signals
In the period following the discovery of VT only molecules dissolved in the ECF and in the CSF, like transmitters and modulators, e.g. gamma-aminobutyric acid (GABA), glutamate, monoamines, acetylcholine, adenosine, neuropeptides and proteins, including trophic factors, were discussed. GABA and glutamate terminals contributed to transmitter spillover originating from synaptic vesicles [28]. This process was regulated mainly by coverage of the synapse by astroglia processes containing, for example, astroglial glutamate transporters restricting extrasynaptic glutamate VT, as illustrated by increased glutamate VT after reduced astrocytic coverage of supraoptic neurons in lactation [29–31]. Thus, increased short distance (micro range) extrasynaptic VT of the classical synaptic transmitters glutamate and GABA develop with reductions in the astroglial barrier. The targets were both glial and neuronal cells with receptors and transporters for glutamate and GABA [16–18,28]. Recently, surface diffusion of astrocytic glutamate transporters were found to shape the kinetics of excitatory postsynaptic currents and thus synaptic transmission [32].
VT mediated by monoamines seems to depend on extrasynaptic release and transmitter spread to extrasynaptic receptors [11,14,15,18,33] since inter alia a large proportion of monoamine terminals lack postjunctional complexes [34,35]. Rice & Cragg [36] have modelled DA extrasynaptic transmission from terminals based on DA spillover after quantal release, considering a large number of experimental data from their own and other groups. In the updated DA synapse, if present since many DA terminals are asynaptic, the DA release is unconstrained by the extrasynapic DA transporter (DAT), the diffusion process being too fast for the DAT. The DA uptake mechanism increases clearance of DA and thus reduces the half-life of its extracellular effects. A gradient of DA is formed and DA can reach the DA receptors which are found extrasynaptically. There are relatively few synaptic DA receptors. Thus, the primary mode of DA communication is VT. The effective radius for high-affinity DA receptors based on diffusion of DA is calculated to be 7–8 µm.
The major mode of communication for neuropeptides is also VT. However, peptide nerve terminals also use long distance VT since neuropeptides often are not metabolized as rapidly as monoamines and can form active fragments that may reach distant neuron–glia networks. When monoamines and peptides are co-stored in terminals, the monoamine neurons communicate via neuropeptides with distant networks by long distance VT involving perivascular ECF channels reaching into the CSF and paraaxonal ECF channels along myelinated fibre bundles as discovered in studies of the diffusion of Texas-red dextran when microinjected into the striatum [37–40]. Striatally microinjected beta-endorphin reaches the CSF as an intact peptide [41]. The beta-endorphin immunoreactivity appears with a typical delay in the CSF taking place via perivascular channels and after extracellular diffusion over the leaky ependymal-CSF barrier along the ventricles [37,40,42]. This delay reflects the time it takes to migrate from the striatum to the CSF. So beta-endorphin may not only employ long distance diffusion in the ECF, but also be carried by the CSF-mediated VT.
(ii). Extracellular vesicle-mediated volume transmission signals
Recently, we have classified ECV-mediated intercellular communication in the CNS as a component of VT [19]. The idea of ECV-mediated VT is based on the significant work of Simons & Raposo [20]. The major type of ECV is the exosome (40–100 nm), which is released from all cell types of the CNS including endothelial cells into the ECF. Exosomes transfer their cargo of multiple molecules (mtDNA, mRNA, miRNA, proteins including receptors, lipids), some of which can exist in a single exosome, to other neuronal and glial cells. The cargo varies from one cell type to another and depends on the functional state of the cell (see also [43]). The exosomes can move via different patterns of cell adhesion molecules and phosphatidylserine to target other neuron–glia networks. The exosomes can be internalized and then release their cargo inside target cells to transiently modify the neurochemical phenotype of the target cell [19]. The exosomes mainly originate from endosomes and are produced when multivesicular bodies are formed. They are released into the ECF when the multivesicular bodies merge with the plasma membrane. The shedding vesicles are another type of ECVs, formed from the plasma membrane through shedding, and of unknown composition. The major form is the so-called microvesicle with a diameter of 100–1000 nm and an origin in most cell types of the CNS.
However, in both synaptic and asynaptic terminals the by far most dominant vesicle is the synaptic vesicle, releasing their transmitters through exocytosis, which in the case of synapses takes place through fusion with the presynaptic membrane. Clear or large granular vesicles may instead undergo exocytosis in the extrasynaptic membrane. Here the ECVs play only a minor role. However, at the soma level they play a significant role in all cell types (see below).
(b). Soma level
(i). Soluble volume transmission signals
The elegant and highly significant work of De-Miguel and colleagues [10,11,18,44] has demonstrated extrasynaptic exocytosis in neuronal soma as a relevant source of 5-HT VT. This leads to activation of 5-HT autoreceptors and is dependent on high-frequency activation, opening of L-type calcium channels and of movement of vesicles to the plasma membrane. In 5-HT immunoreactive neurons, exocytosis is sustained by a feedback loop dependent on 5-HT receptor activation causing an increase in intracellular calcium levels. The available evidence also supports the existence of synaptic exocytosis.
(ii). Extracellular vesicle-mediated volume transmission signals
Studies on cultured cortical nerve cells demonstrate that extracellular membrane vesicles (EMVs) can be released from nerve cells [45], and exosomes were proposed to be a new way of interneuronal communication [19,46–48]. In EMV fractions from the above cultures, GluR2 subunits of the AMPA receptors were found together inter alia with cell adhesion molecule L1 (LICAM). Therefore, it seems possible that AMPA receptor subunits are transferred to other neurons to modulate AMPA receptor-mediated glutamate signalling of acceptor nerve cells. Evidence for intercellular G protein-coupled receptor (GPCR) transfer at the protein and mRNA level has been demonstrated in HEK-293 cell cultures involving adenosine A2A and DA D2 receptors [46]. The ECV-mediated GPCR transfer resulted in the incorporation of functional receptors which also could produce and undergo A2A-D2 receptor heteromerization, as shown with photo-bleaching fluorescence resonance energy transfer. Thus, EMV receptor-mediated transfer among nerve cells may take place in the CNS probably involving mainly release of EMVs from soma, which have a significant capacity to release exosomes in view of their origin in endosomes–multivesicular bodies. The EMV release appears to represent a complimentary mechanism to synaptic and extrasynaptic exocytosis of transmitters at the soma level in view of their contents of proteins, including receptors, lipids, mRNA, miRNA and mtDNA. Their specificity, however, remains to be determined. The ECV-mediated VT increases neuronal plasticity but could also lead to dysfunction as a result of receptor transfer. This can produce pathological heteroreceptor complexes in the neurons, contributing to development of mental disease [23].
All the glial cells can also communicate via EMV release. Microglial cells are here of special interest in view of their role in neuroinflammation [49,50] which can elicit inter alia schizophrenia [51–53]. The transfer compounds are inter alia interleukin-1beta, caspase1 and P2X7 receptors (ionotropic purinoceptors for ATP). P2X7 stimulation induces release of interleukin-1beta from the ECVs into the ECF and spread of neuroinflammation in the CNS [54]. The microglial EMVs under inflammatory conditions also contain different sets of chemokine and cytokine receptor populations [55] which may be transferred to neurons and lead to malfunction of neurons due to their participation in, for example, NMDA heteroreceptor complexes [23]. The relative role of diffusion and flow of soluble peptides and proteins versus their transport in EMVs in VT remain to be determined.
3. Integration of synaptic and volume transmission in the trophic units, the building blocks of the central nervous system
The term trophic unit was used to indicate the smallest set of cells within the CNS that act in a complementary way to support the trophism of one another [21,33]. The CNS trophic unit consists of neurons, glial cells (mainly astroglia, microglia), blood vessels with endothelial cells and extracellular matrix. The theory of trophic units in the CNS is here extended to include the neuro-vascular unit (blood–brain barrier). This unit consists of endothelial cells, pericytes and astrocytic perivascular end feet which form the blood–brain barrier. The trophic unit is where the integration of the various forms of VT and wiring transmission (synapses and gap junctions) takes place mediating neuron–glia interactions. Both soluble VT signals and ECV-mediated VT signals participate.
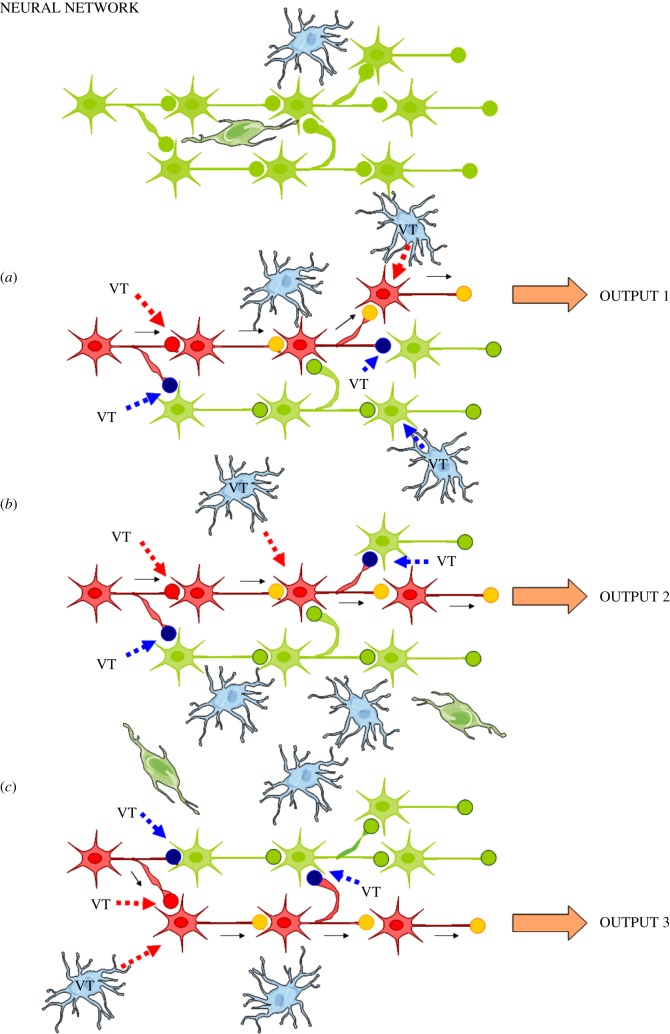
We propose that the trophic units are the building blocks of the CNS forming its neural–glial networks. The trophic units support their neuronal network component and significantly modulate its firing patterns and function (figure 1).
Figure 1.
Balancing and integration of VT and synaptic transmission through receptor–receptor interactions. Relevance for the control of brain circuits. Changes in the modulation by VT of synaptic transmission have a fundamental role in the control of brain circuits. The same neural network (upper panel, shown in green) can in this way give different outputs. As shown in this schematic, VT signals via up (in red) and down (in blue) regulation of the synaptic strength of discrete synapses of the glial–neural network can give rise to three types of outputs through changes in the integrative interactions of the VT and synaptic signalling of the network (a–c). The VT signals may significantly arise from glial cells of the local trophic unit, from extrasynaptic release of transmitters of neural afferents to the trophic unit, and from local collaterals and soma–dendrites of nerve cells of the trophic unit. The neural–glial network of trophic units may also be reached by long distance VT signals, e.g. peptides and proteins from CSF and/or surrounding networks. The major mechanism for the balance and integration of VT and synaptic transmission is likely the receptor–receptor interactions in synaptic and extrasynaptic heteroreceptor complexes located both at the prejunctional and postjunctional level and their signalling cascades. Red intermittent arrow, VT signal upregulating synaptic strength; blue intermittent arrow, VT signal downregulating synaptic strength; filled red circle, upregulated synapse; filled blue circle, downregulated synapse; filled yellow circle, active synapse; filled green circle, inactive synapse. The blue cells illustrate glial cells. Active glial cells providing VT signals are labelled VT. (Online version in colour.)
Immune cells also exist in the trophic units and use VT, including CSF VT, as demonstrated in the exciting work of Schwartz & Baruch [56,57] on inflammation-resolving leucocyte trafficking over the epithelial cells of the choroid plexus (CP) into the CSF. The trophic units are a major site in neuroinflammation. Activated microglia show phagocytic activity and release pro-inflammatory cytokine and chemokine soluble VT signals in the trophic units. They also produce ECVs which travel and flow in the ECF and may inter alia contain sets of chemokine and cytokine ligands as well as sets of chemokine and cytokine receptors which internalize into recipient microglia and nerve cells. This can lead to dysfunction of the affected neuronal networks of these trophic unit assemblies.
From these trophic units with inflammation, VT signals including ECVs can reach the CP via diffusion and flow through inter alia perivascular channels and CSF, and activate inter alia cytokine receptors on the CP epithelium. These epithelial cells have tight junctions (e.g. claudin-1,-2 and -5) regulated by protein kinase C [58]. Interleukin-1beta can also reach CSF through diffusion and flow over the leaky ventricular ependymal cells possessing only adherens junctions [42,59].
The activation of cytokine receptors on CP epithelial cells will initiate a trafficking cascade for T cells and monocytes into the CSF, where they become biased towards the anti-inflammation-suppressor phenotype [56,57]. They arrive at the inflamed trophic units of the brain via the Virchow–Robin spaces and the perivascular channels. The immune cells within the trophic units will exert their anti-inflammatory actions on the other cells of the trophic units via soluble or ECV-VT signals.
(a). The trophic units and the brain circuits
Changes in the modulation by VT of synaptic transmission in the assemblies of trophic units have a fundamental role in the control of brain circuits (figure 1). Thus, the neuronal network, built up of assemblies of trophic units which connect with each other, can produce different outputs dependent on the VT modulation of the synaptic transmission and of the intrinsic firing properties of neurons, which may transit from silent to tonic or bursting firing. As shown in figure 1, VT signals give rise to three types of outputs. This takes place through changes in the integrative interactions of the VT and synaptic signalling of the neuronal network.
(b). On the renewal and clearance of volume transmission signals of the trophic units
The clearance of VT signals in the extracellular space of the CNS was suggested to take place over the blood–brain barrier and the brain-CSF leaky barrier of the ependymal layer, as well as via catabolic enzymes of the extracellular matrix and transporter systems of the neuronal and glial cells [42]. New mechanisms for clearance of VT signals have been proposed. As mentioned, the brain uses pressure waves (oscillations in the CSF induced by the arterial pulse) [40], temperature gradients (rapid macro-gradients in the order of 1–3°C due e.g. to changes in cerebral blood flow and micro-gradients due to activation of brain uncoupling proteins) [60–62] and concentration gradients for diffusion and flow of signalling molecules. These clearance systems also allow renewal of the extracellular fluid, and thus the homeostasis of the brain internal milieu, while at the same making possible the renewal of VT signals at an energy cost much lower than classical synaptic transmission [40]. Nevertheless, other components of extrasynaptic VT have a high energy cost, since release from the soma and maybe dendrites occurs by active transport that uses ATP. Therefore, this may compensate for the low energy expenses of transmitter clearance in VT. In 2005, the ‘tide hypothesis’ of CSF VT-signal migration and clearance was introduced [40]. The arterial pulse and the ‘piston-like’ movement of the brain induced by the pulse cause cyclic pressure oscillations within the subarachnoidal space (SAS). The cyclic pressure oscillations within the SAS induce ‘tide’ movements of the CSF into the Virchow–Robin spaces, hence in the pericapillary spaces and, eventually, in the ECF channels of the trophic units. These fluid push–pull movements contribute to the long distance VT flow of signalling molecules along perivascular and paraaxonal channels [37,63]. In addition, it helps the maintenance of the internal milieu of the brain by increasing the flow of nutrients to and clearance of catabolites from the trophic units. Thus, these fluid push–pull movements induced by the arterial pulse favour both the migration of signalling molecules of VT and the extracellular fluid renewal, especially in the cerebral cortex [40].
In 2012 came the pioneering work of Nedergaard and colleagues with the discovery of the glymphatic system of the brain [64,65]. In this brain-wide pathway, CSF enters the brain along para-arterial routes, whereas ECF is cleared from the brain along paravenous routes. The astroglia possess aquaporin 4 channels especially in their perivascular end feet but also in other branches. This permits increased water flow from the pericapillary space by passing inside the astrocytes to reach the ECF of the trophic unit. This allows an efficient CSF influx from arterial paravascular CSF channels into the ECF, followed by an efficient efflux from the ECF into paravascular channels around the veins into the CSF. An increased understanding of the homeostasis of the internal milieu of the brain developed with the demonstration that astrocytes support water transport. Previously, through similar principles, astrocytes were shown to remove potassium ions from the ECF by having large numbers of potassium ion channels in the plasma membrane. The potassium ions passed into the astrocytic cytoplasm and via gap junctions into other astrocytes preventing the accumulation of extracellular potassium ions leading to depolarization and failure of neurons to propagate action potentials [66–68].
These efficient routes can also contribute to the renewal and clearance of the VT signals in the ECF of the trophic units together with the push–pull movements along the perivascular–pericapillary channels. It should be noted that pericytes on capillaries contribute to regulation of blood flow [69] and likely also flow along pericapillary channels. Interestingly, contractile signals can propagate from one pericyte to another, possibly spreading back to upstream arterioles and their perivascular channels [70].
Furthermore, a large increase in exchange of CSF with ECF was observed during sleep through an increase in ECF volume. This led to substantial increases in convective exchange and clearance of ECF [71]. Thus, sleep drives metabolite clearance. These observations may also suggest an increased clearance of VT signalling molecules from the ECF during sleep. The functional relevance of this phenomenon is not clear. One interpretation may be that signalling molecules of VT are fundamental for information handling in the awake state, and therefore an increased clearance of these molecules and their breakdown products mainly occurs in sleep.
4. Molecular integration of synaptic and volume transmission through heteroreceptor complexes in the plasma membrane
(a). On the existence of heteroreceptor complexes in the central nervous system
The receptor heteromer field started with the discovery of neuropeptide–monoamine receptor–receptor interactions in 1980–1981 as a way to understand integration of peptide and monoamine signals [72]. The concept of direct receptor–receptor interactions in receptor heteromers was born expanding the receptor field from indirect receptor crosstalk to direct interactions in receptor heterodimers and higher order receptor heteromers [15,73]. The term receptor heteromer covers both heterodimer and higher order heteromer. The term heteroreceptor complex is usually used when studying tissue, e.g. CNS, since the stoichiometry is unknown and adaptor proteins and other types of proteins (e.g. ion channels, transporters) are known to participate through direct interactions with the receptors [24,25,27,74–76].
The allosteric receptor–receptor interaction increases the repertoire of GPCR recognition (affinity, density), pharmacology, trafficking and signalling of the protomers [17,24,74,77–79]. Jeffery in 1999 [80,81] introduced moonlighting proteins as a class of multifunctional proteins in which a single polypeptide chain performs multiple functions not linked to splicing, posttranslational modifications, etc. Over 300 such proteins have been identified, including enzymes and receptors. Some moonlighting proteins change their function in response to a changing environment, while others can perform two functions at the same time.
This concept was introduced into the GPCR heteromer field to explain the marked increase in GPCR plasticity observed [82]. Thus, GPCR protomers can moonlight through the allosteric receptor–receptor interactions [83,84] through changes in recognition, G protein selectivity and signalling cascades with, among other things, switching from activating G proteins to beta-arrestin or calmodulin through conformational changes in single or several strands of amino acids of intracellular loops and the C-terminal tail [85,86]. It is hypothesized that GPCR protomer function may also change by becoming linked to receptor tyrosine kinase (RTK) signalling in GPCR-RTK heteroreceptor complexes and involved in trophic functions [22,27,87–91], and through formation of GPCR-ion channel heteroreceptor complexes like NMDA-D1 and D2-NR2B complexes, a field pioneered by Fang Liu and colleagues [92–94]. GPCRs may become capable of modulating neuronal excitability and firing patterns in a novel way through changes in NMDA receptor function [92,95–97].
The structure of the receptor interface (the border-zone between the two protomers) in heteromers is fundamental to understand the formation of heteromers and is a target for drug development. The receptor interface between two GPCRs can involve α-helix–helix interactions between transmembrane domains (e.g. [98]), and intracellular electrostatic interactions between intracellular loop 3 (IC3) of one protomer and the C-terminal tail of the other play a major role [99,100]. Often, positively charged arginins in the IC3 segment of one protomer interact with negatively charged residues in the C-terminal tail of the other, especially phosphorylated serine and glutamate residues.
Based on a mathematical approach, Tarakanov & Fuxe [101] have deduced, based on 48 pairs of receptors that do or do not form heterodimers, a set of triplet amino acid homologies that may be critically involved in receptor–receptor interactions and in forming the receptor heterodimer. We call it the triplet puzzle. Such amino acid triplet homologies may construct a code that helps determine which receptors form heterodimers. Triplet homologies may act as necessary guides for the heteromerization of the two receptors.
The origin of the triplet puzzle of homologies in receptor heteromers is phylogenetically old in view of the existence of integrin triplets in marine sponges and in human brain receptor heteromers [102]. Toll-like receptor triplets and immunoglobulin triplets belonging to phylogenetically old recognition systems are also found in different types of human receptor heteromers [103,104].
(b). The integration of synaptic and volume transmission signals
Such an integration can involve receptor—receptor interactions in heteroreceptor complexes in the pre- and postsynaptic membranes of neurons and in their signalling cascades, but also, extrasynaptic homoreceptor and heteroreceptor complexes may change the responsiveness of neurons without changing the synaptic responses [84]. The VT signals can produce allosteric receptor–receptor interactions in GPCR-NMDAR, GPCR-GABAAR and putative GPCR-kainate receptor (KAR) heteroreceptor complexes, which in many cases can be located extrasynaptically in dendrites and soma regions. Again the corresponding extrasynaptic and synaptic homoreceptor complexes participate and are in balance with the signalling of the heteroreceptor complexes. This can modulate the electrical activity of neurons in the circuit and also the strength of the synaptic transmission mediated by glutamate and GABAA receptor protomers in such complexes [92,93,95,105].
These allosteric receptor–receptor interactions generate a tremendous diversity and bias [84,106–109]. Biased signalling also gives selectivity. In our view, this novel principle in biology [24] supports the Neural Darwinism theory of Gerald M. Edelman, which builds on diversity and selection. The demand for diversity is in part provided by the dynamic formation of heteroreceptor complexes, each with multiple functions, through the allosteric receptor–receptor interactions. Thus, a novel diversity is provided for synaptic modifications in the brain circuits. The selection is provided by choosing out of all these signalling responses the optimal ones from multiple heteroreceptor complexes in the single neuron to enhance synaptic efficacy in the selected network.
(c). The fibroblast growth factor 1(FGFR1)-5-HT1A heteroreceptor complex in the raphe-hippocampal system as a novel target for treatment of depression
In 1967, 5-HT uptake was shown to occur in the cell bodies, axons and terminals of 5-HT neurons [110], and then together with Arvid Carlsson we studied the actions of classic antidepressant drugs on this 5-HT reuptake mechanism. This led to observations that the antidepressant drug imipramine can block 5-HT uptake that later led to the introduction of the concept of using serotonin selective reuptake inhibitors (SSRIs) in the treatment of depression [111].
Much later on, the 5-HT isoreceptor disbalance hypothesis of depression was introduced [112] based inter alia on our observations in 1977, for example that amitryptilin blocks head twitches correlating with its ability to displace d-LSD from its binding sites. However, amitryptilin did not displace 5-HT from its binding sites. The reviewers did not allow the suggestion that these findings may indicate the existence of two types of 5-HT receptors [113].
This suggested that depression may be produced by a disbalance in the activity of 5-HT receptor subtypes some of which contribute to the development of depression, like 5-HT2A and 5-HT7, while others, like 5-HT1A and 5-HT4, contribute to antidepressant actions [112].
During recent years, a number of 5-HT1A heteroreceptor complexes have been identified [27,88,90,114], the first one discovered being the galanin receptor1(GalR1)-5-HT1A heteromer. They are hypothesized to represent novel targets for antidepressant drugs; previously, only 5-HT1A had been considered as a target. For other new strategy developments based on targets other than monoamine transporters for treatment of depression, see Hamon & Blier [115].
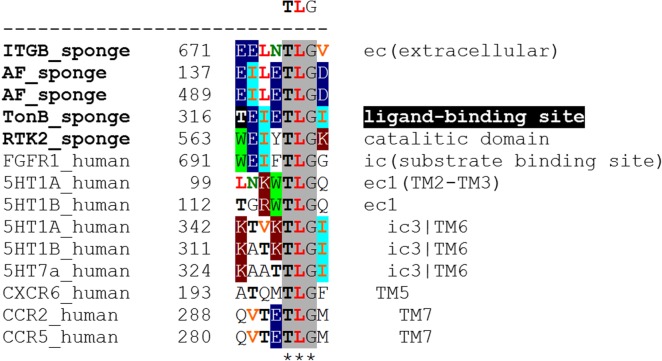
The most interesting heteroreceptor complex as a novel target for treatment of depression is the FGFR1-5-HT1A [27,90]. The potential receptor interface of the human heterodimer has been analyzed through bioinformatics (table 1 and figure 2). A protriplet TLG (Thr-Leu-Gly) amino acid homology was discovered in the intracellular domains of the two receptors which can participate in the mediation of the interaction. This homology was also found in the intracellular domains of an indicated FGFR1-5-HT1B heterodimer (table 1 and figure 2) [27] but was absent in two non-heteromers (FGFR1-5-HT2A, FGFR1-5-HT2C; table 1). The TLG homology was also present in a FGFR1-5-HT7 pair near the border between intracellular domain and TM6 (see ic3|TM6 in figure 2). Note that 5-HT1A and 5-HT1B also possess TLGs in extracellular domains (figure 2).
Table 1.
Examples of integrin triplets TLG, QVT and TVS of marine sponges in the protomers of human receptor heteromers. In particular, their participation as homologies in 5-HT1A receptor containing heteromers is shown. The bioinformatics analysis also indicates the existence of a couple of novel heteromers based on the TLG homology, the 5-HT1A-CXCR6 and 5-HT7-FGFR1 heteromers. Symbol ‘#’ denotes present in both receptors and may contribute to their direct interaction and ‘−’ no homologies found.
| receptor heteromer | references | TLG | QVT | TVS |
|---|---|---|---|---|
| ITGA-ITGB sponge | [116] | − | # | # |
| CCR2-CCR5 | [117] | # | # | − |
| 5HT1A-GalR1 | [118] | − | − | # |
| 5HT1A-5HT7 | [119] | # | − | − |
| 5HT1A-FGFR1 | [27,88,90] | # | − | − |
| 5HT1B-FGFR1 | [27] | # | − | − |
| 5HT1A-CXCR6 | possible heteromer | # | − | − |
| 5-HT7-FGFR1 | possible heteromer | # | − | − |
| 5HT2A-FGFR1 | possible non-heteromer | − | − | − |
| 5HT2C-FGFR1 | possible non-heteromer | − | − | − |
Figure 2.
Examples of the triplet TLG (Thr-Leu-Gly) which in TM6 and extracellular domains may mediate interactions between serotonin receptors 5-HT1A, 5HT1B and 5HT7 and in intracellular domains between FGFR1, 5-HT1A, 5-HT1B and 5-HT7. TLG is shown to be present in receptors of sponges and other sponge proteins and is therefore phylogenetically old. TonB, TonB-dependent receptors are a family of beta-barrel proteins from the outer membrane of Gram-negative bacteria; ITGB, integrin beta; RTK2, receptor tyrosine kinase 2; AF, aggregation factor proteins. (Online version in colour.)
Postjunctional FGFR1-5-HT1A heteroreceptor complexes in the hippocampus are mainly located in the pyramidal nerve cell layer of CA1–CA3 regions [27]. These complexes may exist both synaptically and extrasynaptically but the dominant form of 5-HT communication is VT [120]. Synergistic allosteric receptor–receptor interactions in this complex are seen upon coactivation with FGF2 and 5-HT1A agonists which increases hippocampal plasticity. This produces rapid and robust antidepressant actions in the rat [27]. It seems possible that the hippocampal atrophy found in major depression may be counteracted through coactivation of the hippocampal FGFR1-5-HT1A heteroreceptor complexes [27].
Furthermore, FGFR1-5-HT1A autoreceptor–heteroreceptor complexes exist in soma and dendrites of large numbers of midbrain 5-HT raphe cells [90]. In the view of the low to modest density of 5-HT terminals present in the midbrain raphe nuclei [121], the majority of these complexes are extrasynaptic and are reached by 5-HT through extrasynaptic exocytosis of 5-HT from the 5-HT cell bodies [11].
Synergistic allosteric receptor–receptor interactions were also observed upon coagonist activation of the above autoreceptor complex leading to increased 5-HT-induced nerve cell plasticity. Such events may contribute to acute antidepressant actions by recruiting 5-HT1A autoreceptors into complexes with FGFR1. It has previously been indicated that some heteroreceptor complexes can be formed in the plasma membrane instead of the endoplasmic reticulum [82,88]. This may reduce the 5-HT1A inhibitory autoreceptor function through its uncoupling from G protein-coupled inwardly rectifying potassium channels [122]. Instead, increased trophism can develop in midbrain raphe 5-HT neurons. Counteraction of atrophy in large numbers of ascending 5-HT neurons from the midbrain raphe can be a major event for long-term antidepressant actions.
The effects of SSRIs on these heteroreceptor complexes are being studied in animal models of depression. We suggest that D2 autoreceptor-FGFR1 and alpha2 adrenoreceptor autoreceptor-FGFR1 heteroreceptor complexes in DA and NA neurons, for example, may be targets for additional antidepressant effects.
(d). Formation of pathological NMDA and GABA heteroreceptor complexes in mild neuroinflammation as a mechanism for schizophrenia development
Current views on schizophrenia indicate the involvement of D2, 5-HT2A, NMDA and GABA A and B receptor subtypes [123]. In the view of the mild encephalitis hypothesis of schizophrenia [52,124], it may be postulated that changes in these receptor systems may develop in mild neuroinflammation. The mild encephalitis hypothesis describes a subgroup of schizophrenias with neuroinflammation as the core pathology.
The pioneering work of Kettenmann et al. [55] demonstrates dynamic changes in microglia in neuroinflammation including upregulation of chemokines and cytokines, cell adhesion molecules, extracellular matrix proteins, transcription factors and proteins of the major histocompatibility complex. The microglia have many types of receptors contributing to many different phenotypes. They communicate with neurons and astroglia via VT through soluble signals or ECVs like exosomes and shedding vesicles, which may contain receptors for transfer to other cells in the trophic units. Through increased release of cytokines and chemokines from microglia but also astroglia operating via VT (soluble or ECVs) they may also induce the expression of receptors for multiple chemokines and cytokines in neurons [49,55,56]. Complex and intense neuron–microglia and microglia–astroglia interactions thus develop in neuroinflammation through VT in the trophic units. In the pathological state, bidirectional signalling between neuron–microglia and astroglia–microglia involve cytokines and chemokines and their receptors [55].
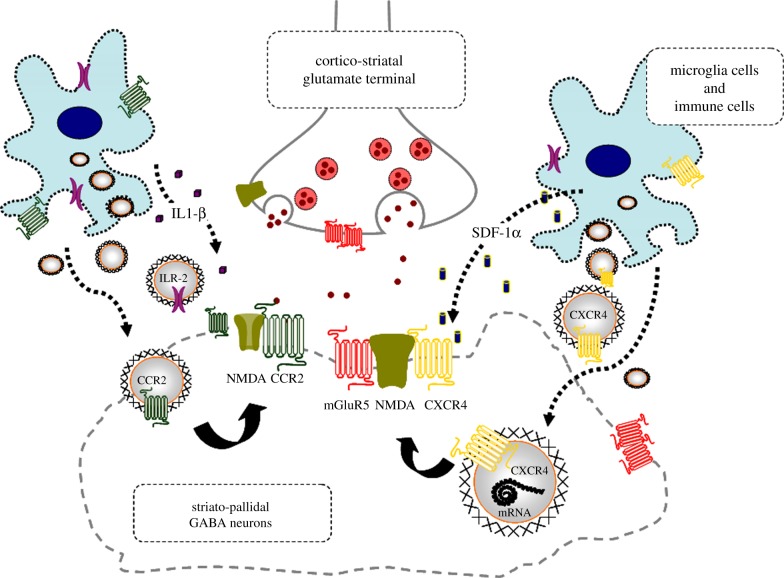
Glial and immune cell-derived ECVs like exosomes are released, which may contain chemokine and/or cytokine receptors and/or their mRNAs (ECV-mediated VT; figure 3) [23]. The ECVs may be internalized into neurons via cell adhesion receptors. Protriplet amino acid homologies ITL, SVS, VST, GLL, LYS and YSG are observed in CXCR4, CCR2 and IL1-R2 on one hand and in NMDA and/or GABAA and/or GABAB1 and B2 receptors on the other hand [23]. Therefore, according to the triplet puzzle theory [101], these chemokine and cytokine receptors may form heteroreceptor complexes with NMDA and GABA receptors upon internalization (figure 3) [23]. The most interesting protriplet is YSG, which is also the protriplet of human integrins.
Figure 3.
Illustration of the potential chemokine and cytokine receptor transfer via ECV-VT from immune and microglial cells to neurons with NMDA receptors in mild inflammation. One mechanism is shown for how chemokine receptors CCR2 and CXCR4 and cytokine receptor interleukin-1 receptor, type II (ILR-2) including their mRNAs can produce schizophrenia-like symptoms in neuroinflammation. These receptors may be transferred via ECV-mediated VT from immune cells, activated microglia and/or astroglia to nerve cells containing NMDA receptors. Upon internalization the receptors CXCR4, CCR2 and ILR-2 can, according to the triplet puzzle theory (figure 2), form complexes with NMDA receptors as illustrated here. Through the development of novel allosteric receptor–receptor interactions in such heteroreceptor complexes NMDA signalling may become pathologically reduced, contributing to schizophrenia-like symptoms. (Online version in colour.)
Through the allosteric receptor–receptor interactions in such pathological chemokine and cytokine receptor-containing heteroreceptor complexes, the neuronal NMDA, GABAA and GABAB1 and B2 protomers may develop dysfunctional activities with, for example, reduced NMDA ion channel signalling and reduced nerve cell activation. This may lead to disturbances in the neuronal networks and brain circuits they control. Such disturbances may contribute to positive, negative and/or cognitive symptoms of schizophrenia in line with the mild encephalitis hypothesis of schizophrenia [52]. As an example, the chemokineR-NMDAR and cytokineR-NMDAR complexes can have synaptic and/or extrasynaptic locations.
In figure 3, we illustrate the receptor transfer mechanism for how CCR2, CXCR4 and IL1-R2 including their mRNAs, which form these receptors not otherwise expressed in these nerve cells, may produce schizophrenia-like symptoms in neuroinflammation. These receptors may be transferred via ECV-mediated VT from immune cells and activated microglia to glutamate nerve cells containing NMDA receptors. Upon internalization, the CXCR4, CCR2 and IL1-R2 can, according to the triplet puzzle theory, form complexes with NMDA receptors. In this way, NMDA signalling in such heteroreceptor complexes may become reduced, contributing to schizophrenia-like symptoms.
5. Reorganization of homo- and heteroreceptor complexes in the postsynaptic membrane as a possible molecular basis for learning and memory
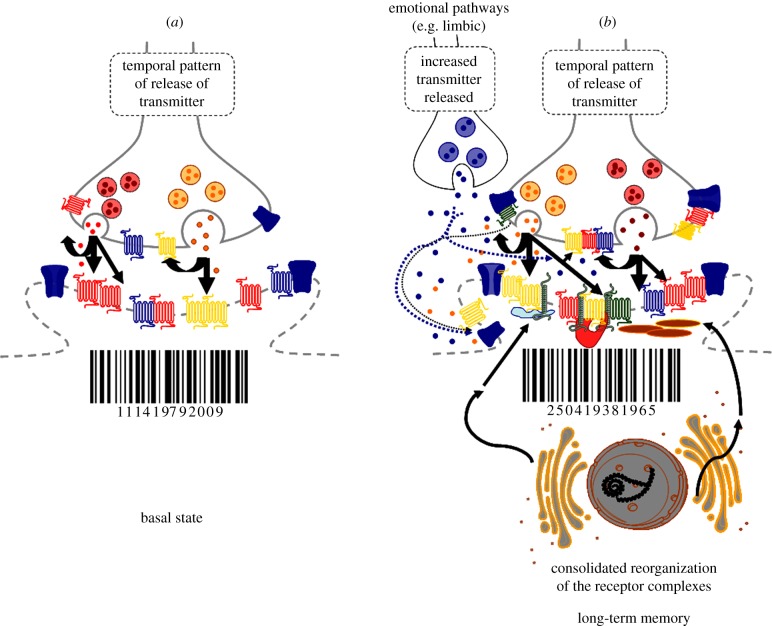
This theory was introduced this year [24]. It is illustrated in figure 4. The basal state with its postsynaptic receptor complexes leads to a defined bar code. The pattern of release is indicated (figure 4a). In learning, a new temporal pattern of release of transmitters is learned through a transient reorganization of sets of homo- and heteroreceptor complexes in postsynaptic and associated extrasynaptic membranes. This receptor reorganization leads to a novel bar code forming a short-term memory of the novel pattern of transmitter release to be learned. The novel pattern of release can be stabilized by the reorganization of the presynaptic hetero- and homoreceptor complexes through changing the formation or disappearance of the receptor complexes via agonist dependent processes (figure 4b). It may be produced by release from the postsynaptic membrane of soluble factors like adenosine and ATP, growth factors and ECVs like exosomes containing proteins (e.g. receptors), and lipids as a result of the new bar code of the postsynaptic membrane. The consolidated reorganization of the postjunctional homo- and heteroreceptor complexes produces the long-term memory (figure 4b). This may be made possible through the transformation of parts of the homo- and heteroreceptor complexes into unique transcription factors. They can then form novel adapter proteins some of which can link the heteroreceptor complexes more strongly together, while others can link the receptor complexes more strongly to the cytoskeleton. In this way, a long-term memory is created. In this process, emotional circuits and their transmitters are involved (figure 4b, upper left). They target their heteroreceptor complexes via VT, which participate in the postsynaptic membrane and adjacent extrasynaptic membrane and are postulated to play a key role in the creation of long-term memory of an exceptionally long duration.
Figure 4.
Illustration of a novel theory on the molecular basis of learning and memory based on the reorganization of homo- and heteroreceptor complexes in the postjunctional membrane of synapses (synaptic and extrasynaptic components) leading also to changes in the prejunctional (synaptic and extrasynaptic components) homo- and heteroreceptor complexes to facilitate the pattern of transmitter release to be learned. Learning is regarded to occur through changes in the synaptic efficacies via changes in synaptic strength. A molecular basis for learning and memory may develop through reorganization of the available homo- and heteroreceptor complexes structurally and/or by resetting the multiple allosteric receptor–receptor interactions in these complexes, as well as by the formation of novel heteroreceptor complexes via alterations in the pattern of synaptic and VT signals. Such multiple molecular changes in the heteromers and their receptor–receptor interactions may be the molecular basis for short-term memory, involving multiple changes in the receptor–protein architecture of the heteroreceptor complexes of the postsynaptic membrane as illustrated by the change of bar code. (a) The basal state with its postjunctional receptor complexes leading to a defined bar code. Two types of transmitters are indicated to be released from different pools of synaptic vesicles shown in red and orange. Short-term memory involves transient learning of a new temporal pattern of release of the two transmitters. This pattern is learned by the transient reorganization of the homo/heteroreceptor complexes into inter alia higher order heteroreceptor complexes with novel signalling features. This receptor reorganization leads to a novel bar code which can represent a short-term memory of the new pattern of transmitter release to be learned. (b) The consolidated reorganization of the postjunctional receptor complexes leading to long-term memory is illustrated. The consolidated memory should result in a bar code similar to one found in short-term memory. The mechanism for this process may involve the transformation of parts of the heteroreceptor complexes into unique transcription factors which can lead to the formation of novel adapter proteins some of which can link the heteroreceptor complexes more strongly together. Others can link the heteroreceptor complexes more strongly to the cytoskeleton. In this way, a stable bar code can be obtained. This is illustrated by the arrows from the rough endoplasmic reticulum onto the newly formed adapter proteins, which have become linked to different types of receptor complexes in the postjunctional membrane. GPCR heteroreceptor complexes with ion channels and receptor activity-modifying proteins are illustrated. The impact of VT signals including monoamines from terminals of emotional circuits is also outlined in (b) (dotted arrows). They are postulated to act especially on postjunctional receptor protomers with a significant ability to contribute to transcriptional activation, which leads to production of adapter proteins with a unique efficacy to consolidate long-term memory traces. (Online version in colour.)
Our theory has been discussed by Smythies [125] in relation to two other hypotheses on the molecular structure of long-term memory linked to electrical activity in the cytoskeleton [126,127] and chemical changes in the cytoskeleton [125].
6. Conclusion
The trophic units of nerve cells, glial cells and endothelial cells are the building blocks of the CNS. At the soma–dendritic level neurons may communicate via VT through both extrasynaptic exocytosis and ECV-mediated transfer of signals. Integration of synaptic and VT in the neuronal networks belonging to assemblies of trophic units is made possible through synaptic up- or downregulation. The molecular mechanism for this integration takes place to a significant degree through heteroreceptor complexes in the plasma membrane (synaptic and extrasynaptic parts) of neurons and probably glia and their signalling cascades. The FGFR1-5-HT1A heteroreceptor complex in the raphe-hippocampal system, likely to be in an extrasynaptic location, is regarded as a novel target for treatment of depression. Potential formation of pathological NMDAR and GABAR heteroreceptor complexes in neurons through ECV-mediated VT in mild neuroinflammation is considered as one mechanism for schizophrenia development. A theory is advanced that reorganization of sets of homo- and heteroreceptor complexes and their receptor–receptor interactions in the postjunctional membrane (synaptic and extrasynaptic components) can be the molecular basis for learning and long-term memory. They are suggested to store the bar code of patterns of synaptic and VT signals learnt after release from the synaptic terminal over the prejunctional (presynaptic and extrasynaptic) membrane and from adjacent asynaptic terminals of emotional systems.
Funding statement
This work has been supported by the Swedish Medical Research Council (62X-00715-50-3) and Telethon TV3′s La Marató Foundation (090131-1) to K.F. and by AFA Försäkring (130328) to D.O.B.-E. and K.F. D.O.B.-E. belongs to Academia de Biólogos Cubanos. A.O.T. has not received any support for this work.
References
- 1.Fuxe K. 1963. Cellular localization of monoamines in the median eminence and in the infundibular stem of some mammals. Acta Physiol. Scand. 58, 383–384. ( 10.1111/j.1748-1716.1963.tb02662.x) [DOI] [PubMed] [Google Scholar]
- 2.Fuxe K. 1965. Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol. Scand. Suppl. (Suppl. 247), 237+. [PubMed] [Google Scholar]
- 3.Fuxe K. 1965. Evidence for the existence of monoamine neurons in the central nervous system. 3. The monoamine nerve terminal. Z. Zellforsch. Mik. Ana. 65, 573–596. ( 10.1007/BF00337069) [DOI] [PubMed] [Google Scholar]
- 4.Dahlstroem A, Fuxe K. 1964. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. (Suppl. 232), 231–255. [PubMed] [Google Scholar]
- 5.Ungerstedt U, Butcher LL, Butcher SG, Anden NE, Fuxe K. 1969. Direct chemical stimulation of dopaminergic mechanisms in the neostriatum of the rat. Brain Res. 14, 461–471. ( 10.1016/0006-8993(69)90122-X) [DOI] [PubMed] [Google Scholar]
- 6.Fuxe K, Ungerstedt U. 1970. Histochemical, biochemical and functional studies on central monoamine neurons after acute and chronic amphetamine administration. In Amphetamines and related compounds (eds Costa E, Garattini S.), pp. 257–288. New York, NY: Raven Press. [Google Scholar]
- 7.Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. 1986. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol. Scand. 128, 201–207. ( 10.1111/j.1748-1716.1986.tb07967.x) [DOI] [PubMed] [Google Scholar]
- 8.Fuxe K, Agnati LF, Zoli M, Cintra A, Harfstrand A, von Euler G, Grimaldi R, Kalia M, Eneroth P. 1988. The opioid peptide systems: their organization and role in volume transmission and neuroendocrine regulation. In Regulatory roles of opioid peptides (eds Illes P, Farsang C.), pp. 33–68. Weinheim, Germany: VCH. [Google Scholar]
- 9.Fuxe K, Hokfelt T, Jonsson G, Ungerstedt U. 1970. Fluorescence microscopy in neuroanatomy. In Contemporary research methods in neuroanatomy (eds Nauta WJH, Ebbesson SOE.), pp. 275–314. Berlin, Germany: Springer. [Google Scholar]
- 10.De-Miguel FF, Trueta C. 2005. Synaptic and extrasynaptic secretion of serotonin. Cell. Mol. Neurobiol. 25, 297–312. ( 10.1007/s10571-005-3061-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trueta C, De-Miguel FF. 2012. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front. Physiol. 3, 319 ( 10.3389/fphys.2012.00319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuxe K, Agnati LF. 1991. Volume transmission in the brain, novel mechanisms for neural transmission. New York, NY: Raven Press. [Google Scholar]
- 13.Agnati LF, Fuxe K, Nicholson C, Sykova E. 2000. Volume transmission revisited. Amsterdam, The Netherlands: Elsevier Science BV. [Google Scholar]
- 14.Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. 2010. Understanding wiring and volume transmission. Brain Res. Rev. 64, 137–159. ( 10.1016/j.brainresrev.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 15.Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. 2010. The discovery of central monoamine neurons gave volume transmission to the wired brain. Progr. Neurobiol. 90, 82–100. ( 10.1016/j.pneurobio.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 16.Fuxe K, et al. 2012. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Front. Physiol. 3, 136 ( 10.3389/fphys.2012.00136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Diaz-Cabiale Z, Agnati LF. 2012. On the role of volume transmission and receptor–receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 1476, 119–131. ( 10.1016/j.brainres.2012.01.062) [DOI] [PubMed] [Google Scholar]
- 18.De-Miguel FF, Fuxe K. 2012. Extrasynaptic neurotransmission as a way of modulating neuronal functions. Front. Physiol. 3, 16 ( 10.3389/fphys.2012.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agnati LF, Fuxe K. 2014. Extracellular-vesicle type of volume transmission and tunnelling-nanotube type of wiring transmission add a new dimension to brain neuro–glial networks. Phil. Trans. R. Soc. B 369, 20130505 ( 10.1098/rstb.2013.0505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons M, Raposo G. 2009. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581. ( 10.1016/j.ceb.2009.03.007) [DOI] [PubMed] [Google Scholar]
- 21.Agnati LF, Cortelli P, Pettersson R, Fuxe K. 1995. The concept of trophic units in the central nervous system. Progr. Neurobiol. 46, 561–574. ( 10.1016/0301-0082(95)00017-P) [DOI] [PubMed] [Google Scholar]
- 22.Fuxe K, Marcellino D, Genedani S, Agnati LF. 2007. Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson's disease. Mov. Disord. 22, 1990–2017. ( 10.1002/mds.21440) [DOI] [PubMed] [Google Scholar]
- 23.Fuxe K, et al. 2013. Understanding the balance and integration of volume and synaptic transmission. Relevance for psychiatry. Fortschr. Neurol. Psyc. 19, 141–158. ( 10.1016/j.npbr.2013.10.002) [DOI] [Google Scholar]
- 24.Fuxe K, Borroto-Escuela DO, Ciruela F, Guidolin D, Agnati LF. 2014. Receptor–receptor interactions in heteroreceptor complexes: a new principle in biology. Focus on their role in learning and memory. Neurosci. Disc. 2, 6 ( 10.7243/2052-6946-2-6) [DOI] [Google Scholar]
- 25.Borroto-Escuela DO, Romero-Fernandez W, Narvaez M, Oflijan J, Agnati LF, Fuxe K. 2014. Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2–5-HT2A heteroreceptor complexes. Biochem. Biophys. Res. Commun. 443, 278–284. ( 10.1016/j.bbrc.2013.11.104) [DOI] [PubMed] [Google Scholar]
- 26.Fuxe K, Borroto-Escuela DO, Tarakanov AO, Romero-Fernandez W, Ferraro L, Tanganelli S, Perez-Alea M, Di Palma M, Agnati LF. 2014. Dopamine D2 heteroreceptor complexes and their receptor–receptor interactions in ventral striatum: novel targets for antipsychotic drugs. Progr. Brain Res. 211, 113–139. ( 10.1016/B978-0-444-63425-2.00005-2) [DOI] [PubMed] [Google Scholar]
- 27.Borroto-Escuela DO, et al. 2012. Fibroblast growth factor receptor 1–5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol. Psychiatry 71, 84–91. ( 10.1016/j.biopsych.2011.09.012) [DOI] [PubMed] [Google Scholar]
- 28.Del Arco A, Segovia G, Fuxe K, Mora F. 2003. Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J. Neurochem. 85, 23–33. ( 10.1046/j.1471-4159.2003.01692.x) [DOI] [PubMed] [Google Scholar]
- 29.Theodosis DT, Chapman DB, Montagnese C, Poulain DA, Morris JF. 1986. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience 17, 661–678. ( 10.1016/0306-4522(86)90038-2) [DOI] [PubMed] [Google Scholar]
- 30.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. 2004. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc. Natl Acad. Sci. USA 101, 2151–2155. ( 10.1073/pnas.0308408100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. 2008. Neuron–glia interactions in the rat supraoptic nucleus. Progr. Brain Res. 170, 109–117. ( 10.1016/S0079-6123(08)00410-X) [DOI] [PubMed] [Google Scholar]
- 32.Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. 2015. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat. Neurosci. 18, 219–226. ( 10.1038/nn.3901) [DOI] [PubMed] [Google Scholar]
- 33.Fuxe K, et al. 2007. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res. Rev. 55, 17–54. ( 10.1016/j.brainresrev.2007.02.009) [DOI] [PubMed] [Google Scholar]
- 34.Descarries L, Mechawar N. 2000. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Progr. Brain Res. 125, 27–47. ( 10.1016/S0079-6123(00)25005-X) [DOI] [PubMed] [Google Scholar]
- 35.Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G. 1996. Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J. Comp. Neurol. 375, 167–186. () [DOI] [PubMed] [Google Scholar]
- 36.Rice ME, Cragg SJ. 2008. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res. Rev. 58, 303–313. ( 10.1016/j.brainresrev.2008.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjelke B, England R, Nicholson C, Rice ME, Lindberg J, Zoli M, Agnati LF, Fuxe K. 1995. Long distance pathways of diffusion for dextran along fibre bundles in brain. Relevance for volume transmission. Neuroreport 6, 1005–1009. ( 10.1097/00001756-199505090-00014) [DOI] [PubMed] [Google Scholar]
- 38.Jansson A, et al. 1999. On the distribution patterns of D1, D2, tyrosine hydroxylase and dopamine transporter immunoreactivities in the ventral striatum of the rat. Neuroscience 89, 473–489. ( 10.1016/S0306-4522(98)00317-0) [DOI] [PubMed] [Google Scholar]
- 39.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Zhang WB, Agnati LF. 2013. Volume transmission and its different forms in the central nervous system. Chin. J. Integr. Med. 19, 323–329. ( 10.1007/s11655-013-1455-1) [DOI] [PubMed] [Google Scholar]
- 40.Agnati LF, Genedani S, Lenzi PL, Leo G, Mora F, Ferre S, Fuxe K. 2005. Energy gradients for the homeostatic control of brain ECF composition and for VT signal migration: introduction of the tide hypothesis. J. Neural Transm. 112, 45–63. ( 10.1007/s00702-004-0180-5) [DOI] [PubMed] [Google Scholar]
- 41.Hoistad M, Samskog J, Jacobsen KX, Olsson A, Hansson HA, Brodin E, Fuxe K. 2005. Detection of beta-endorphin in the cerebrospinal fluid after intrastriatal microinjection into the rat brain. Brain Res. 1041, 167–180. ( 10.1016/j.brainres.2005.02.014) [DOI] [PubMed] [Google Scholar]
- 42.Jansson A, Lippoldt A, Mazel T, Bartfai T, Ogren SO, Sykova E, Agnati LF, Fuxe K. 2000. Long distance signalling in volume transmission. Focus on clearance mechanisms. Progr. Brain Res. 125, 399–413. ( 10.1016/S0079-6123(00)25028-0) [DOI] [PubMed] [Google Scholar]
- 43.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. 2012. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64, 676–705. ( 10.1124/pr.112.005983) [DOI] [PubMed] [Google Scholar]
- 44.Leon-Pinzon C, Cercos MG, Noguez P, Trueta C, De-Miguel FF. 2014. Exocytosis of serotonin from the neuronal soma is sustained by a serotonin and calcium-dependent feedback loop. Front. Cell. Neurosci. 8, 169 ( 10.3389/fncel.2014.00169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faure J, et al. 2006. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648. ( 10.1016/j.mcn.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 46.Guescini M, et al. 2012. Microvesicle and tunneling nanotube mediated intercellular transfer of g-protein coupled receptors in cell cultures. Exp. Cell Res. 318, 603–613. ( 10.1016/j.yexcr.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 47.Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. 2013. Exosomes as a novel way of interneuronal communication. Biochem. Soc. Trans. 41, 241–244. ( 10.1042/BST20120266) [DOI] [PubMed] [Google Scholar]
- 48.Lachenal G, et al. 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 46, 409–418. ( 10.1016/j.mcn.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 49.Pocock JM, Kettenmann H. 2007. Neurotransmitter receptors on microglia. Trends Neurosci. 30, 527–535. ( 10.1016/j.tins.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 50.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. 2011. Physiology of microglia. Physiol. Rev. 91, 461–553. ( 10.1152/physrev.00011.2010) [DOI] [PubMed] [Google Scholar]
- 51.Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. 2010. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 44, 321–330. ( 10.1016/j.jpsychires.2009.08.008) [DOI] [PubMed] [Google Scholar]
- 52.Bechter K. 2013. Updating the mild encephalitis hypothesis of schizophrenia. Progr. Neuro-psychopharmacol. Biol. Psychiatry 42, 71–91. ( 10.1016/j.pnpbp.2012.06.019) [DOI] [PubMed] [Google Scholar]
- 53.Bechter K. 2013. [Schizophrenia—a mild encephalitis?]. Fortschr. Neurol. Psychiatr. 81, 250–259. ( 10.1055/s-0033-1335253) [DOI] [PubMed] [Google Scholar]
- 54.Prada I, Furlan R, Matteoli M, Verderio C. 2013. Classical and unconventional pathways of vesicular release in microglia. Glia 61, 1003–1017. ( 10.1002/glia.22497) [DOI] [PubMed] [Google Scholar]
- 55.Kettenmann H, Kirchhoff F, Verkhratsky A. 2013. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18. ( 10.1016/j.neuron.2012.12.023) [DOI] [PubMed] [Google Scholar]
- 56.Schwartz M, Baruch K. 2014. Breaking peripheral immune tolerance to CNS antigens in neurodegenerative diseases: boosting autoimmunity to fight-off chronic neuroinflammation. J. Autoimmunity 54C, 8–14. ( 10.1016/j.jaut.2014.08.002) [DOI] [PubMed] [Google Scholar]
- 57.Schwartz M, Baruch K. 2014. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 33, 7–22. ( 10.1002/embj.201386609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lippoldt A, Liebner S, Andbjer B, Kalbacher H, Wolburg H, Haller H, Fuxe K. 2000. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2 and -5 expression by protein kinase C. Neuroreport 11, 1427–1431. ( 10.1097/00001756-200005150-00015) [DOI] [PubMed] [Google Scholar]
- 59.Lippoldt A, Jansson A, Kniesel U, Andbjer B, Andersson A, Wolburg H, Fuxe K, Haller H. 2000. Phorbol ester induced changes in tight and adherens junctions in the choroid plexus epithelium and in the ependyma. Brain Res. 854, 197–206. ( 10.1016/S0006-8993(99)02355-0) [DOI] [PubMed] [Google Scholar]
- 60.Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. 1999. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J. Neurosci. 19, 10 417–10 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuxe K, Rivera A, Jacobsen KX, Hoistad M, Leo G, Horvath TL, Staines W, De la Calle A, Agnati LF. 2005. Dynamics of volume transmission in the brain. Focus on catecholamine and opioid peptide communication and the role of uncoupling protein 2. J. Neural Transm. 112, 65–76. ( 10.1007/s00702-004-0158-3) [DOI] [PubMed] [Google Scholar]
- 62.Wang H, et al. 2014. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front. Neurosci. 8, 307 ( 10.3389/fnins.2014.00307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. 1999. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 20, 142–150. ( 10.1016/S0165-6147(99)01343-7) [DOI] [PubMed] [Google Scholar]
- 64.Iliff JJ, et al. 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111 ( 10.1126/scitranslmed.3003748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nedergaard M. 2013. Neuroscience. Garbage truck of the brain. Science 340, 1529–1530. ( 10.1126/science.1240514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walz W, Hertz L. 1983. Comparison between fluxes of potassium and of chloride in astrocytes in primary cultures. Brain Res. 277, 321–328. ( 10.1016/0006-8993(83)90940-X) [DOI] [PubMed] [Google Scholar]
- 67.Walz W. 2000. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 36, 291–300. ( 10.1016/S0197-0186(99)00137-0) [DOI] [PubMed] [Google Scholar]
- 68.Kofuji P, Newman EA. 2004. Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056. ( 10.1016/j.neuroscience.2004.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peppiatt CM, Howarth C, Mobbs P, Attwell D. 2006. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704. ( 10.1038/nature05193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall CN, et al. 2014. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60. ( 10.1038/nature13165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie L, et al. 2013. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. ( 10.1126/science.1241224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuxe K, Agnati LF, Benfenati F, Celani M, Zini I, Zoli M, Mutt V. 1983. Evidence for the existence of receptor–receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J. Neural Transm. Suppl. 18, 165–179. [PubMed] [Google Scholar]
- 73.Zoli M, Agnati LF, Hedlund PB, Li XM, Ferre S, Fuxe K. 1993. Receptor–receptor interactions as an integrative mechanism in nerve cells. Mol. Neurobiol. 7, 293–334. ( 10.1007/BF02769180) [DOI] [PubMed] [Google Scholar]
- 74.Fuxe K, Agnati LF, Borroto-Escuela DO. 2014. The impact of receptor–receptor interactions in heteroreceptor complexes on brain plasticity. Expert Rev. Neurother. 14, 719–721. ( 10.1586/14737175.2014.922878) [DOI] [PubMed] [Google Scholar]
- 75.George SR, Kern A, Smith RG, Franco R. 2014. Dopamine receptor heteromeric complexes and their emerging functions. Progr. Brain Res. 211, 183–200. ( 10.1016/B978-0-444-63425-2.00008-8) [DOI] [PubMed] [Google Scholar]
- 76.Perreault ML, O'Dowd BF, George SR. 2014. Dopamine D(1)-D(2) receptor heteromer regulates signaling cascades involved in addiction: potential relevance to adolescent drug susceptibility. Dev. Neurosci. 36, 287–296. ( 10.1159/000360158) [DOI] [PubMed] [Google Scholar]
- 77.Fuxe K, et al. 2003. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson's disease. Neurology 61(Suppl. 6), S19–S23. ( 10.1212/01.WNL.0000095206.44418.5C) [DOI] [PubMed] [Google Scholar]
- 78.Agnati LF, Ferre S, Lluis C, Franco R, Fuxe K. 2003. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol. Rev. 55, 509–550. ( 10.1124/pr.55.3.2) [DOI] [PubMed] [Google Scholar]
- 79.Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernandez-Duenas V, Tanganelli S, Rivera A, Ciruela F, Agnati LF. 2010. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Therap. 16, e18–e42. ( 10.1111/j.1755-5949.2009.00126.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeffery CJ. 1999. Moonlighting proteins. Trends Biochem. Sci. 24, 8–11. ( 10.1016/S0968-0004(98)01335-8) [DOI] [PubMed] [Google Scholar]
- 81.Jeffery CJ. 2014. An introduction to protein moonlighting. Biochem. Soc. Trans. 42, 1679–1683. ( 10.1042/BST20140226) [DOI] [PubMed] [Google Scholar]
- 82.Borroto-Escuela DO, Tarakanov AO, Guidolin D, Ciruela F, Agnati LF, Fuxe K. 2011. Moonlighting characteristics of G protein-coupled receptors: focus on receptor heteromers and relevance for neurodegeneration. IUBMB Life 63, 463–472. ( 10.1002/iub.473) [DOI] [PubMed] [Google Scholar]
- 83.Fuxe K, et al. 2012. GPCR heteromers and their allosteric receptor–receptor interactions. Curr. Med. Chem. 19, 356–363. ( 10.2174/092986712803414259) [DOI] [PubMed] [Google Scholar]
- 84.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Palkovits M, Tarakanov AO, Ciruela F, Agnati LF. 2014. Moonlighting proteins and protein–protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology 39, 131–155. ( 10.1038/npp.2013.242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Ciruela F, Agnati LF, Fuxe K. 2011. On the existence of a possible A2A-D2-beta-Arrestin2 complex: A2A agonist modulation of D2 agonist-induced beta-arrestin2 recruitment. J. Mol. Biol. 406, 687–699. ( 10.1016/j.jmb.2011.01.022) [DOI] [PubMed] [Google Scholar]
- 86.Hasbi A, O'Dowd BF, George SR. 2011. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol. Brain 4, 26 ( 10.1186/1756-6606-4-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borroto-Escuela DO, Flajolet M, Agnati LF, Greengard P, Fuxe K. 2013. Bioluminescence resonance energy transfer methods to study g protein-coupled receptor–receptor tyrosine kinase heteroreceptor complexes. Methods Cell Biol. 117, 141–164. ( 10.1016/B978-0-12-408143-7.00008-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borroto-Escuela DO, Corrales F, Narvaez M, Oflijan J, Agnati LF, Palkovits M, Fuxe K. 2013. Dynamic modulation of FGFR1–5-HT1A heteroreceptor complexes. Agonist treatment enhances participation of FGFR1 and 5-HT1A homodimers and recruitment of beta-arrestin2. Biochem. Biophys. Res. Commun. 441, 387–392. ( 10.1016/j.bbrc.2013.10.067) [DOI] [PubMed] [Google Scholar]
- 89.Borroto-Escuela DO, Romero-Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K. 2013. G protein-coupled receptor heterodimerization in the brain. Methods Enzymol. 521, 281–294. ( 10.1016/B978-0-12-391862-8.00015-6) [DOI] [PubMed] [Google Scholar]
- 90.Borroto-Escuela DO, et al. 2015. Evidence for the existence of FGFR1–5-HT1A heteroreceptor complexes in the midbrain raphe 5-HT system. Biochem. Biophys. Res. Commun. 456, 489–493. ( 10.1016/j.bbrc.2014.11.112) [DOI] [PubMed] [Google Scholar]
- 91.Flajolet M, et al. 2008. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat. Neurosci. 11, 1402–1409. ( 10.1038/nn.2216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee FJ. et al. 2002. Dual regulation of NMDA receptor functions by direct protein–protein interactions with the dopamine D1 receptor. Cell 111, 219–230. ( 10.1016/S0092-8674(02)00962-5) [DOI] [PubMed] [Google Scholar]
- 93.Liu XY, et al. 2006. Modulation of D2R-NR2B interactions in response to cocaine. Neuron 52, 897–909. ( 10.1016/j.neuron.2006.10.011) [DOI] [PubMed] [Google Scholar]
- 94.Wang M, Wong AH, Liu F. 2012. Interactions between NMDA and dopamine receptors: a potential therapeutic target. Brain Res. 1476, 154–163. ( 10.1016/j.brainres.2012.03.029) [DOI] [PubMed] [Google Scholar]
- 95.Lee FJ, Wang YT, Liu F. 2005. Direct receptor cross-talk can mediate the modulation of excitatory and inhibitory neurotransmission by dopamine. J. Mol. Neurosci. 26, 245–252. ( 10.1385/JMN:26:2-3:245) [DOI] [PubMed] [Google Scholar]
- 96.Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F. 2005. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J. Neurosci. 25, 4385–4395. ( 10.1523/JNEUROSCI.5099-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nai Q, Li S, Wang SH, Liu J, Lee FJ, Frankland PW, Liu F. 2010. Uncoupling the D1-N-methyl-D-aspartate (NMDA) receptor complex promotes NMDA-dependent long-term potentiation and working memory. Biol. Psychiatry 67, 246–254. ( 10.1016/j.biopsych.2009.08.011) [DOI] [PubMed] [Google Scholar]
- 98.Borroto-Escuela DO, et al. 2010. Characterization of the A2AR-D2R interface: focus on the role of the C-terminal tail and the transmembrane helices. Biochem. Biophys. Res. Commun. 402, 801–807. ( 10.1016/j.bbrc.2010.10.122) [DOI] [PubMed] [Google Scholar]
- 99.Borroto-Escuela DO, Marcellino D, Narvaez M, Flajolet M, Heintz N, Agnati L, Ciruela F, Fuxe K. 2010. A serine point mutation in the adenosine A2AR C-terminal tail reduces receptor heteromerization and allosteric modulation of the dopamine D2R. Biochem. Biophys. Res. Commun. 394, 222–227. ( 10.1016/j.bbrc.2010.02.168) [DOI] [PubMed] [Google Scholar]
- 100.O'Dowd BF, Ji X, Nguyen T, George SR. 2012. Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation. Biochem. Biophys. Res. Commun. 417, 23–28. ( 10.1016/j.bbrc.2011.11.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tarakanov AO, Fuxe KG. 2010. Triplet puzzle: homologies of receptor heteromers. J. Mol. Neurosci. 41, 294–303. ( 10.1007/s12031-009-9313-5) [DOI] [PubMed] [Google Scholar]
- 102.Tarakanov AO, Fuxe KG, Borroto-Escuela DO. 2012. Integrin triplets of marine sponges in human brain receptor heteromers. J. Mol. Neurosci. 48, 154–160. ( 10.1007/s12031-012-9793-6) [DOI] [PubMed] [Google Scholar]
- 103.Tarakanov AO, Fuxe KG, Borroto-Escuela DO. 2012. On the origin of the triplet puzzle of homologies in receptor heteromers: immunoglobulin triplets in different types of receptors. J. Mol. Neurosci. 46, 616–621. ( 10.1007/s12031-011-9649-5) [DOI] [PubMed] [Google Scholar]
- 104.Tarakanov AO, Fuxe KG, Borroto-Escuela DO. 2012. On the origin of the triplet puzzle of homologies in receptor heteromers: Toll-like receptor triplets in different types of receptors. J. Neural Transm. 119, 517–523. ( 10.1007/s00702-011-0734-2) [DOI] [PubMed] [Google Scholar]
- 105.Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB. 2000. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature 403, 274–280. ( 10.1038/35002014) [DOI] [PubMed] [Google Scholar]
- 106.Kenakin T, Miller LJ. 2010. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 62, 265–304. ( 10.1124/pr.108.000992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maudsley S. 2012. G protein-coupled receptor biased agonism: development towards future selective therapeutics. Mini Rev. Med. Chem. 12, 803 ( 10.2174/138955712800959161) [DOI] [PubMed] [Google Scholar]
- 108.Maudsley S, Patel SA, Park SS, Luttrell LM, Martin B. 2012. Functional signaling biases in G protein-coupled receptors: game theory and receptor dynamics. Mini Rev. Med. Chem. 12, 831–840. ( 10.2174/138955712800959071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuxe K. et al. 2014. Diversity and bias through receptor–receptor interactions in GPCR heteroreceptor complexes. Focus on examples from dopamine D2 receptor heteromerization. Front. Endocrinol. 5, 71 ( 10.3389/fendo.2014.00071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuxe K, Ungerstedt U. 1967. Localization of 5-hydroxytryptamine uptake in rat brain after intraventricular injection. J. Pharmacy Pharmacol. 19, 335–337. ( 10.1111/j.2042-7158.1967.tb08097.x) [DOI] [PubMed] [Google Scholar]
- 111.Carlsson A, Fuxe K, Ungerstedt U. 1968. The effect of imipramine on central 5-hydroxytryptamine neurons. J. Pharmacy Pharmacol. 20, 150–151. ( 10.1111/j.2042-7158.1968.tb09706.x) [DOI] [PubMed] [Google Scholar]
- 112.Fuxe K, Hedlund P, Von Euler G, Lundgren K, Martire M, Ogren SO, Eneroth P, Agnati L. 1991. Galanin/5-HT interactions in the rat central nervous system. Relevance for depression. In Galanin: A new multifunctional peptide in the neuroendocrine system (eds Hokfelt T, Bartfai T, Jocobowitz DM, Ottoson D.), pp. 221–235. London, UK: MacMillan Press. [Google Scholar]
- 113.Fuxe K, Ogren SO, Agnati L, Gustafsson JA, Jonsson G. 1977. On the mechanism of action of the antidepressant drugs amitriptyline and nortriptyline. Evidence for 5-hydroxytryptamine receptor blocking activity. Neurosci. Lett. 6, 339–343. ( 10.1016/0304-3940(77)90095-7) [DOI] [PubMed] [Google Scholar]
- 114.Borroto-Escuela DO, Narvaez M, Marcellino D, Parrado C, Narvaez JA, Tarakanov AO, Agnati LF, Diaz-Cabiale Z, Fuxe K. 2010. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 393, 767–772. ( 10.1016/j.bbrc.2010.02.078) [DOI] [PubMed] [Google Scholar]
- 115.Hamon M, Blier P. 2013. Monoamine neurocircuitry in depression and strategies for new treatments. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 45, 54–63. ( 10.1016/j.pnpbp.2013.04.009) [DOI] [PubMed] [Google Scholar]
- 116.Pancer Z, Kruse M, Muller I, Muller WE. 1997. On the origin of Metazoan adhesion receptors: cloning of integrin alpha subunit from the sponge Geodia cydonium. Mol. Biol. Evol. 14, 391–398. ( 10.1093/oxfordjournals.molbev.a025775) [DOI] [PubMed] [Google Scholar]
- 117.El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. 2005. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol. Pharmacol. 67, 460–469. ( 10.1124/mol.104.003624) [DOI] [PubMed] [Google Scholar]
- 118.Fuxe K, et al. 2008. Receptor–receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Rev. 58, 415–452. ( 10.1016/j.brainresrev.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 119.Renner U, et al. 2012. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 125, 2486–2499. ( 10.1242/jcs.101337) [DOI] [PubMed] [Google Scholar]
- 120.Jansson A, Tinner B, Bancila M, Verge D, Steinbusch HW, Agnati LF, Fuxe K. 2001. Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2A receptor-immunoreactive neuronal processes in the rat forebrain. J. Chem. Neuroanat. 22, 185–203. ( 10.1016/S0891-0618(01)00133-8) [DOI] [PubMed] [Google Scholar]
- 121.Steinbusch HW. 1981. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6, 557–618. ( 10.1016/0306-4522(81)90146-9) [DOI] [PubMed] [Google Scholar]
- 122.Albert PR, Le Francois B, Millar AM. 2011. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol. Brain 4, 21 ( 10.1186/1756-6606-4-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuxe K, Marcellino D, Woods AS, Giuseppina L, Antonelli T, Ferraro L, Tanganelli S, Agnati LF. 2009. Integrated signaling in heterodimers and receptor mosaics of different types of GPCRs of the forebrain: relevance for schizophrenia. J. Neural Transm. 116, 923–939. ( 10.1007/s00702-008-0174-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bechter K. 2011. The peripheral cerebrospinal fluid outflow pathway—physiology and pathophysiology of CSF recirculation: a review and hypothesis. Fortschr. Neurol. Psychiatr. 17, 51–66. ( 10.1016/j.npbr.2011.06.003) [DOI] [Google Scholar]
- 125.Smythies J. 2015. Off the beaten track: the molecular structure of long-term memory: three novel hypotheses—electrical, chemical and anatomical (allosteric). Front. Integr. Neurosci. 9, 4 ( 10.3389/fnint.2015.00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Priel A, Tuszynski JA, Woolf NJ. 2010. Neural cytoskeleton capabilities for learning and memory. J. Biol. Phys. 36, 3–21. ( 10.1007/s10867-009-9153-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Plankar M, Brezan S, Jerman I. 2013. The principle of coherence in multi-level brain information processing. Progr. Biophys. Mol. Biol. 111, 8–29. ( 10.1016/j.pbiomolbio.2012.08.006) [DOI] [PubMed] [Google Scholar]