Abstract
The long-term replacement therapy with the dopamine (DA) precursor 3,4-dihydroxy-l-phenylalanine (L-DOPA) is a milestone in the treatment of Parkinson's disease (PD). Although this drug precursor can be metabolized into the active neurotransmitter DA throughout the brain, its therapeutic benefit is due to restoring extracellular DA levels within the dorsal striatum, which lacks endogenous DA as a consequence of the neurodegenerative process induced by the disease. In the early phases of PD, L-DOPA treatment is able to restore both long-term depression (LTD) and long-term potentiation (LTP), two major forms of corticostriatal synaptic plasticity that are altered by dopaminergic denervation. However, unlike physiological DA transmission, this therapeutic approach in the advanced phase of the disease leads to abnormal peaks of DA, non-synaptically released, which are supposed to trigger behavioural sensitization, namely L-DOPA-induced dyskinesia. This condition is characterized by a loss of synaptic depotentiation, an inability to reverse previously induced LTP. In the advanced stages of PD, L-DOPA can also induce non-motor fluctuations with cognitive dysfunction and neuropsychiatric symptoms such as compulsive behaviours and impulse control disorders. Although the mechanisms underlying the role of L-DOPA in both motor and behavioural symptoms are still incompletely understood, recent data from electrophysiological and imaging studies have increased our understanding of the function of the brain areas involved and of the mechanisms implicated in both therapeutic and adverse actions of L-DOPA in PD patients.
Keywords: synaptic plasticity, Parkinson's disease, animal models, Levodopa treatment
1. Introduction
Parkinson's disease (PD) is caused by the progressive degeneration of dopamine (DA) neurons in the substantia nigra pars compacta, resulting in a deficiency of DA in the striatum. PD patients develop a triad of motor symptoms: akinesia, rigidity and tremor. The aetiology of neuronal death in PD is still unclear. Several possible mechanisms of degeneration in dopaminergic neurons might occur in addition to genetic factors: formation of free radicals, oxidative stress, mitochondrial dysfunction, excitotoxicity, calcium-induced neurodegeneration, altered production of neurotrophic factors, inflammatory processes, environmental factors and toxic action of nitric oxide [1–3]. These multiple factors reciprocally interact inducing a vicious cycle of toxicity that causes altered synaptic plasticity, morphological changes and finally cell death.
The current therapeutic approaches only alleviate the clinical symptoms, but they cannot cure the disease by changing the natural course of this disorder. In fact, until now, no therapy has been developed to stop or at least slow down the neurodegeneration occurring in dopaminergic neurons in PD patients.
Levodopa (3,4-dihydroxy-l-phenylalanine, L-DOPA) is a precursor of DA and it represents the first and most successful breakthrough in the symptomatic treatment of PD. In fact, DA replacement therapy with L-DOPA is still the gold standard for symptomatic treatment of PD. However, patients usually experience severe side effects after several years of L-DOPA treatment, such as the L-DOPA-induced dyskinesias (LIDs) [4,5].
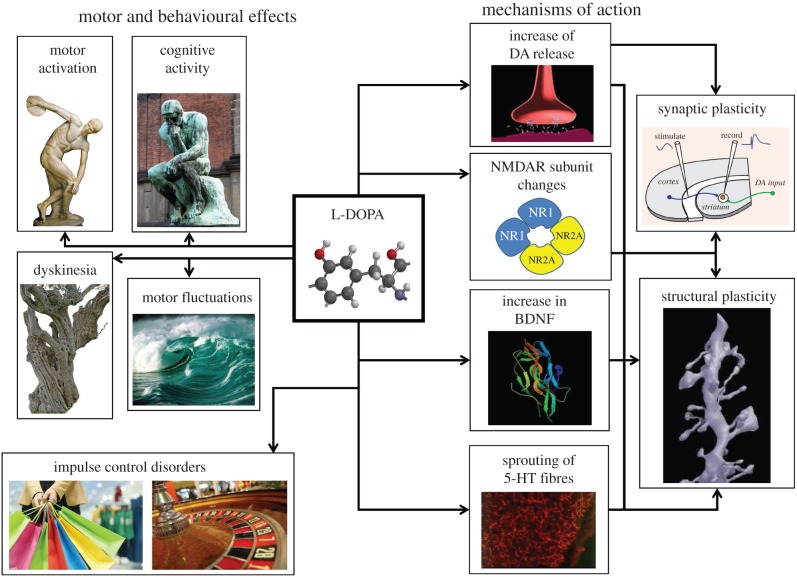
Although L-DOPA treatment is more than 50 years old, the exact mechanism of action of L-DOPA is still controversial [6]. In fact, it has been reported in preclinical studies that while acute treatment with L-DOPA might not be able to restore motor activity, chronic treatment is more effective in reducing parkinsonian symptoms and in restoring striatal synaptic plasticity [7]. A possible explanation for this difference is that long-term L-DOPA treatment is able to restore physiological synaptic plasticity in the DA-denervated striatum because it is able to ensure a tonic dopaminergic level that is not reached following an acute treatment. It has also been proposed that chronic treatment might stimulate the production of trophic factors able to compensate for the structural alterations of dendritic spines induced by DA denervation [8,9]. Chronic L-DOPA treatment, however, might also cause, by itself, aberrant structural plasticity in the dendrites and the spines of striatal medium spiny neurons causing further functional short- and long-term alterations of glutamatergic and dopaminergic transmission [10,11] (figure 1).
Figure 1.
Schematic representation of the possible mechanisms of action, the motor and behavioural effects induced by a long-term treatment with L-DOPA in PD patients.
2. Possible mechanisms of adverse effects
L-DOPA also seems to act through non-canonical modes of action besides its well-known effect as a DA precursor. These effects could involve not only the striatum, considered the established target area for the actions of DA, but also other nuclei of the basal ganglia circuits. For example, it has been recently reported that L-DOPA has dual effects on nigral dopaminergic neurons [12]. An ‘early’ effect is associated with a membrane depolarization and is reduced by an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist. A ‘late’ phase of the excitation is not mediated by glutamate receptors but is sensitive to carbidopa, a dopa-decarboxylase inhibitor, demonstrating its dependence on the conversion of L-DOPA to DA. These dual excitatory effects are associated with an intracellular calcium increase and they might influence the survival of the dopaminergic neurons as well as the release of DA from the residual axon terminals.
An increasing number of findings support the view that, in the advanced phase of PD and in the presence of a massive degeneration of nigrostriatal terminals, DA is released as a false neurotransmitter from the striatal serotonin terminals and this might act as a critical presynaptic factor underlying LIDs [13]. Experimental data from animal models and human clinical studies support this view, which indicates the serotonin system as a promising target for anti-dyskinetic therapy in PD patients under L-DOPA medication. Accordingly, it has been reported that L-DOPA treatment induces sprouting of serotonin axon terminals, with an increased incidence of synaptic contacts, and a larger activity-dependent potentiation of DA release in the denervated striatum [14]. It is interesting to note that the authors hypothesize that an increased activity of brain-derived neurotrophic factor (BDNF) could be involved in this sprouting. Accordingly, an increase in mRNA for BDNF has been previously reported following repeated L-DOPA administrations [15].
According to this hypothesis, serotonin terminals, which lack an efficient reuptake machinery to control synaptically released DA, can contribute to abnormal pulsatile stimulation of DA receptors that causes aberrant responses to long-term L-DOPA treatment. In this regard, L-DOPA-induced plasticity of the striatal serotonin innervation might represent an important factor underlying abnormal presynaptic DA dynamics contributing to the development of LIDs.
In addition to the multiple therapeutic motor effects of L-DOPA, possible toxic effects induced by this drug have to be taken into account. These effects might induce maladaptive plasticity in the striatum and in other brain structures (figure 1 and table 1).
Table 1.
Forms of brain plasticity modulated by L-DOPA in experimental models of PD and PD patients. Deep brain stimulation, DBS; high-frequency stimulation, HFS; low-frequency stimulation, LFS; long-term depression, LTD; long-term potentiation, LTP; paired-associative stimulation, PAS; spike timing-dependent plasticity, STDP; transcranial direct current stimulation, tDCS; transcranial magnetic stimulation, TMS; internal globus pallidus, Gpi; intraperitoneal, i.p.
| brain area | type of plasticity | effect | methods | route of administration | experimental/clinical conditions | references |
|---|---|---|---|---|---|---|
| dorsal striatum | LTD | recovery | ex vivo, intracellular recordings with sharp electrodes, HFS, LFS | subchronic and chronic administration, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, non-dyskinetic rats | [7,16] |
| LTD | recovery | ex vivo, sharp electrodes and whole-cell patch clamp recordings, HFS | chronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, dyskinetic rats | [17] | |
| depotentiation | loss | ex vivo, intracellular recordings with sharp electrodes, HFS, LFS | chronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, dyskinetic rats | [16] | |
| LTD (striatopallidal) | becomes LTP | in vivo extracellular recordings, HFS | chronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, dyskinetic rats | [18] | |
| LTD (striatonigral) | loss of reversibility | in vivo extracellular recordings, HFS, LFS | chronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, dyskinetic rats | [18] | |
| bidirectional plasticity | recovery | ex vivo, whole-cell patch clamp recordings, STDP protocol | subchronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, non-rats | [19] | |
| bidirectional plasticity | segregated | ex vivo, whole-cell patch clamp recordings, STDP protocol | chronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, dyskinetic rats | [19] | |
| hippocampus, dentate gyrus | LTD | recovery | ex vivo, extracellular and whole-cell patch clamp recordings, LFS | subchronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, non-dyskinetic rats | [20] |
| hippocampus, CA1 | LTD | recovery | ex vivo, extracellular and whole-cell patch clamp recordings, HFS | subchronic, systemic (i.p. injection) | unilateral 6-OHDA-induced lesion, parkinsonian transgenic mice, non-dyskinetic rats | [21] |
| substantia nigra/GPi | LTP | recovery | in vivo, DBS | chronic, systemic, oral | PD patients | [22] |
| depotentiation | loss | in vivo, DBS | chronic, systemic, oral | PD patients, dyskinetic | [23] | |
| cortex | LTD | recovery | tDCS and TMS | prolonged, systemic, oral | PD patients | [24] |
| LTP and LTD | none | TMS | acute, systemic, oral | de novo PD patients | [25] | |
| LTP | recovery | PAS | prolonged, systemic, oral | PD patients, non-dyskinetic | [26] | |
| LTP and depotentiation | recovery | TMS | prolonged, systemic, oral, full dose | PD patients, non-dyskinetic | [27] | |
| LTP | no recovery | TMS | prolonged, systemic, oral, half dose | PD patients, non-dyskinetic | [27] | |
| LTP | recovery | TMS | prolonged, systemic, oral, half dose | PD patients, dyskinetic | [27] |
3. Non-motor effects
(a). Cognition
The influence of L-DOPA on the cognitive and behavioural status of PD patients has gained increasing attention. It is now widely recognized that in PD patients, cognitive impairment and psychiatric symptoms cause a great worsening in the quality of life of the patients and their carers [28,29].
Original studies contributed to the concept of subcortical dementia associated with bradyphrenia and cognitive rigidity resulting from basal ganglia dysfunction in PD [30]. However, it is now clear that cognitive deficits in PD might be caused not only by fronto-executive dysfunctions, but also by functional deficits in anatomical structures other than basal ganglia, such as the hippocampus [31]. Moreover, it is now clear that not only dopaminergic dysregulation, appearing as deficits in flexibility, planning, working memory and reinforcement learning, but also non-dopaminergic, and in particular cholinergic cortical dysfunction, might cause mild cognitive impairment and dementia in PD. Thus, DA and acetylcholine might interact in a critical manner in the regulation of cognitive function resulting in diverse clinical phenotypes [32]. Accordingly, recent clinical, neuropathological, imaging and genetic studies have revealed a great heterogeneity in the features of cognitive deficits in PD patients [33–35].
An important clinical issue is represented by the influence that L-DOPA exerts on cognitive function in PD patients. Despite the fact that L-DOPA improves motor function in PD patients, this DA precursor can ameliorate cognition in some patients while worsening this function in others. To explain this apparent discrepancy, some authors [36] have postulated that dopaminergic medications, and in particular L-DOPA, impair the activity of those neural structures receiving dopaminergic innervations, with less denervation than the striatum, and alter the behavioural outcomes depending on these structures. According to this hypothesis, supported by imaging studies, the regional topography of DA denervation becomes a critical factor. Functions such as reversal learning and memory of motor sequences might be significantly affected by L-DOPA. In particular, patients can express either positive or negative cognitive responses to L-DOPA according to the mesolimbic and prefrontal cortical dopaminergic status and to the specific gene polymorphisms [36].
A heterogeneous cognitive response to L-DOPA can also be predicted according to the ‘dual syndrome’ hypothesis for cognitive deficits in PD. This hypothesis postulates that while an executive syndrome results from the frontostriatal dysfunction and is caused by dopaminergic deficits, a posterior cortical syndrome involves visuospatial, mnemonic and semantic functions and correlates with Lewy body pathology and cholinergic loss [37].
(b). Impulse control disorders
Another spectrum of non-motor disorders observed in PD and possibly related to dopaminergic treatments is represented by the impulse control disorders, a complex variety of psychiatric manifestations such as compulsive eating, pathological gambling, hypersexuality and compulsive buying [38]. Patients treated with dopaminergic medications may also develop repetitive, purposeless behaviours known as punding [39,40]. These disabling non-motor symptoms appear with frequencies of 13–35% among patients receiving DA replacement therapy [41]. Although impulse control disorders in PD are strongly associated with the use of DA agonists, these disturbances can be observed following L-DOPA treatment as well as in naive PD patients [28]. Individual susceptibility and synaptic alterations progressively observed are also critical factors for the development of impulse control disorders. In particular, differences between nigrostriatal and mesolimbic dopaminergic denervation, coupled to non-physiological pulsatile administration of dopaminergic drugs may induce abnormal ‘hyperstimulation’ of the mesolimbic system. This ‘hyper-dopaminergic state’ alters reward-learning behaviours in PD patients. In addition, L-DOPA can increase impulsivity of PD patients during decision-making and favour risk-taking behaviours [42,43]. Abnormal DA intake might also correlate with an excessive seeking of rewards, a condition closely mimicking drug-seeking behaviour observed in drug addiction [44].
4. Dorsal striatum
(a). Evoked and spontaneous excitatory glutamatergic synaptic transmission
Unilateral 6-hydroxy-dopamine (6-OHDA)-induced lesion of the substantia nigra is commonly used as an experimental model for PD. The activity of striatal neurons from parkinsonian rats has been recorded intracellularly in an ex vivo slice preparation [45]. Intracellular recordings were obtained at different time points after the denervation. In DA-denervated slices, unlike naive slices, most of the neurons showed spontaneous depolarizing postsynaptic potentials characterized as glutamate-mediated synaptic events. The percentage of cells showing increased spontaneous depolarizing postsynaptic potentials was maximal at four months after the denervation and was not associated with changes in intrinsic membrane properties such as resting membrane potential or input resistance. Thus, it was proposed that abnormal excitability of striatal neurons in this model of PD was not caused by changes of the intrinsic membrane properties, but was the result of increased glutamatergic cortical inputs to the striatum. Using this model of PD, it has also been shown that a dose of L-DOPA able to reverse motor deficits in about half of the parkinsonian animals, reversed glutamatergic overactivity and hypersensitivity of presynaptic D2 DA receptors controlling glutamate release from corticostriatal terminals [46]. Surprisingly, no change was detected in the sensitivity of presynaptic D2 DA receptors modulating striatal γ-amino butyric acid (GABA) transmission in both parkinsonian and L-DOPA-treated rats. These findings indicate that the reversal of striatal glutamatergic overactivity and the normalization of hypersensitive D2 DA receptors modulating excitatory transmission might underlie some of the therapeutic actions of L-DOPA in PD.
An interesting field of research has been represented by the characterization of the effects of stereotaxic neurosurgery to reverse the motor symptoms of PD and ameliorate LIDs. The subthalamic nucleus is a target of choice for the neurosurgical treatment of PD. The therapeutic effect of subthalamic lesion in PD is classically ascribed to the rescue of physiological activity in the output structures of the basal ganglia and a consequent reduction in the L-DOPA equivalent dose necessary to exert a therapeutic action.
Stimulation of the subthalamic nucleus also improves the majority of non-motor symptoms, such as mood, impulse control disorders, sleep and some autonomic dysfunctions. Nevertheless, little is known about the possible adaptive changes of the striatal medium spiny neurons following this procedure in comparison with L-DOPA treatment. Interestingly, it has been reported that the beneficial motor effects produced in parkinsonian rats by subthalamic lesion or L-DOPA therapy are paralleled by the normalization of the overactive frequency and amplitude of striatal glutamate-mediated spontaneous excitatory postsynaptic currents. Thus, the reversal of these abnormalities in striatal excitatory synaptic transmission can be attributable to the normalization of glutamate release [47].
Another promising field of investigation is the link between glutamatergic synaptic transmission, L-DOPA and the endocannabinoid system. This system is highly expressed at different levels in the basal ganglia where it bidirectionally interacts with the dopaminergic and glutamatergic signalling systems. In particular, at synapses linking cortical and striatal neurons, endocannabinoids modulate synaptic transmission [48,49]. In the 6-OHDA model of PD, striatal levels of the endocannabinoid anandamide are increased, while the activity of its membrane transporter and hydrolase (fatty-acid amide hydrolase) are reduced [50]. Interestingly, both L-DOPA treatment and the pharmacological inhibition of fatty-acid amide hydrolase were able to reverse the anomalies in the endocannabinoid system. These biochemical changes were paralleled by a normalization of glutamatergic activity suggesting that inhibition of fatty-acid amide hydrolase, in association with L-DOPA, might represent a possible strategy to decrease the abnormal cortical glutamatergic activity observed in PD [50].
(b). Long-term depression
Among the various mechanisms of action that have been postulated to underlie the beneficial motor and cognitive effects of L-DOPA is the restoration of physiological forms of synaptic plasticity such as long-term depression (LTD), long-term potentiation (LTP) and synaptic depotentiation in different brain areas (figure 2). In fact, cognition and memory are generally believed to involve the adjustment of synaptic strength in networks of connected neurons. Accordingly, LTD and LTP are processes whereby synaptic strength is rapidly decreased or increased, respectively, and they are currently the best candidates as cellular substrates of cognition and memory. Similarly, maladaptive synaptic plasticity might mediate the adverse motor effects such as LIDs and wearing-off as well as non-motor symptoms such as cognitive dysfunction and impulse control disorders observed following long-term L-DOPA treatment [51] (figure 2). Moreover, different brain areas can be implicated in specific therapeutic and adverse events induced by this drug.
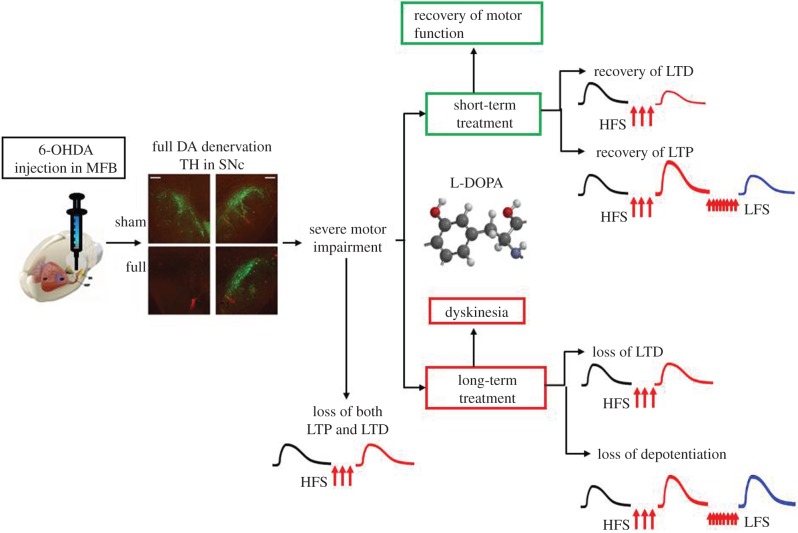
Figure 2.
Scheme of the experimental procedure to investigate L-DOPA-dependent striatal synaptic plasticity in a rat model of PD induced by unilateral DA denervation by 6-OHDA. The unilateral injection of 6-OHDA in the medial forebrain bundle (MFB) causes a full DA denervation in the ipsilateral substantia nigra pars compacta (SNc) as detected by the staining for tyrosine hydroxylase (TH). This full denervation induces severe motor impairment and loss of both LTD and LTP induced by high-frequency stimulation (HFS) protocols. Short-term treatment with L-DOPA (3 days) allows the improvement of motor function as well as the recovery of both LTP and LTD. Conversely, a long-term treatment with L-DOPA (21 days) induces dyskinesia in the majority of the rats. Diskinetic animals show a loss of both LTD after HFS and depotentiation (reversal of LTP) after a low-frequency stimulation (LFS) protocol. For further experimental details, see Picconi et al. [16,17].
The dorsal striatum is a major station of the basal ganglia circuit receiving dopaminergic inputs from the substantia nigra and excitatory glutamatergic afferents from the cortex and the thalamus [52]. The cortico-basal ganglia-cortical circuit functions as a complex, integrated network with multiple feedback and feed-forward loops. The inputs from motor cortical areas send a somatotopically organized, glutamatergic signal to the striatal GABAergic medium spiny neurons. The medium spiny neurons are connected to the output nuclei either indirectly, through the external globus pallidus and the subthalamic nucleus, or directly through striatonigral projections. The internal globus pallidus and the substantia nigra pars reticulata are the output nuclei of the basal ganglia and project to the thalamic nuclei, which project to the striatum and back to the cortex.
Postsynaptically, DA regulates the excitability of striatal D2-bearing and D1-bearing neurons [53]. DA also regulates the plasticity of striatal neurons by modulating glutamate-mediated LTD, LTP and synaptic depotentiation [54,55]. This latter form of synaptic plasticity represents a homeostatic mechanism that returns a potentiated synapse to its pre-potentiated state [16]. LTD and LTP are important for motor learning, while depotentiation is thought to be necessary for removing unnecessary motor information [16]. The activation of D1 receptors is necessary for induction of both LTP and depotentiation, while the co-activation of D1 and D2 receptors is required for induction of LTD [56].
An activity-dependent and N-methyl-d-aspartate (NMDA)-independent form of LTD was originally described in medium spiny neurons recorded from striatal slices following high-frequency stimulation (HFS) of glutamatergic afferents [57,58]. Interestingly, a critical involvement of striatal cholinergic interneurons in this form of synaptic plasticity observed in medium spiny neurons was also demonstrated [59]. While these studies suggested that LTD was expressed in the large majority of medium spiny neurons, another study has proposed that this form of synaptic plasticity is only expressed in the medium spiny neurons of the indirect pathway [60]. In a more recent investigation, an immunohistochemical analysis combined with whole-cell recordings to identify direct and indirect medium spiny neurons pathways, demonstrated the presence of a DA-dependent LTD in both direct and indirect pathways. This suggests that differences in experimental settings might account for the apparent discrepancies among the results obtained by different research groups [61].
The hypothesis that an altered cell-type-specific induction of plasticity can be expressed in distinct striatal neuronal subtypes as an effect of L-DOPA treatment has been investigated as a possible cellular basis for LIDs by a study conducted using in vivo extracellular recordings [18].
Electrophysiological studies associated with pharmacological analyses have demonstrated a critical role of the nitric oxide/cyclic guanosin monophosphate (GMP) pathway in the induction of corticostriatal LTD [62]. Experiments have been made to target striatal phosphodiesterases and thereby regulate the intracellular levels of cyclic GMP to reduce LIDs [17]. Behavioural measurements of LIDs were performed before and after the treatment with two phosphodiesterase inhibitors, zaprinast and UK-343664. LIDs were associated with the loss of LTD expression at glutamatergic striatal synapses in medium spiny neurons. Both zaprinast and UK-343664 rescued this form of synaptic plasticity via the modulation of intracellular cyclic GMP levels. The rescue of LTD was associated with a decrease of LIDs following intrastriatal injection of phosphodiesterase inhibitors [17]. This analysis reveals the need for future studies to investigate the possible therapeutic effects of phosphodiesterase inhibitors in non-human primate models of PD.
Repetitive transcranial magnetic stimulation in humans increases DA levels in the proximity of active corticostriatal terminals [63,64], suggesting its use to alleviate PD symptoms. A single-session of cortical repetitive transcranial magnetic stimulation using intermittent theta-burst pattern rescued LTD of glutamate-mediated field excitatory postsynaptic potentials recorded from hemiparkinsonian rats [65]. These findings show that cortical intermittent theta-burst stimulation affects neuronal activity of subcortical regions and suggest that this technique, in conjunction with L-DOPA treatment, might concur to ameliorate PD symptoms.
(c). Long-term potentiation
HFS of glutamatergic afferents to striatal medium spiny neurons induces an NMDA-dependent LTP when these glutamate receptors are active [66]. This form of synaptic plasticity is also DA-dependent, as it is blocked by unilateral 6-OHDA-induced lesion on the medial forebrain bundle [67] as well as by D1 receptor antagonism [68]. The requirement of a rise in intracellular calcium [69] and the concomitant activation of both glutamate and dopaminergic inputs [70] for the induction of striatal LTP was further supported by in vivo electrophysiological studies.
While the complete depletion of striatal DA, mimicking advanced stages of the disease, results in the loss of LTP and LTD, a partial denervation, causing mild motor deficits, selectively affects the NMDA-dependent LTP, but not the LTD. Moreover, partial and full dopaminergic lesions alter the NMDA receptor subunit composition in the postsynaptic density in different manners, indicating that decreasing the striatal DA levels has distinct effects on corticostriatal synaptic plasticity depending on the level of depletion [71].
DA is not only released in the striatum, but also from ventrally projecting dendrites of the substantia nigra pars compacta on the substantia nigra pars reticulata, a major output structure of the basal ganglia. The synaptic plasticity in the substantia nigra pars reticulata using field-evoked potentials induced with a nearby microelectrode has been analysed in PD patients undergoing implantation of deep brain stimulation electrodes in the subthalamic nucleus [22]. Tetanic stimulation in the substantia nigra pars reticulata failed to induce a lasting change in test field-evoked potential amplitudes in PD patients OFF medication. However, after oral L-DOPA administration, HFS was able to induce a potentiation of the field-evoked potentials amplitudes. This clinical finding clearly confirms that L-DOPA, by increasing the basal ganglia DA tone, modulates the activity-dependent synaptic plasticity inducing LTP-like synaptic changes.
(d). Depotentiation (reversal of long-term potentiation)
Various manipulations can reverse LTP when applied shortly after its induction. This kind of reversal of synaptic strength from the potentiated state to pre-LTP levels is termed synaptic depotentiation. Depotentiation of HFS-induced LTP in striatal medium spiny neurons is effectively induced by low-frequency stimulation of afferent corticostriatal fibres [16]. The mechanisms responsible for this phenomenon have not been fully characterized. Nevertheless, NMDA receptor activation, the increases in intracellular calcium concentrations and altered states of protein kinases and phosphatases seem to play a role. Importantly, this phenomenon has been implicated in the mechanisms of ‘forgetting’ unessential information [72].
Thus, depotentiation can be considered a homeostatic synaptic downscaling operating as a negative feedback response to an abnormally increased network activity to reduce the firing rate of neurons. This form of synaptic plasticity decreases the strength of individual synapses. Moreover, synaptic downscaling might counteract the possible run-away excitation due to Hebbian-type LTP and maintain an appropriate degree of synaptic efficacy linked to memory information [73].
Control and L-DOPA-treated non-dyskinetic rats express synaptic depotentiation in response to a subsequent low-frequency stimulation protocol, while dyskinetic rats do not. The depotentiation observed in both L-DOPA-treated non-dyskinetic rats and intact animals is prevented by activation of the D1-like DA receptors as well as by inhibition of protein phosphatases. The striata of dyskinetic rats contain abnormally high levels of phospho[Thr34]-DARPP-32, an inhibitor of protein phosphatase 1. These findings show that the development of LIDs is associated with abnormal information storage in corticostriatal synapses [16].
Another factor influencing the induction and expression of LIDs is the molecular composition of NMDA receptor subunits in striatal medium spiny neurons. Molecular studies have shown that dyskinetic rats have significantly higher levels of GluN2A subunit in the postsynaptic compartment than naive parkinsonian rats or parkinsonian rats showing therapeutic benefit following L-DOPA treatment. Moreover, the GluN2B subunit is significantly reduced in the postsynaptic density of DA-denervated and dyskinetic rats [74]. These molecular alterations are associated with changes of NMDA receptor GluN2B subunit association with scaffolding elements, i.e. members of the membrane-associated guanylate kinase (MAGUK) protein family, such as postsynaptic density-95, synapse-associated protein-97 and synapse-associated protein-102. Interestingly, treatment of non-dyskinetic animals with a cell-permeable synthetic peptide (TAT2B), able to affect GluN2B binding to MAGUK proteins, as well as synaptic localization of this subunit, induces a shift in treated rats towards a dyskinetic phenotype. This finding indicates abnormal GluN2B redistribution between synaptic and extrasynaptic membranes as an important molecular disturbance of the glutamatergic synapse involved in dyskinesia.
In line with this hypothesis, the manipulation of the composition of synaptic NMDA receptors by using a cell-permeable peptide targeting the GluN2A subunit during the induction of LIDs reduces the percentage of parkinsonian rats developing LIDs [75].
Another issue in the characterization of the mechanisms underlying the occurrence of LIDs is the comparison of the molecular and motor effects of L-DOPA with those of D2 receptor agonists. These drugs are usually preferred to L-DOPA in the early phases of the disease for their lower risk of developing dyskinesia [76]. However, D2 receptor agonists can also be used in the advanced stages of PD, in conjunction with reduced doses of L-DOPA, to delay the expression of the motor complications. Electrophysiological experiments and behavioural analysis have compared the effects of L-DOPA to those of pramipexole, a D2 receptor agonist widely used in PD therapy [76]. These experiments have shown that the striatal NMDA/AMPA receptor ratio and the AMPA receptor subunit composition are altered in experimental parkinsonism. Surprisingly, while L-DOPA in dyskinetic subjects fails to restore these critical synaptic alterations, chronic treatment with pramipexole, a D2-agonist, is associated not only with a reduced risk of dyskinesia development but also with a dose-dependent rebalance of the synaptic properties. However, high-dose pramipexole fails to rescue the physiological NMDAR/AMPAR ratio and, similarly to L-DOPA, induces dyskinesia.
The Ras-extracellular signal-regulated kinase (Ras-ERK) pathway, a signal transduction cascade implicated in behavioural plasticity, regulates synaptic activity in striatal medium spiny neurons and it can be considered as an important transduction signal downstream of the D1 receptor [77]. A recent molecular and electrophysiological study has reported that the Ras-ERK pathway is not only essential for activity-dependent striatal LTP, but also for the synaptic depotentiation. Ablation of Ras protein-specific guanine nucleotide-releasing factor 1 (Ras-GRF1), a neuronal activator of Ras proteins, causes a specific loss of LTP in the medium spiny neurons in the direct pathway without affecting LTP in the indirect pathway. Analysis of LTP in 6-OHDA-lesioned animals showing LIDs revealed a complex Ras-GRF1 and pathway-independent, apparently stochastic involvement of ERK [77].
Another molecular study has identified several hundred genes modulated in a similar model of LIDs. The expression of these genes correlated with the dose of L-DOPA and many of them are under the control of activator protein-1 and ERK signalling [78]. The authors propose that, although homeostatic adaptations involve several signalling modulators, activator protein-1-dependent gene expression is significantly altered especially in the medium spiny neurons of the direct pathway following chronic L-DOPA treatment.
The paradigm of spike-timing-dependent plasticity has been recently applied to the study of PD models. In this type of protocol, the order and the precise temporal interval between pre- and postsynaptic spikes determine the sign and magnitude of LTP or LTD. Spike-timing-dependent plasticity is widely used in models of circuit-level plasticity, development and learning [79].
Using this model in brain slices from DA receptor transgenic mice, it has been reported that DA plays distinct and complementary roles in medium spiny neurons of the direct and indirect pathways to ensure synaptic plasticity in a bidirectional and Hebbian manner [80]. Interestingly, in models of PD, this system is placed out of balance, leading to unidirectional changes in plasticity that might cause network dysregulation and motor symptoms.
The molecular mechanisms of LIDs were also investigated using spike-timing-dependent plasticity protocols applied to corticostriatal synapses in slices from 6-OHDA-lesioned mouse models of parkinsonism and LIDs. These disease models were generated in transgenic mice in which transfection with a bacterial artificial chromosome allows the expression of the enhanced Green Fluorescent Protein selectively on the direct or the indirect output pathways [19]. In control mice, bidirectional synaptic plasticity (LTD and LTP) was induced, resulting in a significant change in the amplitude of excitatory postsynaptic potentials in each direction in both striatal output pathways. In parkinsonism and LIDs, both pathways exhibited only unidirectional plasticity, irrespective of stimulation paradigm. In fact, in parkinsonian animals, a symptomatic dose of L-DOPA restored bidirectional plasticity on both pathways to levels comparable to control animals. In dyskinetic animals, in the presence of L-DOPA, the indirect pathway exhibited only LTD, whereas in the direct pathway, only LTP could be induced. Thus, this study further confirms the concept that while normal motor control requires bidirectional plasticity of both striatal outputs, LIDs are caused by a switch from bidirectional to unidirectional plasticity.
Another study of Surmeier's group suggests that changes of spike-timing-dependent plasticity in the PD state and in LIDs are cell-type specific. In fact, the intrinsic excitability and corticostriatal synaptic connectivity of medium spiny neurons of the indirect pathway are lower in PD models than in the healthy condition. Conversely, these properties in medium spiny neurons of the direct pathway are elevated in tissues from PD models and suppressed in LIDs models [81].
The concept of a distinct pattern of abnormal bidirectional synaptic plasticity as a functional marker of PD and LIDs has been recently confirmed in PD patients undergoing deep brain stimulation [23]. The authors investigated whether low-frequency stimulation of the internal globus pallidus and the substantia nigra pars reticulata could induce depotentiation at synapses that had already undergone LTP. They measured the field-evoked potentials induced by stimulation from a nearby microelectrode in patients undergoing implantation of deep brain stimulation electrodes in the subthalamic nucleus and internal globus pallidus. According to studies in experimental models, synapses of internal pallidus and substantia nigra pars reticolata in patients with less severe LIDs underwent greater depotentiation following low-frequency stimulation than in patients with more severe dyskinesia. This demonstration of impaired depotentiation in basal ganglia output nuclei in PD patients with dyskinesia is an important validation of the animal models of LIDs.
5. Ventral striatum
A brain area to be investigated to understand the cognitive effects of L-DOPA in PD patients is the ventral striatum (nucleus accumbens). This structure plays an important role in the cognitive processing of motivation, pleasure, reward, and reinforcement learning in physiological conditions [82]. The ventral striatum is also extensively involved in the neural mechanisms of conditions [83]. Moreover, it has been implicated in the development of emotions like fear and impulsivity [84] as well as in the placebo effect [85].
The ventral striatum receives DA innervation mainly from ventral tegmental area dopaminergic neurons. Studies using functional magnetic resonance imaging (fMRI) in humans have shown that some healthy old adults have an abnormal signature of expected value, resulting in an incomplete reward prediction error signal in the ventral striatum. Moreover, structural connectivity between ventral tegmental area and accumbens, measured by diffusion tensor imaging, is associated to inter-individual differences in the expression of this expected reward value signal in the ventral striatum. Interestingly, L-DOPA augments the task-based learning rate and task performance in old adults to the level of young adults. This pharmacological effect is linked to the recovery of a normal neural reward prediction error suggesting that in old adults there is a neurochemical failure of the dopaminergic system underlying reward processing operating in the ventral striatum [86].
L-DOPA does not only induce restorative positive therapeutic effects in the ventral striatum. In fact, in PD patients this DA precursor improves task-switching performance but impairs certain aspects of cognitive function, such as reversal learning [87,88]. In particular, it has been postulated that the beneficial effect of L-DOPA on task-switching reflects a compensation of DA levels in depleted dorsal frontostriatal circuitry, whereas the impairing effect of L-DOPA on reversal learning reflects a detrimental ‘over-dosing’ of intact ventral frontostriatal circuitry [87,88]. According to this hypothesis, functional imaging studies in PD patients have revealed that the beneficial effect of L-DOPA on working memory is accompanied by modulation of the dorsolateral prefrontal cortex (PFC), which is strongly connected with the severely depleted dorsal striatum. Conversely, it has been shown, by performing neuroimaging analysis in PD patients scanned in both ON and OFF condition during a reversal learning task, that L-DOPA modulates reversal-related activity in the nucleus accumbens, but not in the dorsal striatum or the PFC [88].
In a study that analysed D2-like DA receptor binding through the use of positron emission tomography, it has been observed that PD patients with a DA dysregulation syndrome exhibit enhanced L-DOPA-induced ventral striatal DA release compared with L-DOPA-treated patients not compulsively taking dopaminergic drugs [89]. The sensitized ventral striatal DA neurotransmission induced by L-DOPA in these patients correlates with self-reported compulsive drug ‘wanting’ but not ‘liking’ and is coupled with an increased psychomotor activation. This clinical finding clearly links sensitization of ventral striatum to compulsive drug use in PD patients.
Although the synaptic and/or neuronal mechanism mediating reversal learning and compulsive drug use in the ventral striatum has not been fully investigated, we can hypothesize that changes in synaptic plasticity might account for this behavioural process. In fact, medium spiny neurons of the ventral striatum express both LTD and LTP with some similarities with the features of these forms of synaptic plasticity in the dorsal striatum [90]. Moreover, it has been reported that drugs of abuse such as cocaine might induce long-lasting changes at excitatory synapses in the nucleus accumbens and ventral tegmental area owing to activation of the mechanisms that underlie LTP and LTD in these structures [91,92]. Thus, it is possible that also L-DOPA, acting in this brain area, might affect motivated behaviour with mechanisms similar to those implicated in the plastic changes induced by drugs of abuse.
Another mechanism potentially implicated in the behavioural effects of L-DOPA and involving the nucleus accumbens emerges from the in vivo electrophysiological studies from Grace's group. These electrophysiological recordings, combined with manipulation of the DA system, have shown that distinct modulation of D1- and D2-like receptors in the nucleus accumbens produces different behavioural effects, such as learning versus set shifting of response strategy. Moreover, the features of the release of DA within the nucleus accumbens regulate the balance between limbic and cortical drive via the activation of distinct DA receptor subtypes. These synaptic and electrophysiological effects can, in turn, control goal-directed behaviour [93]. Thus, L-DOPA might play a critical role in this regulation of the limbic system.
It is also useful to consider that L-DOPA administration might shift the release of DA from a more physiological continuous release to a phasic and pulsatile presence of DA in the synaptic cleft. This phasic release of DA in conjunction with the glutamatergic inputs might be important for structural plasticity in both physiological and pathological conditions. Accordingly, it is known that animal behaviours are reinforced by subsequent rewards within a narrow time window. In line with this hypothesis, in an elegant study, dopaminergic and glutamatergic inputs to medium spiny neurons of the ventral striatum were optically stimulated in a separate manner, demonstrating that DA promotes structural plasticity measured as a spine enlargement only during a narrow time window (0.3 to 2 s) after activation of the glutamatergic inputs [94]. This study further emphasizes the role of the medium spiny neurons dendritic spine as the locus of reinforcement plasticity in the ventral striatum and it might also shed light on the mechanism of action of L-DOPA in the ventral striatum.
6. Hippocampus
Preclinical studies have shown that DA critically favours the cellular consolidation of hippocampal-dependent memories by inducing protein synthesis in hippocampal neurons [95,96]. Activation of the hippocampus is required to encode memories for new events. Thus, it has been hypothesized that a release of DA is required for the persistence of hippocampal-dependent memory beyond 4 to 6 h after its induction. This observation suggests that dopaminergic enhancement might improve human episodic memory persistence also for events encoded with weak hippocampal activation.
A recent study using fMRI in an elderly population displaying a loss of DA neurons as part of normal aging, seems to confirm this hypothesis. In this population, L-DOPA induced a dose-related persistent episodic memory amelioration for images of scenes [97], suggesting a role for DA in human episodic memory consolidation.
In line with this observation in human subjects, a pioneering in vivo electrophysiological study has shown that oral administration of L-DOPA is able to induce LTP in a subregion of the hippocampus, the dentate gyrus of the hippocampus, even after a sub-threshold tetanic stimulation of the perforant pathway, which usually fails to elicit LTP [98].
Another interesting field of research dealing with DA hippocampal function and memory is represented by the analysis of the role of DA and L-DOPA in experimental models of PD [31]. It has been reported that LTP in the CA1 area of the hippocampus is altered in both 6-OHDA-lesioned rats and transgenic models of PD (mice expressing a truncated form of human α-synuclein 1–120). This plastic alteration is associated with an impaired dopaminergic transmission and a decrease of GluN2A/N2B subunit ratio in synaptic NMDA receptors [21]. In the 6-OHDA-lesioned animals as well as in these mutant animals, the alterations of the CA1 LTP were paralleled by deficits in hippocampal-dependent learning. Interestingly, L-DOPA was able to restore hippocampal LTP activating D1/D5 receptors and to ameliorate the cognitive deficit in parkinsonian animals suggesting that DA-dependent impairment of hippocampal LTP may contribute to cognitive deficits in patients with PD.
The restorative effects of L-DOPA on hippocampal synaptic plasticity have also been analysed in the dentate gyrus, in the unilateral 6-OHDA-lesion model [20]. Here, the electrophysiological analysis was coupled to a neurochemical detection of catecholamines and the form of synaptic plasticity investigated was the LTD induced by low-frequency stimulation of the medial perforant path/dentate gyrus synapses. In vivo microdialysis measurements revealed that the 6-OHDA injection disrupts dopaminergic and noradrenergic transmission in dentate gyrus. Ex vivo electrophysiological recordings have shown that these neurochemical alterations are accompanied by impairment of LTD. This alteration was reversed by subchronic L-DOPA treatment. Surprisingly, however, the therapeutic effect of L-DOPA on LTD was blocked by the antagonism of β-noradrenergic receptors, but not by DA D1 or D2 receptor antagonists. Thus, while the dopaminergic transmission does not seem to be implicated in this therapeutic effect of L-DOPA, the noradrenergic system plays a central role in the synaptic dysfunction of the dentate gyrus in experimental PD. This work further supported the complex role of the catecholaminergic control on hippocampal synaptic plasticity as well as on the possible synaptic mechanisms underlying cognitive deficits in PD, suggesting that L-DOPA exerts a therapeutic effect on the parkinsonian brain through different and coexistent mechanisms.
7. Cortex
In the striatum, activation of the D1-like family of DA receptor increases the expression of several molecular markers [4]. In particular, the members of the immediate-early gene (IEG) family, a class of genes rapidly transcribed in response to an external stimulus, are modulated by L-DOPA. These IEG include ΔFosB, ARC, FRA2 and Zif268 and their expression patterns are specific for LIDs not only in the dorsal striatum, but also in other dopaminoceptive structures of the brain such as the motor cortex [99].
As several experimental and clinical studies have reported that L-DOPA, acting as a DA precursor, exerts dose-dependent effects with an inverted U-shaped profile on both cognitive and motor functions, the possibility of a nonlinear dose-dependent effect of L-DOPA on human cortical plasticity was investigated by analysing transcranial direct current stimulation-induced plasticity in healthy human subjects [24]. In particular, the primary motor cortex was investigated as a model system, and plasticity was monitored by motor-evoked potential amplitudes elicited by transcranial magnetic stimulation. Surprisingly, acute low and high dosages of L-DOPA abolished facilitatory as well as inhibitory plasticity, whereas the medium dosage prolonged inhibitory plasticity, and switched facilitatory plasticity into an inhibitory form. Thus, these results confirm the hypothesis that L-DOPA exerts nonlinear, dose-dependent effects on both forms of cortical plasticity supporting the view that a specific dosage of L-DOPA is required to ameliorate plasticity.
Reduced levels of endogenous DA in untreated PD patients are present not only in the striatum as a consequence of degeneration of the mesostriatal pathway but also in the cortex, resulting from a parallel impairment of the mesocortical projection. This latter deficiency affects synaptic plasticity in various cortical areas. In particular, it has been proposed that reduction of DA disrupts neural interactions between prefrontal and premotor areas. This alteration might underlie the impairment of motor control observed in patients with PD. In fact, DA denervation observed in PD patients impairs the ability to establish oscillatory coupling between prefrontal and premotor areas during an externally paced motor task [100]. Interestingly, administration of L-DOPA in these PD patients restores physiological prefrontal–premotor coupling and additionally favours the occurrence of a frequency-specific connectivity between these areas, which is not expressed in healthy subjects.
In another electrophysiological study, plasticity of primary motor cortices (M1) in de novo PD patients and age-matched healthy controls in response to a single dose of L-DOPA has been tested using intermittent versus continuous theta-burst stimulation protocols to induce respectively LTP- and LTD-like plasticity on both M1 cortices [25]. These protocols induced plasticity in M1 of controls while in de novo PD patients no plasticity was measured. Surprisingly, acute L-DOPA administration did not improve these forms of plasticity, although motor signs of PD improved. The differential response to acute L-DOPA response between motor signs and M1 plasticity observed in this study might be explained by possible distinct effects of acute DA replacement on circuits recruited by specific plasticity-induction techniques.
Different results from the latter study were obtained in another experimental setting used to investigate cortical plasticity in PD patients and healthy controls. In fact, paired-associative stimulation to the contralateral peripheral nerve and cerebral cortex to enhance the M1 excitability with two synchronously arriving inputs has been used to analyse the action of L-DOPA on cortical plasticity in PD patients and, in particular, to investigate its possible contribution to the associative LTP-like effect in the M1 in PD patients [26]. The paired-associative stimulation comprises a single electric stimulus to the right median nerve at the wrist and subsequent transcranial magnetic stimulation of the left M1. The motor-evoked potential amplitude in the right abductor pollicis brevis muscle is increased by paired-associative stimulation in healthy volunteers, but not in PD patients. However, the ratio of the motor-evoked potential amplitude before and after paired-associative stimulation in PD patients in the off-state increased after L-DOPA replacement therapy, suggesting that DA influences human cortical plasticity and this action might be required for motor learning.
Electrophysiological studies using corticostriatal slices obtained from parkinsonian rats with LIDs have shown that loss of depotentiation is a key feature of this disabling condition [16]. To address this issue, an elegant clinical electrophysiological study has investigated depotentiation of pre-existing LTP-like synaptic facilitation in the motor cortex of PD patients with and without LIDs [27]. The authors found that patients with PD without LIDs have normal LTP- and depotentiation-like effects when they took their full dose of L-DOPA, but there was no LTP-like effect when they were on half dose of this drug. Conversely, patients with LIDs could be successfully potentiated when they were on half their usual dose of L-DOPA. However, in striking similarity with the observations in preclinical studies, they were unresponsive to the depotentiation protocol, further supporting the view that reversal of LTP is also abnormal in the motor cortex of patients with PD and LIDs.
The role of specific cortical areas in the pathophysiology of LIDs has been further addressed by a recent study that has combined fMRI and repetitive transcranial magnetic stimulation [101]. The authors have analysed resting-state fMRI on patients with PD with LIDs and patients without LIDs, before and after administration of L-DOPA. The resting-state imaging analysis has shown that in patients with LIDs, the connectivity of the right inferior frontal cortex was decreased with the left motor cortex and increased with the right putamen when compared with patients without LIDs. This abnormal pattern of connectivity was evident only during the ON phase of L-DOPA treatment. Interestingly, the degree of this alteration correlated with the motor disability. In a second experiment, the authors applied different protocols of repetitive transcranial magnetic stimulation over the right inferior frontal cortex in another group of patients with LIDs. These experiments demonstrated that continuous but not intermittent theta-burst stimulation applied at the inferior frontal cortex decreased LIDs induced by a single dose of L-DOPA. These elegant experiments indicate that this cortical area plays a key role in the pathophysiology of LIDs.
Maladaptive plasticity in response to L-DOPA in the cortex of advanced PD patients is also suggested by another neurophysiological study investigating the effects of an acute non-physiological DA boost. The authors propose that the loss of the long-duration clinical response to L-DOPA and the negative effect of acute doses on cortical plasticity with progression of disease may contribute to the pathophysiology of motor complications [102]. They also postulate that repeated non-physiological pulsatile elevations in synaptic DA during acute L-DOPA dosing could potentially lead to persistent dysfunction of key enzymes of the intracellular signalling cascade that are involved in the control of cortical plasticity.
It is interesting to note that cortical plasticity in advanced PD patients can be modulated not only by L-DOPA but also by drugs enhancing the pharmacological effect of this drug, such as entacapone, a peripheral inhibitor of catechol-O-methyltransferase (COMT). Treatment with entacapone, in fact, reduces wearing-off, one of the most frequent motor complications observed in L-DOPA-treated patients with advanced PD, consisting of a progressive shortening of L-DOPA therapeutic effect duration. In a clinical imaging study, it has been reported that patients significantly improved under COMT-inhibitor treatment, and the fMRI findings have shown that while at baseline patients present a bilateral activation of the primary motor, contralateral premotor cortex and supplementary motor area, as well as ipsilateral cerebellum, during treatment with entacapone, PD patients show reductions in the activations of these cortical areas and a decreased activation in the ipsilateral cerebellum [103]. These findings suggest an increased specificity for the activation of task-related cortical areas during this treatment. These findings also indicate that fMRI is able to detect cortical activation changes during long-term modulation of dopaminergic treatment drug-induced cortical plasticity.
Altogether, human studies using repetitive transcranial magnetic stimulation, as well as other clinical electrophysiological techniques, indicate that cortex shows synaptic abnormalities in PD patients with LIDs similar to the plastic alterations observed in the basal ganglia of experimental animal models. For this reason, the morphology of dendritic spines of pyramidal neurons in the motor cortex has been recently analysed in a rat model of LIDs [104]. Using the 6-OHDA-induced unilateral dopaminergic lesion in the rat and a chronic treatment with L-DOPA, the authors have measured the density and size of dendritic spines in pyramidal neurons in M1 cortex that project to the medium spiny neurons in the direct pathway. The spine density was not different among control animals and parkinsonian rats with and without LIDs. Conversely, spine size was enlarged both in parkinsonian rats and animals showing LIDs. However, the enlargement of the spines was greater in the LIDs model than in the PD model. This enlargement of the spines suggests that these pyramidal neurons express a reduced threshold to excitatory stimuli. According to this hypothesis, the amplitude of the miniature excitatory postsynaptic currents in the pyramidal neurons recorded from the M1 cortex using whole-cell patch clamps was augmented in the LIDs model compared with naive animals. Thus, it could be hypothesized that spine enlargement and the subsequent overexcitability of pyramidal neurons in M1 cortex contribute to the altered cortical synaptic plasticity in LIDs.
8. Conclusion
Recent experimental and clinical studies have provided a contribution to the understanding of the mechanisms underlying both the beneficial and the detrimental effects of L-DOPA in the parkinsonian brain. Concerning the pathophysiology of LIDs, the most widely accepted theories assume that the culprit for this adverse pharmacological effect are the non-physiological synthesis, release and reuptake of DA synthesized by exogenously administered L-DOPA in the striatum, and the aberrant plasticity in the cortico-basal ganglia systems (figure 1).
Physiological motor activity requires a correct integration among the primary motor cortex M1, other cortical areas and basal ganglia. DA-dependent plasticity expressed at these different brain levels is of crucial importance for this integrative activity and this delicate balance is disrupted in PD as well as in LIDs. When tested with the regular pulsatile L-DOPA doses, PD patients with LIDs show impairment of the cortical M1 plasticity, and in particular the inability to depotentiate an already facilitated synapse.
Dyskinetic patients have also severe impairment of the associative, sensorimotor plasticity of M1 attributed to deficient cerebellar modulation of sensory afferents to this cortical area. In line with this evidence, recently described bidirectional connections between the cerebellum and the basal ganglia support the hypothesis of a key role of the cerebellum in the generation of LIDs. Accordingly, some clinical studies suggest that stimulation of the cerebellum can reduce LIDs [105].
Motor complications, gait and balance disturbance as well as cognitive impairment are problems often present in PD patients. Motor complications can be reduced, or at least delayed, by the appropriate use of L-DOPA and/or the administration of other dopaminergic agents to spare L-DOPA use.
Moreover, recent clinical research has concentrated on non-dopaminergic neurotransmitter systems, which may also have applicability in the management of gait and balance as well as in cognitive impairment in PD. In particular, cognitive alterations also include deficits in inhibitory control, ranging from subclinical alterations in decision-making to severe impulse control disorders [42]. Preclinical studies on impulsivity factors suggest the possibility of behavioural disturbances with distinct psychological profiles, as well as differential anatomical and pharmacological features can be present in PD. Thus, not only the DA, but also other transmitters such as noradrenaline and serotonin, might be implicated in the cognitive dysfunction present in PD. These systems might represent interesting additional therapeutic targets.
Acknowledgements
We thank C. Spaccatini for excellent technical support.
Funding statement
This work was supported by grants from the Italian Ministry of Education, Universities and Research, Progetto di Ricerca di Interesse Nazionale (PRIN) 2010-AHHP5H (to P.C.) and from the Italian Ministry of Health, Ricerca Finalizzata - Giovani Ricercatori GR-2010–2316671 (to V.G.)
Conflict of interests
P.C. is a member of the editorial boards of Lancet Neurology and Synapse and receives research support from Bayer Schering, Biogen, Boehringer Ingelheim, Eisai, Lundbeck, Merck Sharp and Dohme, Novartis, Sanofi-Aventis, Sigma Tau, UCB, Fondazione Santa Lucia IRCCS, Ministero della Salute and Agenzia Italiana del Farmaco. All other authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Calabresi P, Di Filippo M, Gallina A, Wang Y, Stankowski JN, Picconi B, Dawson VL, Dawson TM. 2013. New synaptic and molecular targets for neuroprotection in Parkinson's disease. Mov. Disord. 28, 51–60. ( 10.1002/mds.25096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubellini P, Picconi B, Di Filippo M, Calabresi P. 2010. Downstream mechanisms triggered by mitochondrial dysfunction in the basal ganglia: from experimental models to neurodegenerative diseases. Biochim. Biophys. Acta 1802, 151–161. ( 10.1016/j.bbadis.2009.08.001) [DOI] [PubMed] [Google Scholar]
- 3.Schapira AH, Jenner P. 2011. Etiology and pathogenesis of Parkinson's disease. Mov. Disord. 26, 1049–1055. ( 10.1002/mds.23732) [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. 2010. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol. 9, 1106–1117. ( 10.1016/S1474-4422(10)70218-0) [DOI] [PubMed] [Google Scholar]
- 5.Jenner P. 2008. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 9, 665–677. ( 10.1038/nrn2471) [DOI] [PubMed] [Google Scholar]
- 6.Mercuri NB, Bernardi G. 2005. The ‘magic’ of L-dopa: why is it the gold standard Parkinson's disease therapy? Trends Pharmacol. Sci. 26, 341–344. ( 10.1016/j.tips.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 7.Picconi B, Paille V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M, Calabresi P. 2008. L-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol. Dis. 29, 327–335. ( 10.1016/j.nbd.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 8.Day M, et al. 2006. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 9, 251–259. ( 10.1038/nn1632) [DOI] [PubMed] [Google Scholar]
- 9.Deutch AY, Colbran RJ, Winder DJ. 2007. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat. Disord. 13(Suppl. 3), S251–S258. ( 10.1016/S1353-8020(08)70012-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishijima H, et al. 2014. Morphologic changes of dendritic spines of striatal neurons in the levodopa-induced dyskinesia model. Mov. Disord. 29, 336–343. ( 10.1002/mds.25826) [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K. 2013. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J. Neurosci. 33, 11 655–11 667. ( 10.1523/JNEUROSCI.0288-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guatteo E, Yee A, McKearney J, Cucchiaroni ML, Armogida M, Berretta N, Mercuri NB, Lipski J. 2013. Dual effects of L-DOPA on nigral dopaminergic neurons. Exp. Neurol. 247, 582–594. ( 10.1016/j.expneurol.2013.02.009) [DOI] [PubMed] [Google Scholar]
- 13.Carta M, Carlsson T, Munoz A, Kirik D, Bjorklund A. 2008. Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog. Brain Res. 172, 465–478. ( 10.1016/S0079-6123(08)00922-9) [DOI] [PubMed] [Google Scholar]
- 14.Rylander D, Parent M, O'Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. 2010. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 68, 619–628. ( 10.1002/ana.22097) [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Andren PE, Svenningsson P. 2006. Repeated L-DOPA treatment increases c-fos and BDNF mRNAs in the subthalamic nucleus in the 6-OHDA rat model of Parkinson's disease. Brain Res. 1095, 207–210. ( 10.1016/j.brainres.2006.04.019) [DOI] [PubMed] [Google Scholar]
- 16.Picconi B, et al. 2003. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci. 6, 501–506. ( 10.1038/nn1040) [DOI] [PubMed] [Google Scholar]
- 17.Picconi B, et al. 2011. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain 134, 375–387. ( 10.1093/brain/awq342) [DOI] [PubMed] [Google Scholar]
- 18.Belujon P, Lodge DJ, Grace AA. 2010. Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Mov. Disord. 25, 1568–1576. ( 10.1002/mds.23245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiele SL, Chen B, Lo C, Gertler TS, Warre R, Surmeier JD, Brotchie JM, Nash JE. 2014. Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and L-DOPA induced dyskinesia in mouse models. Neurobiol. Dis. 71, 334–344. ( 10.1016/j.nbd.2014.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pendolino V, et al. 2014. L-DOPA reverses the impairment of Dentate Gyrus LTD in experimental parkinsonism via beta-adrenergic receptors. Exp. Neurol. 261, 377–385. ( 10.1016/j.expneurol.2014.07.006) [DOI] [PubMed] [Google Scholar]
- 21.Costa C, et al. 2012. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental Parkinson's disease. Brain 135, 1884–1899. ( 10.1093/brain/aws101) [DOI] [PubMed] [Google Scholar]
- 22.Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. 2009. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients. Brain 132, 309–318. ( 10.1093/brain/awn322) [DOI] [PubMed] [Google Scholar]
- 23.Prescott IA, Liu LD, Dostrovsky JO, Hodaie M, Lozano AM, Hutchison WD. 2014. Lack of depotentiation at basal ganglia output neurons in PD patients with levodopa-induced dyskinesia. Neurobiol. Dis. 71, 24–33. ( 10.1016/j.nbd.2014.08.002) [DOI] [PubMed] [Google Scholar]
- 24.Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA. 2010. Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J. Physiol. 588, 3415–3424. ( 10.1113/jphysiol.2010.190181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishore A, Joseph T, Velayudhan B, Popa T, Meunier S. 2012. Early, severe and bilateral loss of LTP and LTD-like plasticity in motor cortex (M1) in de novo Parkinson's disease. Clin. Neurophysiol. 123, 822–828. ( 10.1016/j.clinph.2011.06.034) [DOI] [PubMed] [Google Scholar]
- 26.Ueki Y, et al. 2006. Altered plasticity of the human motor cortex in Parkinson's disease. Ann. Neurol. 59, 60–71. ( 10.1002/ana.20692) [DOI] [PubMed] [Google Scholar]
- 27.Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS. 2011. Abnormal bidirectional plasticity-like effects in Parkinson's disease. Brain 134, 2312–2320. ( 10.1093/brain/awr158) [DOI] [PubMed] [Google Scholar]
- 28.de la Riva P, Smith K, Xie SX, Weintraub D. 2014. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 83, 1096–1103. ( 10.1212/WNL.0000000000000801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins TW, Cools R. 2014. Cognitive deficits in Parkinson's disease: a cognitive neuroscience perspective. Mov. Disord. 29, 597–607. ( 10.1002/mds.25853) [DOI] [PubMed] [Google Scholar]
- 30.Kehagia AA, Barker RA, Robbins TW. 2010. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 9, 1200–1213. ( 10.1016/S1474-4422(10)70212-X) [DOI] [PubMed] [Google Scholar]
- 31.Calabresi P, Castrioto A, Di Filippo M, Picconi B. 2013. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson's disease. Lancet Neurol. 12, 811–821. ( 10.1016/S1474-4422(13)70118-2) [DOI] [PubMed] [Google Scholar]
- 32.Calabresi P, Picconi B, Parnetti L, Di Filippo M. 2006. A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 5, 974–983. ( 10.1016/S1474-4422(06)70600-7) [DOI] [PubMed] [Google Scholar]
- 33.Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D. 2014. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain 137, 2493–2508. ( 10.1093/brain/awu193) [DOI] [PubMed] [Google Scholar]
- 34.Muller ML, et al. 2014. Clinical markers for identifying cholinergic deficits in Parkinson's disease. Mov. Disord. 30, 269–273. ( 10.1002/mds.26061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nombela C, et al. 2014. Genetic impact on cognition and brain function in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain 137, 2743–2758. ( 10.1093/brain/awu201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kehagia AA, Barker RA, Robbins TW. 2013. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener. Dis. 11, 79–92. ( 10.1159/000341998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. 2013. Dopamine overdose hypothesis: evidence and clinical implications. Mov. Disord. 28, 1920–1929. ( 10.1002/mds.25687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weintraub D, Nirenberg MJ. 2013. Impulse control and related disorders in Parkinson's disease. Neurodegener. Dis. 11, 63–71. ( 10.1159/000341996) [DOI] [PubMed] [Google Scholar]
- 39.Evans AH, Katzenschlager R, Paviour D, O'Sullivan JD, Appel S, Lawrence AD, Lees AJ. 2004. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov. Disord. 19, 397–405. ( 10.1002/mds.20045) [DOI] [PubMed] [Google Scholar]
- 40.Friedman JH. 1994. Punding on levodopa. Biol. Psychiatry 36, 350–351. ( 10.1016/0006-3223(94)90636-X) [DOI] [PubMed] [Google Scholar]
- 41.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. 2010. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch. Neurol. 67, 589–595. ( 10.1001/archneurol.2010.65) [DOI] [PubMed] [Google Scholar]
- 42.Nombela C, Rittman T, Robbins TW, Rowe JB. 2014. Multiple modes of impulsivity in Parkinson's disease. PLoS ONE 9, e85747 ( 10.1371/journal.pone.0085747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obeso I, et al. 2011. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp. Brain Res. 212, 371–384. ( 10.1007/s00221-011-2736-6) [DOI] [PubMed] [Google Scholar]
- 44.Everitt BJ, Robbins TW. 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489. ( 10.1038/nn1579) [DOI] [PubMed] [Google Scholar]
- 45.Calabresi P, Mercuri NB, Sancesario G, Bernardi G. 1993. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson's disease. Brain 116, 433–452. [PubMed] [Google Scholar]
- 46.Picconi B, Centonze D, Rossi S, Bernardi G, Calabresi P. 2004. Therapeutic doses of L-dopa reverse hypersensitivity of corticostriatal D2-dopamine receptors and glutamatergic overactivity in experimental parkinsonism. Brain 127, 1661–1669. ( 10.1093/brain/awh190) [DOI] [PubMed] [Google Scholar]
- 47.Centonze D, Gubellini P, Rossi S, Picconi B, Pisani A, Bernardi G, Calabresi P, Baunez C. 2005. Subthalamic nucleus lesion reverses motor abnormalities and striatal glutamatergic overactivity in experimental parkinsonism. Neuroscience 133, 831–840. ( 10.1016/j.neuroscience.2005.03.006) [DOI] [PubMed] [Google Scholar]
- 48.Di Filippo M, Picconi B, Tozzi A, Ghiglieri V, Rossi A, Calabresi P. 2008. The endocannabinoid system in Parkinson's disease. Curr. Pharm. Des. 14, 2337–2347. ( 10.2174/138161208785740072) [DOI] [PubMed] [Google Scholar]
- 49.Lovinger DM. 2008. Presynaptic modulation by endocannabinoids. Handb. Exp. Pharmacol. 184, 435–477. ( 10.1007/978-3-540-74805-2_14) [DOI] [PubMed] [Google Scholar]
- 50.Maccarrone M, et al. 2003. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 85, 1018–1025. ( 10.1046/j.1471-4159.2003.01759.x) [DOI] [PubMed] [Google Scholar]
- 51.Calabresi P, Giacomini P, Centonze D, Bernardi G. 2000. Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann. Neurol. 47, S60–S68; discussion S8–9. [PubMed] [Google Scholar]
- 52.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. 2014. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 17, 1022–1030. ( 10.1038/nn.3743) [DOI] [PubMed] [Google Scholar]
- 53.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. 2007. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30, 228–235. ( 10.1016/j.tins.2007.03.008) [DOI] [PubMed] [Google Scholar]
- 54.Calabresi P, Picconi B, Tozzi A, Di Filippo M. 2007. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 30, 211–219. ( 10.1016/j.tins.2007.03.001) [DOI] [PubMed] [Google Scholar]
- 55.Lovinger DM. 2010. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58, 951–961. ( 10.1016/j.neuropharm.2010.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabresi P, et al. 2000. Dopamine and cAMP-regulated phosphoprotein 32kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 20, 8443–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. 1992. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J. Neurosci. 12, 4224–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovinger DM, Tyler EC, Merritt A. 1993. Short- and long-term synaptic depression in rat neostriatum. J. Neurophysiol. 70, 1937–1949. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. 2006. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50, 443–452. ( 10.1016/j.neuron.2006.04.010) [DOI] [PubMed] [Google Scholar]
- 60.Kreitzer AC, Malenka RC. 2007. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445, 643–647. ( 10.1038/nature05506) [DOI] [PubMed] [Google Scholar]
- 61.Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampa C, Calabresi P. 2011. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease. J. Neurosci. 31, 12 513–12 522. ( 10.1523/JNEUROSCI.2236-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calabresi P, et al. 1999. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J. Neurosci. 19, 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strafella AP, Paus T, Barrett J, Dagher A. 2001. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21, RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strafella AP, Paus T, Fraraccio M, Dagher A. 2003. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126, 2609–2615. ( 10.1093/brain/awg268) [DOI] [PubMed] [Google Scholar]
- 65.Ghiglieri V, Pendolino V, Sgobio C, Bagetta V, Picconi B, Calabresi P. 2012. Theta-burst stimulation and striatal plasticity in experimental parkinsonism. Exp. Neurol. 236, 395–398. ( 10.1016/j.expneurol.2012.04.020) [DOI] [PubMed] [Google Scholar]
- 66.Calabresi P, Pisani A, Mercuri NB, Bernardi G. 1992. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur. J. Neurosci. 4, 929–935. ( 10.1111/j.1460-9568.1992.tb00119.x) [DOI] [PubMed] [Google Scholar]
- 67.Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G. 1999. Unilateral dopamine denervation blocks corticostriatal LTP. J. Neurophysiol. 82, 3575–3579. [DOI] [PubMed] [Google Scholar]
- 68.Kerr JN, Wickens JR. 2001. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 85, 117–124. [DOI] [PubMed] [Google Scholar]
- 69.Charpier S, Deniau JM. 1997. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc. Natl Acad. Sci. USA 94, 7036–7040. ( 10.1073/pnas.94.13.7036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reynolds JN, Hyland BI, Wickens JR. 2001. A cellular mechanism of reward-related learning. Nature 413, 67–70. ( 10.1038/35092560) [DOI] [PubMed] [Google Scholar]
- 71.Paille V, et al. 2010. Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J. Neurosci. 30, 14 182–14 193. ( 10.1523/JNEUROSCI.2149-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang CC, Hsu KS. 2001. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev. Neurosci. 12, 51–68. ( 10.1515/REVNEURO.2001.12.1.51) [DOI] [PubMed] [Google Scholar]
- 73.Siddoway B, Hou H, Xia H. 2014. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology 78, 38–44. ( 10.1016/j.neuropharm.2013.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gardoni F, et al. 2006. A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J. Neurosci. 26, 2914–2922. ( 10.1523/JNEUROSCI.5326-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardoni F, Sgobio C, Pendolino V, Calabresi P, Di Luca M, Picconi B. 2012. Targeting NR2A-containing NMDA receptors reduces L-DOPA-induced dyskinesias. Neurobiol. Aging 33, 2138–2144. ( 10.1016/j.neurobiolaging.2011.06.019) [DOI] [PubMed] [Google Scholar]
- 76.Bagetta V, et al. 2012. Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson's disease. J. Neurosci. 32, 17 921–17 931. ( 10.1523/JNEUROSCI.2664-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cerovic M, et al. 2014. Derangement of Ras-Guanine nucleotide-releasing factor 1 (Ras-GRF1) and extracellular signal-regulated kinase (ERK) dependent striatal plasticity in L-DOPA-induced dyskinesia. Biol. Psychiatry 77, 106–115. ( 10.1016/j.biopsych.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 78.Heiman M, et al. 2014. Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc. Natl Acad. Sci. USA 111, 4578–4583. ( 10.1073/pnas.1401819111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feldman DE. 2012. The spike-timing dependence of plasticity. Neuron 75, 556–571. ( 10.1016/j.neuron.2012.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen W, Flajolet M, Greengard P, Surmeier DJ. 2008. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321, 848–851. ( 10.1126/science.1160575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fieblinger T, et al. 2014. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat. Commun. 5, 5316 ( 10.1038/ncomms6316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. 2013. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J. Neurosci. 33, 17 569–17 576. ( 10.1523/JNEUROSCI.3250-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. 2010. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33, 267–276. ( 10.1016/j.tins.2010.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abraham AD, Neve KA, Lattal KM. 2014. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol. Learn. Mem. 108, 65–77. ( 10.1016/j.nlm.2013.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zubieta JK, Stohler CS. 2009. Neurobiological mechanisms of placebo responses. Ann. NY Acad. Sci. 1156, 198–210. ( 10.1111/j.1749-6632.2009.04424.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Duzel E, Dolan RJ. 2013. Dopamine restores reward prediction errors in old age. Nat. Neurosci. 16, 648–653. ( 10.1038/nn.3364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cools R, Barker RA, Sahakian BJ, Robbins TW. 2001. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb. Cortex 11, 1136–1143. ( 10.1093/cercor/11.12.1136) [DOI] [PubMed] [Google Scholar]
- 88.Cools R, Lewis SJ, Clark L, Barker RA, Robbins TW. 2007. l-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology 32, 180–189. ( 10.1038/sj.npp.1301153) [DOI] [PubMed] [Google Scholar]
- 89.Evans AH, et al. 2006. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann. Neurol. 59, 852–858. ( 10.1002/ana.20822) [DOI] [PubMed] [Google Scholar]
- 90.Thomas MJ, Malenka RC. 2003. Synaptic plasticity in the mesolimbic dopamine system. Phil. Trans. R. Soc. Lond. B 358, 815–819. ( 10.1098/rstb.2003.1288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Creed MC, Luscher C. 2013. Drug-evoked synaptic plasticity: beyond metaplasticity. Curr. Opin. Neurobiol. 23, 553–558. ( 10.1016/j.conb.2013.03.005) [DOI] [PubMed] [Google Scholar]
- 92.Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. 2010. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr. Top. Behav. Neurosci. 3, 3–27. ( 10.1007/7854_2009_23) [DOI] [PubMed] [Google Scholar]
- 93.Goto Y, Grace AA. 2005. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 8, 805–812. ( 10.1038/nn1471) [DOI] [PubMed] [Google Scholar]
- 94.Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H. 2014. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620. ( 10.1126/science.1255514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frey U, Morris RG. 1997. Synaptic tagging and long-term potentiation. Nature 385, 533–536. ( 10.1038/385533a0) [DOI] [PubMed] [Google Scholar]
- 96.O'Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RG. 2006. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn. Mem. 13, 760–769. ( 10.1101/lm.321006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Duzel E. 2012. Dopamine modulates episodic memory persistence in old age. J. Neurosci. 32, 14 193–14 204. ( 10.1523/JNEUROSCI.1278-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kusuki T, Imahori Y, Ueda S, Inokuchi K. 1997. Dopaminergic modulation of LTP induction in the dentate gyrus of intact brain. Neuroreport 8, 2037–2040. ( 10.1097/00001756-199705260-00046) [DOI] [PubMed] [Google Scholar]
- 99.Bastide MF, Dovero S, Charron G, Porras G, Gross CE, Fernagut PO, Bézard E. 2014. Immediate-early gene expression in structures outside the basal ganglia is associated to L-DOPA-induced dyskinesia. Neurobiol. Dis. 62, 179–192. ( 10.1016/j.nbd.2013.09.020) [DOI] [PubMed] [Google Scholar]
- 100.Herz DM, Siebner HR, Hulme OJ, Florin E, Christensen MS, Timmermann L. 2014. Levodopa reinstates connectivity from prefrontal to premotor cortex during externally paced movement in Parkinson's disease. Neuroimage 90, 15–23. ( 10.1016/j.neuroimage.2013.11.023) [DOI] [PubMed] [Google Scholar]
- 101.Cerasa A, et al. 2014. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain 138, 414–427. ( 10.1093/brain/awu329) [DOI] [PubMed] [Google Scholar]
- 102.Kishore A, Popa T, Velayudhan B, Joseph T, Balachandran A, Meunier S. 2012. Acute dopamine boost has a negative effect on plasticity of the primary motor cortex in advanced Parkinson's disease. Brain 135, 2074–2088. ( 10.1093/brain/aws124) [DOI] [PubMed] [Google Scholar]
- 103.Tambasco N, et al. 2014. Entacapone reduces cortical activation in Parkinson's disease with wearing-off: a f-MRI study. PLoS ONE 9, e96806 ( 10.1371/journal.pone.0096806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ueno T, et al. 2014. Morphological and electrophysiological changes in intratelencephalic-type pyramidal neurons in the motor cortex of a rat model of levodopa-induced dyskinesia. Neurobiol. Dis. 64, 142–149. ( 10.1016/j.nbd.2013.12.014) [DOI] [PubMed] [Google Scholar]
- 105.Kishore A, Popa T. 2014. Cerebellum in levodopa-induced dyskinesias: the unusual suspect in the motor network. Front. Neurol. 5, 157 ( 10.3389/fneur.2014.00157) [DOI] [PMC free article] [PubMed] [Google Scholar]