Abstract
Certain neurodegenerative diseases are thought to be initiated by the aggregation of amyloidogenic proteins. However, the mechanism underlying toxicity remains obscure. Most of the suggested mechanisms are generic in nature and do not directly explain the neuron-type specific lesions observed in many of these diseases. Some recent reports suggest that the toxic aggregates impair the synaptic vesicular machinery. This may lead to an understanding of the neuron-type specificity observed in these diseases. A disruption of the vesicular machinery can also be deleterious for extra-synaptic, especially somatic, neurotransmission (common in serotonergic and dopaminergic systems which are specifically affected in Alzheimer's disease (AD) and Parkinson's disease (PD), respectively), though this relationship has remained unexplored. In this review, we discuss amyloid-induced damage to the neurotransmitter vesicular machinery, with an eye on the possible implications for somatic exocytosis. We argue that the larger size of the system, and the availability of multi-photon microscopy techniques for directly visualizing monoamines, make the somatic exocytosis machinery a more tractable model for understanding the effect of amyloids on all types of vesicular neurotransmission. Indeed, exploring this neglected connection may not just be important, it may be a more fruitful route for understanding AD and PD.
Keywords: Alzheimer's, Parkinson's, multiphoton microscopy, monoamine, neurodegeneration, protein aggregation
1. Introduction
Aggregation of intrinsically disordered ‘amyloid’ proteins in the brain is suspected to be a major contributing factor to neurodegenerative diseases, such as Parkinson's (PD) and Alzheimer's (AD). Emerging evidence suggests that soluble oligomers are the key toxic species in a large number of such diseases [1]. However, no consensus mechanism for toxicity has emerged yet for any of these. Identification of the toxic pathway is of paramount importance, as it will settle lingering doubts about the amyloid hypothesis of disease causation, isolate the exact species of aggregates (if any) responsible for toxicity and help design effective strategies to tackle these diseases.
Accumulation of cerebral amyloid beta (Aβ) has been hypothesized to play a key role in the development of synaptic deficits and neuronal loss in AD while alpha synuclein (α-syn) is a key component of the pathological Lewy bodies observed in PD. α-Syn is an intracellular protein while Aβ is predominantly extracellular, although there is evidence to suggest that Aβ can also accumulate inside neurons in human brain tissues [2–6] and in Aβ transgenic mice models [7–19]. Various pathways have been suggested for amyloid toxicity, such as impairment of the ubiquitin proteasomal system [20,21], mitochondrial deficits [22,23], oxidative stress [24], impaired stress-induced protective response [25], membrane permeabilization and/or ion channel formation [26–33], calcium dyshomeostasis [34,35], alterations in lipid/sterol biosynthesis [25], and changes in gene expression [36]. It is possible that any or a combination of these are responsible to varying degrees for a given disease. However, these mechanisms are generic in nature, and they fail to directly explain why particular types of neurons are specifically affected by particular protein aggregates, while others are spared for years. For example, in AD at initial stages there is loss of basal forebrain cholinergic cells [37,38] and major loss of serotonergic neurons in the caudate part of dorsal raphe nucleus [39,40], while in PD there is selective loss of dopaminergic (DA) neurons [41].
2. Somatic monoamine neurotransmission and amyloid diseases
It has been established over the last two decades that monoaminergic neurons take part in volume neurotransmission, and especially somatic exocytosis [42–50], in addition to carrying out synaptic exocytosis. Somatic release has been observed in multiple systems, including serotonergic neurons such as the Retzius neurons of leech [45,51,52], mesencephalic trigeminal nucleus of rat [53], primary cultures of raphe neurons and raphe slices of rats [49,50,54], and serotonergic neurons obtained from differentiated human embryonic stem cells [55]. It has also been observed in DA neurons of rats and mice [56–58]. While the precise role of somatic exocytosis is unclear, it is hypothesized to mediate responses which persist for long durations, for example, mood changes [46]. The synaptic and somatic release may not be completely unrelated. Somatic exocytosis involves vesicular movement over long spatial scales, and relatively long-term storage of neurotransmitters in vesicles which are much larger in size and have higher neurotransmitter content than the usual synaptic ones [50]. Proper vesicular packaging of these monoamines is important, since free monoamines can be neurotoxic due to their propensity to produce reactive oxygen species (ROS) [59,60]. Volume transmission also requires elaborate and well-regulated reuptake transport machinery. A failure of any of these components can undermine neurotransmission and induce toxicity, and so it makes these neurons especially vulnerable to insults targeted towards the vesicular machinery.
It has been suggested that at least some of the neurodegenerative diseases originate in synaptic failure [61], and so the functioning of the neurotransmitter vesicular machinery may be a target of the toxic amyloid species. Indeed, experiments over the past decade have established the deleterious effects of the amyloids on various steps of vesicular neurotransmission. Many of these steps are identical in somatic exocytosis, and their impairment can have at least as large an impact. However, investigations on amyloid toxicity have not focused on this aspect so far, potentially missing out an important piece of the neurodegeneration puzzle.
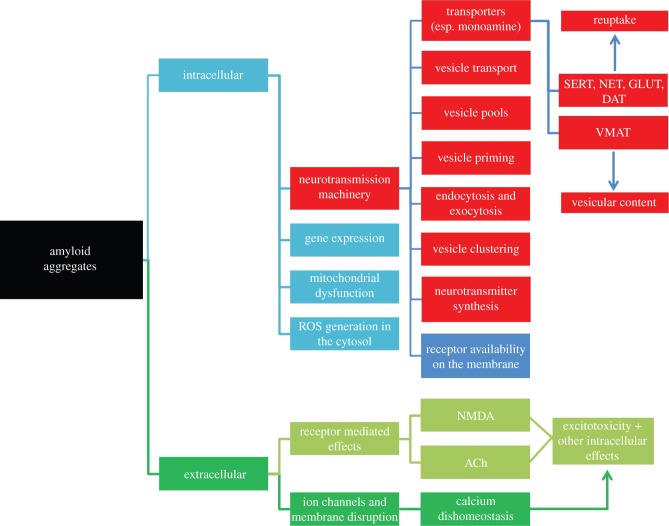
Mechanism of synaptic neurotransmission can be broadly divided into the following steps: (i) synthesis, (ii) sequestration into vesicles, (iii) vesicular transport, (iv) vesicle storage in different pools, (v) vesicle priming for exocytosis, (vi) endocytosis and exocytosis and (vii) reuptake. There is evidence, sometimes contradictory, that each of these stages may be affected by amyloid proteins, such as Aβ and α-syn. A schematic diagram describing the various pathways of amyloid toxicity which are relevant to our present context is given in figure 1. The boxes marked red pertain to the aspects involving vesicular neurotransmission, which this review is centred on. In the following sections, we consider each of these aspects and their possible correlation with somatic exocytosis. In the concluding part, we also argue that in addition to the physiological relevance of the studies of amyloid aggregation on somatic exocytosis, the larger size offered by the cell body can potentially make optical microscopy-based studies much easier and more precise in this system than what is possible for its synaptic counterpart. Therefore, studying the effects of amyloids on somatic exocytosis has the potential to uncover additional mechanisms of amyloid toxicity which have not been apparent till now.
Figure 1.
A schematic depicting the putative effects of amyloid aggregates on the different parts and processes in a neuron. The boxes marked red constitute the effects on vesicular machinery, the focus of this review. Transporters: VMAT, vesicular monoamine; SERT, serotonin; NET, norepinephrine; GLUT, glutamate; DAT, dopamine.
(a). Amyloids and monoamine neurotransmitter synthesis
α-Syn significantly affects the activity of enzymes involved in the synthesis of dopamine. Dopamine is synthesized in the cytoplasm with the help of tyrosine hydroxylase (TH) and amino acid decarboxylase (AADC). Interestingly, α-syn affects TH activity in two known ways. Endogenous α-syn directly binds to TH as observed in rat brain homogenates and reduces TH activity as seen in cell-free assay. This was also observed in MN9D cells (a DA cell line) along with reduced TH phosphorylation and dopamine synthesis [62]. Secondly, in MN9D cells and in PC12 cells stably transfected with α-syn, it activates protein phosphatase 2A which eventually reduces TH phosphorylation and lowers its activity [63]. Overexpression of α-syn in MES23.5 DA cells reduces not only the activity of the TH promoter [64] but also reduces levels of TH mRNA (in neuroblastoma cell lines transfected with α-syn [65]) and the level of TH proteins (in α-syn overexpressed MES23.5 DA cells [66]). Conversely, silencing of α-syn expression in MN9D cells by vector-mediated RNA interference leads to increased phosphorylated TH, enhancing TH activity and dopamine biosynthesis [67]. Similar observations were made in animal models where overexpression of human α-syn in mice under the platelet-derived growth factor-β (PDGF-β) promoter [68] and in rats expressing α-syn in the nigrostriatal DA neurons [69] leads to reduced TH activity. Overexpression of α-syn in transfected DA and pheochromocytomic cell lines also reduces the activity of l-aromatic AADC, an enzyme which helps in the conversion of l-DOPA to dopamine, thereby reducing dopamine levels [70].
We can infer that one of the effects of α-syn is to regulate dopamine homeostasis by directly affecting the enzymes involved in the dopamine biosynthesis pathway. It is possible that the aggregation of α-syn leads to a loss of this regulatory function and increased levels of dopamine, thereby affecting neurotransmission and cellular health.
(b). Amyloids and sequestration into vesicles
There have been reports that the concentration of VGLUT1, a vesicle-specific neurotransmitter transporter, is reduced in the cortices of AD brain [71–73]. Reduced VGLUT1 levels will lead to defects in packaging of neurotransmitters into vesicles. On the other hand, Aβ has the potential to permeabilize membranes through its lipid binding properties as observed in artificially prepared phospholipid vesicles [74]. Aβ may therefore directly affect the integrity of synaptic vesicles and hence the levels of neurotransmitters inside and outside the vesicles.
In addition, α-syn forms multimers in transfected hippocampal neurons which associate with synaptic vesicles and form vesicular clusters, thereby restricting their motility [75]. One of the possible outcomes of such clustering could be to restrict synaptic vesicle recycling and affect neurotransmission. Thus, amyloids seem to affect vesicles either directly or indirectly, affecting sequestration of neurotransmitters in vesicles.
(c). Amyloids and vesicle transport within the neuron
Vesicles need to be transported to appropriate cellular locations for exocytosis and are sorted there into different pools, such as the reserve pool and the readily releasable pool [76,77]. Appropriate transport of vesicles is therefore a crucial part of the exocytosis machinery. There is strong evidence that in yeast and in lower animals (worms and drosophila), α-syn affects endoplasmic reticulum to Golgi transport [78]. Similar effect on transport and docking of vesicles to the Golgi is also seen in rat DA neurons in culture [79]. The effect manifests itself in an abnormal accumulation of vesicles in the soma, which may ultimately prove to be cytotoxic. The pathway through which α-syn affects the transport is not clear. However, there is clear evidence that microtubule assembly can be affected in AD [80]. In addition to Aβ aggregation, another hallmark of AD is the hyperphosphorylation of tau, a microtubule-associated protein. Tau hyperphosphorylation is possibly a downstream event of Aβ aggregation, and the microtubules may be affected through tau, in turn affecting vesicular transport. However, it has also been shown that 1 µM of exogenously added Aβ oligomers can affect the microtubule assembly directly, independent of tau as observed in NIH3T3 cells [80]. Transport impairment will have obvious consequences for vesicle storage near the location of exocytosis, and observations consistent with this expectation have been reported (vide infra). Photo-bleaching experiments have clearly shown that vesicle traffic between adjacent boutons is impaired in hippocampal neurons cultured from α-syn overexpressing mice [81]. However, it is difficult to directly track vesicle movement at or near the synaptic boutons, owing to the small spatial and temporal scales involved. So it has remained difficult to quantify these effects. Another important unsolved question is whether this is a primary effect, with the oligomers directly interacting with the microtubules, or a secondary one. A colocalization study of labelled amyloids with the transport machinery would be required to settle this question. Some of these issues will be discussed later in the context of somatic exocytosis.
(d). Amyloids and vesicular distribution in different vesicle pools
It has been observed that externally added 2 µM mixture of insoluble fibrillar and soluble oligomeric Aβ in cultured hippocampal cells causes ultra-structural changes which indicate impairment of synaptic vesicle endocytosis [82]. Electron microscopy experiments revealed a diminished pool of docked synaptic vesicles in terminals of squid giant synapse microinjected with 10–100 nM of Aβ42 oligomer [83].
On the other hand, it appears that α-syn at endogenous levels helps in regulating synaptic vesicle mobilization at nerve terminals. Microscopic examination of hippocampal synapses in mice lacking α-syn suggests that there exists a deficiency in the number of undocked vesicles while the number of docked vesicles remain unaffected. Moreover, replenishment of docked vesicles by the reserve pool was slower in these synapses [84]. Suppression of α-syn using antisense oligonucleotides in primary cultured hippocampal neurons decreased the availability of a ‘distal’ or reserve synaptic vesicle pool [85]. On the other hand, overexpression of α-syn in dissociated embryonic hippocampal cultures affects reclustering of synaptic vesicles post endocytosis, thereby reducing the size of the synaptic vesicle recycling pool [86]. However, in mice lacking α-syn, an increased rate of replenishment of the readily releasable pool has been reported in DA neurons, which tends to keep the pool size unchanged [87]. This is consistent with the recovery from paired-pulse depression (PPD) reported earlier in striatal slices of α-syn lacking mice [88]. Therefore, existing data from different brain regions, taken together, suggests that amyloids affect the size of the vesicle pools, but the effects may be system-dependent.
(e). Amyloids and priming of vesicles for exocytosis
The docked pools of vesicles are the first ones to undergo exocytosis upon depolarization. However, there is a complex array of steps which make this last step possible, a process generally termed ‘priming’. It involves the assembly of the SNARE proteins which operate on the vesicle as well as on the plasma membrane at the active zone where the vesicle is about to fuse. It has been suggested that this assembly and/or its response to the Ca2+ trigger for fusion is modified in neurodegenerative diseases. Overexpression of α-syn in PC12 and chromaffin cells leads to marked increase in the number of docked vesicles [89]. This effect was interpreted as a consequence of defects in the vesicle priming machinery. However, α-syn overexpression in primary hippocampal cultures did not change the number of docked vesicles [85]. Interestingly, co-localization of synaptophysin and α-syn, and an increase in the number of vesicles in the distal pool were also observed [85]. On the other hand, a decrease in the number of vesicles in the distal undocked pool in mice lacking α-syn has been reported [84], which was interpreted in terms of an increased release probability. Aβ seems to have similar effects on neurons. There have been reports of changes in the vesicle release probability on exogenous administration of Aβ oligomers (200 nM in concentration), though the data are contradictory [90,91]. There is an increase in the number of vesicles in the storage pool, and a decrease in the readily releasable pool, again consistent with an increase in the release probability [91]. Interestingly, exogenously added 50 nM oligomeric Aβ disrupts the complex formed between synaptophysin and vesicle-associated protein 2 (VAMP2) in cultured rat hippocampal neurons, thereby increasing the amount of primed vesicles [92]. Evidence points to α-syn increasing the release probability of the vesicles, perhaps by positively regulating the vesicle priming process.
(f). Effects of amyloids on endocytosis and exocytosis
It has been reported that levels of several presynaptic proteins are altered in the hippocampus and other areas of AD patient brains [93], presumably due to Aβ aggregation. External addition of a mixture of 2 µM solution of insoluble fibrillar and soluble oligomeric Aβ to cultured rat hippocampal neurons depletes the levels of dynamin-1, a key GTPase presynaptic protein which pinches off synaptic vesicles from the plasma membrane, leading to a disruption of synaptic vesicle endocytosis [82]. Aβ oligomers not only impair endocytosis but also impair regeneration of fusion-competent synaptic vesicles [91]. Both short- and long-term treatments of primary cortical neurons with 200 nM Aβ oligomers lowers the levels of PtDIns(4,5)P2, a phospholipid that maintains key neuronal function such as signal transduction, regulation of actin cytoskeleton, exocytosis, endocytosis and permeability of ion channels [94–98], and this may lead to alteration in the endocytosis process [99]. Expression of several proteins known to regulate endocytosis is affected by Aβ, and therefore, Aβ can also indirectly affect the endocytosis process.
When human α-syn is expressed in mice, it impairs synaptic vesicle exocytosis in hippocampal neurons [86]. Similar results are obtained in the case of rat ventral midbrain DA neurons transfected with α-syn. Overexpression of α-syn in hippocampal neurons affects the reclustering of synaptic vesicles following endocytosis, causing a reduction in the size of the synaptic vesicle recycling pool. Additionally, overexpression of α-syn carrying familial Parkinson's mutations causes an inhibition of exocytosis [86]. A reduction in the rate of dopamine release has been reported in cell culture and animal model systems overexpressing α-syn [100]. The existing data therefore collectively suggest that amyloids may have substantial effects on the endocytotic and exocytotic processes.
(g). Amyloids and reuptake
The monoamines, serotonin, dopamine and norepinephrine, have separate but related reuptake transporters. α-Syn, when cotransfected with dopamine transporter (DAT) in different cell lines, shows physical interaction with DAT and causes a dose-dependent reduction of its cell surface availability [101–104]. α-Syn also physically interacts with the serotonin transporter (SERT) in cotransfected cultured cells as well as in rat brain tissue, and negatively modulates its cell surface availability and uptake activity [105,106].
It appears that this lower availability is not due to a reduction in protein expression, but rather due to an increase in the endocytosis rates for these transporters. A decrease of dopamine reuptake by α-syn overexpressing nigral neurons has also been reported [107]. A lower reuptake rate may translate into a lower availability of these neurotransmitters for sequestration into vesicles. This would be in addition to any inhibition of dopamine synthesis mentioned earlier. However, it is difficult to ascertain whether that translates into a lower amount of neurotransmitters per vesicle. Direct imaging methods which can relate the signal observed from the vesicles with the concentration of the neurotransmitters contained in them (though not absolutely quantitative) would help answer some of these questions. This can potentially settle the question whether the amyloids have an effect on the neurotransmitter packaging.
3. Implication for somatic exocytosis
The results discussed above have mostly been worked out in the context of synaptic exocytosis. However, some of these are also equally applicable for somatic exocytosis, though the mechanisms and effects may not be completely equivalent. Impairment of the somatic exocytosis in neurons (such as monoaminergic ones) can play a major role in the toxicity pathway. Also, in many cases, the verification of the effect on somatic exocytosis machinery would actually be easier, though it may not have been achieved experimentally yet. Here we outline the possible effects on somatic exocytosis, and experiments that can be performed to verify them.
Somatic exocytosis observed in the rat raphe neurons involves transport of vesicles over large distances (many micrometres) from inside the cell to the plasma membrane for exocytosis [49,50]. So in the context of somatic exocytosis, any transport defect could have a severe effect. A dynamic real-time measurement of vesicular transport is, in principle, possible using vesicle marker dyes, such as the FM dyes [108,109]. It is even possible to track the movement of individual vesicles (or vesicular clusters) using the native fluorescence of serotonin [50]. The latter has the advantage of only showing the movements of the serotonin-filled vesicles, suppressing the background from others. Such measurements can yield the speed and directionality of vesicle movement, providing a quantitative understanding of the effects of amyloids. It should also be possible to perform a colocalization study of labelled Aβ, which can easily be observed inside cells and the microtubules. An example of the colocalization of FM1–43, which marks the vesicular membrane (visualized by confocal microscopy), and serotonin (visualized by three-photon microscopy) is shown in figure 2d [50]. Such experiments under external Aβ insult can help us understand whether Aβ directly associates with the transport assembly or unleashes its deleterious effects through secondary players. We note that, in principle, externally added Aβ can be different from the endogenous species, owing to possible differences in spatial distributions, aggregation states, conformation and post-translational modifications. However, external addition also has some advantages. It is exactly quantifiable, its aggregation state before addition can be determined, and the time of its introduction can be precisely controlled.
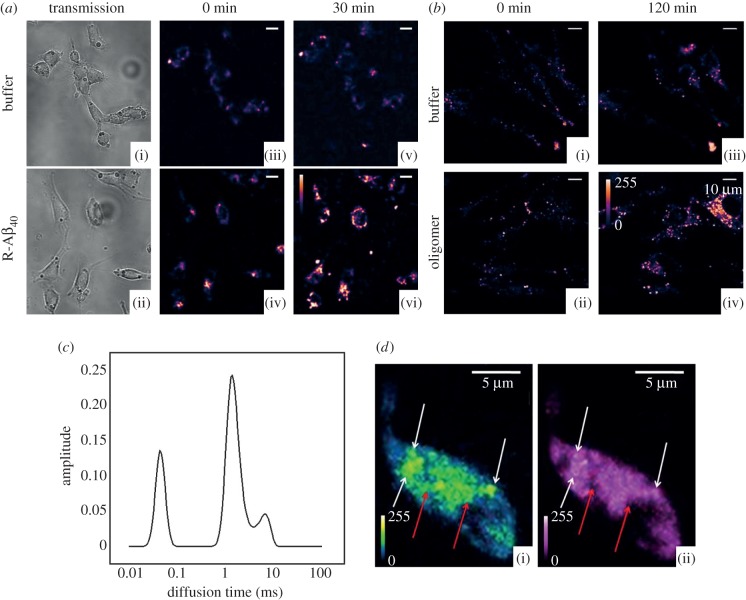
Figure 2.
Membrane interaction and internalization of amyloid β1–40 (Aβ40) incubated with RN46A cells. (a) Binding of Aβ40 oligomers to cell membranes of serotonergic neuron-derived cell line RN46A. ((i),(ii)) Transmission and ((iii),(iv)) confocal sections of two sets of cells with similar initial cell densities (excitation: 543 nm, emission: 550–700 nm) at time zero. ((v),(vi)) The same cells after 30 min of incubation with (v) buffer and (vi) 250 nM solution of rhodamine-labelled Aβ40(R-Aβ40). (b) Internalization of Aβ40 oligomers upon longer R-Aβ40 exposure: ((i),(ii)) confocal sections of two sets of cells with similar initial cell densities (excitation: 543 nm, emission: 550–700 nm) at time zero. ((iii),(iv)). The same cells after 120 min of incubation with (iii) buffer and (iv) 250 nM solution of R-Aβ40. (c) Aggregation of Aβ40 as seen in cultured neuronal cell membranes: a maximum entropy method based model-free data analysis (using the MEMFCS algorithm [109]) of diffusion of R-Aβ40 on RN46A cell membranes incubated with 1 µM Aβ40 solution for 1 h, determined by FCS. Diffusion time is proportional to size. Methods have been described elsewhere [111,112]. (d) Colocalization of serotonin and vesicles labelled with FM1–43. Quasi-simultaneous imaging of (i) serotonin (5-HT) using three-photon excitation and (ii) FM1–43 (a vesicle labelling dye), using one photon excitation in cultured serotonergic neurons depolarized by high K+ exocytosis buffer. In both the images, white arrows show colocalized vesicular structures, and red arrows mark spots visible in the serotonin channel only, most probably vesicles which were not recycled. (Adapted from Sarkar et al. [50].)
An impairment of the synthesis of monoamines can lower the vesicular content, lower the number of vesicles or both. Since both serotonin and dopamine fluorescence can be imaged directly using multiphoton excitation, it is possible to measure both the content and the population of monoamine-containing somatic vesicles in a given cell [49,50,54,58]. This presents a unique opportunity compared to studying synaptic exocytosis, as vesicles at a typical synapse cannot be visualized in live cells. Impairment of sequestration, including leakage from the somatic vesicles, can have strong effects on the health of the cells, as free neurotransmitters can be toxic. The somatic vesicles can be much larger than the synaptic vesicles and together hold as much or more monoamines than their synaptic counterparts, as was shown by a brain-slice wide mapping of serotonin in the rat brain [54]. These somatic storage sites are also close to vital housekeeping machinery and the nucleus, increasing the potential for damage.
Vesicle transport defects can also cause major disruption to the process of somatic exocytosis. We have shown previously that within a few minutes of persistent depolarization, vesicles from the interior perinuclear region of the cell are transported to the plasma membrane where they start getting released [49,110]. A single vesicle can move out from a cluster of vesicles at the interior of the cell, while other members of the same cluster remain immobile. This would suggest that a transport mechanism, and not random diffusion, takes vesicles from their storage areas to the place of exocytosis, frequently several micrometres away. More recent work has shown that vesicle transport can be diffusive, but only over a small distance range—indicating the presence of ‘tethers' or barriers which keep the vesicles near their original place, until they are moved by an external machinery [50]. If amyloid aggregates affect this tethering process, it may actually make the vesicles free to diffuse. It is difficult to predict whether such uncontrolled diffusion would increase the rate of exocytosis, but it remains an interesting possibility.
Organization of vesicles into different pools, such as the readily releasable pool and the recycling pool, has not been clearly identified in somatic exocytosis. However, experiments with colocalized FM1–43 and serotonin imaging have shown that even after 10 min of depolarization, there are pools of serotonin-rich vesicles which do not get stained with FM1–43 (figure 2d) [50]. This suggests the existence of a storage pool which does not easily participate in the exocytosis process. Conversely, there are FM1–43 labelled vesicles which do not contain serotonin. This latter pool may consist of freshly recycled vesicles, which are in the process of getting refilled. Therefore, it is, in principle, possible to generate separate statistics for these different pools. The majority of the vesicles, however, contain serotonin and are also stained by the FM dye, and probably belong to the recycling pool, at least at the relatively long time scale of somatic exocytosis. Though less evidence is available for somatic DA vesicles, the overall organization and the kinetics of exocytosis appear to be similar to those observed for the somatic serotonergic machinery [58]. While the effect of amyloids on the relative size of these somatic vesicular pools is not known, such experiments are, in principle, doable with the technology we have developed.
A lack of proper characterization of the nature of aggregates is a lacuna in most of the experiments discussed here. It is believed that neuronal damage is mostly caused by the soluble oligomeric intermediates of aggregation. However, there is no consensus about which of these oligomers is toxic. It is possible that the different, sometimes inconsistent, effects observed by different laboratories originate from differences between the aggregate species. There are rather precise fluorescence-based methods to characterize the different oligomers. Forster resonance energy transfer (FRET)-based experiments yield the conformational state of the oligomers, while fluorescence correlation spectroscopy (FCS)-based studies can measure the size of the aggregates [111,113–115]. It is important to compare the effects of amyloid aggregates correctly, after duly considering the type of aggregates used in each of these experiments. It is possible to track the key amyloid species which cause toxicity. For example, starting with extracellular fluorescently labelled Aβ40 as a model peptide, we have shown that the oligomers of fluorescently labelled Aβ40 peptides (whose sizes are characterized by FCS in solution) attach to the membrane of serotonergic RN46A cells (for example, see figure 2a [114,116]). These oligomers form large assemblies on the membrane, perhaps indicating the formation of ion channel-like structures, which can also be characterized by FCS (for example, see figure 2c; experimental details are similar to those described elsewhere [112]). It is also possible to visualize the Aβ40 oligomers eventually entering the cells (figure 2b). Such imaging can, in principle, be combined with direct multi-photon imaging of serotonin [117] or dopamine [58]. Thus, some of the experimental limitations of the synaptic experiments can be overcome if a similar set of experiments are attempted in a cell carrying out somatic exocytosis. Such experiments may provide valuable inputs for understanding the mechanism of amyloid-induced toxicity.
4. Summary
Somatic exocytosis has remained an unexplored but potentially significant player in determining the toxic effects of amyloids on neurons. It can help explain why serotonergic and DA neurons are especially vulnerable in Alzheimer's and Parkinson's diseases, respectively. We now have a wealth of information on how amyloids affect the synaptic exocytosis process. Some of these toxic processes are unlikely to spare components of the somatic exocytosis process. Optical microscopic and spectroscopic techniques are now available which have the power to unveil the damage wrought by amyloids on the somatic exocytosis machinery. These can, in principle, yield data with a level of detail not easily attainable in experiments performed on the much smaller synaptic machinery. Somatic exocytosis may yet prove to be one of the most significant pieces of the neurodegeneration puzzle, especially for AD and PD.
Funding statement
This work is partially supported by Department of Biotechnology grant no. BT/53/NE/TBP/2010 to S.M.
Authors' contributions
A.K.D. and R.P. co-wrote the manuscript, and S.M. conceptualized the manuscript, decided on its organization and content, and edited it.
Competing interests
The authors declare no competing interests.
References
- 1.Benilova I, Karran E, De Strooper B. 2001. 2012 The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat. Neurosci. 15, 349–357. ( 10.1038/nn.3028) [DOI] [PubMed] [Google Scholar]
- 2.Cataldo AM, et al. 2004. Aβ localization in abnormal endosomes: association with earliest Aβ elevations in AD and Down syndrome. Neurobiol. Aging 25, 1263–1272. ( 10.1016/j.neurobiolaging.2004.02.027) [DOI] [PubMed] [Google Scholar]
- 3.D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. 2004. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology 38, 120–134. ( 10.1046/j.1365-2559.2001.01082.x) [DOI] [PubMed] [Google Scholar]
- 4.Echeverria V, Cuello AC. 2002. Intracellular A-β amyloid, a sign for worse things to come? Mol. Neurobiol. 26, 299–316. ( 10.1385/MN:26:2-3:299) [DOI] [PubMed] [Google Scholar]
- 5.Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. 2001. Intraneuronal aβ-amyloid precedes development of amyloid plaques in Down syndrome. Arch. Pathol. Lab. Med. 125, 489–492. [DOI] [PubMed] [Google Scholar]
- 6.Tabira T, Chui DH, Kuroda S. 2002. Significance of intracellular Aβ42 accumulation in Alzheimer's disease. Front. Biosci. 7, a44–a49. ( 10.2741/tabira) [DOI] [PubMed] [Google Scholar]
- 7.Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. 2001. Intraneuronal Aβ accumulation precedes plaque formation in β-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. 306, 116–120. ( 10.1016/S0304-3940(01)01876-6) [DOI] [PubMed] [Google Scholar]
- 8.Wirths O, Multhaup G, Czech C, Feldmann N, Blanchard V, Tremp G, Beyreuther K, Pradier L, Bayer TA, 2002. Intraneuronal APP/Aβ trafficking and plaque formation in β-amyloid precursor protein and presenilin-1 transgenic mice. Brain Pathol. 12, 275–286. ( 10.1111/j.1750-3639.2002.tb00442.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi RH, et al. 2002. Intraneuronal Alzheimer aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161, 1869–1879. ( 10.1016/S0002-9440(10)64463-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oddo S, et al. 2003. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421. ( 10.1016/S0896-6273(03)00434-3) [DOI] [PubMed] [Google Scholar]
- 11.Casas C, et al. 2004. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am. J. Pathol. 165, 1289–1300. ( 10.1016/S0002-9440(10)63388-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. 2007. Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J. Neurosci. 27, 7648–7653. ( 10.1523/JNEUROSCI.0395-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN. 2006. The Arctic Alzheimer mutation facilitates early intraneuronal Aβ aggregation and senile plaque formation in transgenic mice. Neurobiol. Aging 27, 67–77. ( 10.1016/j.neurobiolaging.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 14.Oakley H, et al. 2006. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10 129–10 140. ( 10.1523/JNEUROSCI.1202-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Broeck B, et al. 2008. Intraneuronal amyloid β and reduced brain volume in a novel APP T714I mouse model for Alzheimer's disease. Neurobiol. Aging 29, 241–252. ( 10.1016/j.neurobiolaging.2006.10.016) [DOI] [PubMed] [Google Scholar]
- 16.Tomiyama T, et al. 2010. A mouse model of amyloid β oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J. Neurosci. 30, 4845–4856. ( 10.1523/JNEUROSCI.5825-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, Laferla FM, Saura CA. 2010. Intraneuronal β-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol. Psychiatry 67, 513–521. ( 10.1016/j.biopsych.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 18.Gandy S, et al. 2010. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-β oligomers. Annu. Neurol. 68, 220–230. ( 10.1002/ana.22052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi O, Dolev I, Belinson H, Michaelson DM. 2007. Intraneuronal amyloid-β plays a role in mediating the synergistic pathological effects of apoE4 and environmental stimulation. J. Neurochem. 103, 1031–1040. ( 10.1111/j.1471-4159.2007.04810.x) [DOI] [PubMed] [Google Scholar]
- 20.Petrucelli L, et al. 2002. Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36, 1007–1019. ( 10.1016/S0896-6273(02)01125-X) [DOI] [PubMed] [Google Scholar]
- 21.Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. 2001. Expression of A53 T mutant but not wild-type α-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J. Neurosci. 21, 9549–9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu LJ, et al. 2000. α-Synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 157, 401–410. ( 10.1016/S0002-9440(10)64553-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka Y, et al. 2001. Inducible expression of mutant α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 10, 919–926. ( 10.1093/hmg/10.9.919) [DOI] [PubMed] [Google Scholar]
- 24.Klein AM, Kowall NW, Ferrante RJ. 1999. Neurotoxicity and oxidative damage of β amyloid 1–42 versus β amyloid 1–40 in the mouse cerebral cortex. Ann. NY Acad. Sci. 893, 314–320. ( 10.1111/j.1749-6632.1999.tb07845.x) [DOI] [PubMed] [Google Scholar]
- 25.Auluck PK, Caraveo G, Lindquist S. 2010. α-Synuclein: membrane interactions and toxicity in Parkinson's disease. Annu. Rev. Cell Dev. Biol. 26, 211–233. ( 10.1146/annurev.cellbio.042308.113313) [DOI] [PubMed] [Google Scholar]
- 26.Arispe N, Rojas E, Pollard HB. 1993. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl Acad. Sci. USA 90, 567–571. ( 10.1073/pnas.90.2.567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lal R, Lin H, Quist AP. 2007. Amyloid β ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta 1768, 1966–1975. ( 10.1016/j.bbamem.2007.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. 2005. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc. Natl Acad. Sci. USA 102, 10 427–10 432. ( 10.1073/pnas.0502066102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demuro A, Smith M, Parker I. 2011. Single-channel Ca2+ imaging implicates Aβ1–42 amyloid pores in Alzheimer's disease pathology. J. Cell Biol. 195, 515–524. ( 10.1083/jcb.201104133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawahara M, Arispe N, Kuroda Y, Rojas E. 1997. Alzheimer's disease amyloid β-protein forms Zn(2+)-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophys. J. 73, 67–75. ( 10.1016/S0006-3495(97)78048-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson KL, Butler L, Ingram VM. 1997. Aggregates of a β-amyloid peptide are required to induce calcium currents in neuron-like human teratocarcinoma cells: relation to Alzheimer's disease. Brain Res. 744, 7–14. ( 10.1016/S0006-8993(96)01060-8) [DOI] [PubMed] [Google Scholar]
- 32.Mason RP, Jacob RF, Walter MF, Mason PE, Avdulov NA, Chochina SV, Igbavboa U, Wood WG. 1999. Distribution and fluidizing action of soluble and aggregated amyloid β-peptide in rat synaptic plasma membranes. J. Biol. Chem. 274, 18 801–18 807. ( 10.1074/jbc.274.26.18801) [DOI] [PubMed] [Google Scholar]
- 33.Bhatia R, Lin H, Lal R. 2000. Fresh and globular amyloid β protein (1–42) induces rapid cellular degeneration: evidence for AβP channel-mediated cellular toxicity. FASEB J. 14, 1233–1243. [DOI] [PubMed] [Google Scholar]
- 34.Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. 2010. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 66, 739–754. ( 10.1016/j.neuron.2010.04.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demuro A, Parker I, Stutzmann GE. 2010. Calcium signaling and amyloid toxicity in Alzheimer disease. J. Biol. Chem. 285, 12 463–12 468. ( 10.1074/jbc.R109.080895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebollela A, et al. 2012. Amyloid-β oligomers induce differential gene expression in adult human brain slices. J. Biol. Chem. 287, 7436–7445. ( 10.1074/jbc.M111.298471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. 2002. Alzheimer's disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Progr. Neurobiol. 68, 209–245. ( 10.1016/S0301-0082(02)00079-5) [DOI] [PubMed] [Google Scholar]
- 38.Mufson EJ, Ginsberg SD, Ikonomovic MD, Dekosky ST. 2003. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 26, 233–242. ( 10.1016/S0891-0618(03)00068-1) [DOI] [PubMed] [Google Scholar]
- 39.Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. 1988. The neuropathology of aminergic nuclei in Alzheimer's disease. Ann. Neurol. 24, 233–242. ( 10.1002/ana.410240210) [DOI] [PubMed] [Google Scholar]
- 40.Aletrino MA, Vogels OJ, Van Domburg PH, Ten Donkelaar HJ. 1992. Cell loss in the nucleus raphes dorsalis in Alzheimer's disease. Neurobiol. Aging 13, 461–468. ( 10.1016/0197-4580(92)90073-7) [DOI] [PubMed] [Google Scholar]
- 41.Brichta L, Greengard P, Flajolet M. 2013. Advances in the pharmacological treatment of Parkinson's disease: targeting neurotransmitter systems. Trends Neurosci. 36, 543–554. ( 10.1016/j.tins.2013.06.003) [DOI] [PubMed] [Google Scholar]
- 42.Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. 1986. A correlation analysis of the regional distribution of central enkephalin and β-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol. Scand. 128, 201–207. ( 10.1111/j.1748-1716.1986.tb07967.x) [DOI] [PubMed] [Google Scholar]
- 43.De-Miguel FF, Fuxe K. 2012. Extrasynaptic neurotransmission as a way of modulating neuronal functions. Front. Physiol. 3, 16 ( 10.3389/fphys.2012.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunin MA, Wightman RM. 1998. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J. Neurosci. 18, 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De-Miguel FF, Trueta C. 2005. Synaptic and extrasynaptic secretion of serotonin. Cell Mol. Neurobiol. 25, 297–312. ( 10.1007/s10571-005-3061-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trueta C, De-Miguel FF. 2012. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front. Physiol. 3, 319 ( 10.3389/fphys.2012.00319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Descarries L, Beaudet A, Watkins KC. 1975. Serotonin nerve terminals in adult rat neocortex. Brain Res. 100, 563–588. ( 10.1016/0006-8993(75)90158-4) [DOI] [PubMed] [Google Scholar]
- 48.Fuxe K, et al. 2007. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res. Rev. 55, 17–54. ( 10.1016/j.brainresrev.2007.02.009) [DOI] [PubMed] [Google Scholar]
- 49.Kaushalya SK, Desai R, Arumugam S, Ghosh H, Balaji J, Maiti S. 2008. Three-photon microscopy shows that somatic release can be a quantitatively significant component of serotonergic neurotransmission in the mammalian brain. J. Neurosci. Res. 86, 3469–3480. ( 10.1002/jnr.21794) [DOI] [PubMed] [Google Scholar]
- 50.Sarkar B, Das AK, Arumugam S, Kaushalya SK, Bandyopadhyay A, Balaji J, Maiti S. 2012. The dynamics of somatic exocytosis in monoaminergic neurons. Front. Physiol. 3, 414 ( 10.3389/fphys.2012.00414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruns D, Riedel D, Klingauf J, Jahn R. 2003. Quantal release of serotonin. Neuron 28, 205–220. ( 10.1016/S0896-6273(00)00097-0) [DOI] [PubMed] [Google Scholar]
- 52.Trueta C, Mendez B, De-Miguel FF. 2003. Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones. J. Physiol. 547, 405–416. ( 10.1113/jphysiol.2002.030684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B, et al. 2012. Action potential-triggered somatic exocytosis in mesencephalic trigeminal nucleus neurons in rat brain slices. J. Physiol. 590, 753–762. ( 10.1113/jphysiol.2011.221051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaushalya SK, Nag S, Ghosh H, Arumugam S, Maiti S. 2008. A high-resolution large area serotonin map of a live rat brain section. Neuroreport 19, 717–721. ( 10.1097/WNR.0b013e3282fd6946) [DOI] [PubMed] [Google Scholar]
- 55.Kumar M, Kaushalya SK, Gressens P, Maiti S, Mani S. 2009. Optimized derivation and functional characterization of 5-HT neurons from human embryonic stem cells. Stem Cells Dev. 18, 615–627. ( 10.1089/scd.2008.0181) [DOI] [PubMed] [Google Scholar]
- 56.Jaffe EH, Marty A, Schulte A, Chow RH. 1998. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J. Neurosci. 18, 3548–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. 2001. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron 30, 211–225. ( 10.1016/S0896-6273(01)00274-4) [DOI] [PubMed] [Google Scholar]
- 58.Sarkar B, Banerjee A, Das AK, Nag S, Kaushalya SK, Tripathy U, Shameem M, Shukla S, Maiti S, 2014. Label-free dopamine imaging in live rat brain slices. ACS Chem. Neurosci. 5, 329–334. ( 10.1021/cn5000138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisaglia M, Filograna R, Beltramini M, Bubacco L. 2014. Are dopamine derivatives implicated in the pathogenesis of Parkinson's disease? Ageing Res. Rev. 13, 107–114. ( 10.1016/j.arr.2013.12.009) [DOI] [PubMed] [Google Scholar]
- 60.Cubells JF, Rayport S, Rajendran G, Sulzer D. 1994. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci. 14, 2260–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selkoe DJ. 2002. Alzheimer's disease is a synaptic failure. Science 298, 789–791. ( 10.1126/science.1074069) [DOI] [PubMed] [Google Scholar]
- 62.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. 2002. A role for α-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 22, 3090–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. 2005. α-Synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J. Cell Sci. 118, 3523–3530. ( 10.1242/jcs.02481) [DOI] [PubMed] [Google Scholar]
- 64.Gao N, Li YH, Li X, Yu S, Fu GL, Chen B. 2007. Effect of α-synuclein on the promoter activity of tyrosine hydroxylase gene. Neurosci. Bull. 23, 53–57. ( 10.1007/s12264-007-0008-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baptista MJ, O'farrell C, Daya S, Ahmad R, Miller DW, Hardy J, Farrer MJ, Cookson MR. 2003. Co-ordinate transcriptional regulation of dopamine synthesis genes by α-synuclein in human neuroblastoma cell lines. J. Neurochem. 85, 957–968. ( 10.1046/j.1471-4159.2003.01742.x) [DOI] [PubMed] [Google Scholar]
- 66.Yu S, Zuo X, Li Y, Zhang C, Zhou M, Zhang YA, Uéda K, Chan P. 2004. Inhibition of tyrosine hydroxylase expression in α-synuclein-transfected dopaminergic neuronal cells. Neurosci. Lett. 367, 34–39. ( 10.1016/j.neulet.2004.05.118) [DOI] [PubMed] [Google Scholar]
- 67.Liu D, et al. 2008. Silencing α-synuclein gene expression enhances tyrosine hydroxylase activity in MN9D cells. Neurochem. Res. 33, 1401–1409. ( 10.1007/s11064-008-9599-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. 2000. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269. ( 10.1126/science.287.5456.1265) [DOI] [PubMed] [Google Scholar]
- 69.Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Björklund A. 2002. Parkinson-like neurodegeneration induced by targeted overexpression of α-synuclein in the nigrostriatal system. J. Neurosci. 22, 2780–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG. 2006. α-Synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J. Neurochem. 99, 1188–1196. ( 10.1111/j.1471-4159.2006.04146.x) [DOI] [PubMed] [Google Scholar]
- 71.Kirvell SL, Esiri M, Francis PT. 2006. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer's disease. J. Neurochem. 98, 939–950. ( 10.1111/j.1471-4159.2006.03935.x) [DOI] [PubMed] [Google Scholar]
- 72.Chen KH, Reese EA, Kim HW, Rapoport SI, Rao JS. 2011. Disturbed neurotransmitter transporter expression in Alzheimer's disease brain. J. Alzheimer's Dis. 26, 755–766. ( 10.3233/JAD-2011-110002) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Kashani A, et al. 2008. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol. Aging 29, 1619–1630. ( 10.1016/j.neurobiolaging.2007.04.010) [DOI] [PubMed] [Google Scholar]
- 74.McLaurin J, Chakrabartty A. 1996. Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. J. Biol. Chem. 271, 26 482–26 489. ( 10.1074/jbc.271.43.26482) [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. 2014. α-Synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 24, 2319–2326. ( 10.1016/j.cub.2014.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murthy VN, Stevens CF. 1999. Reversal of synaptic vesicle docking at central synapses. Nat. Neurosci. 2, 503–507. ( 10.1038/9149) [DOI] [PubMed] [Google Scholar]
- 77.Sudhof TC. 2000. The synaptic vesicle cycle revisited. Neuron 28, 317–320. ( 10.1016/S0896-6273(00)00109-4) [DOI] [PubMed] [Google Scholar]
- 78.Cooper AA, et al. 2006. α-Synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324–328. ( 10.1126/science.1129462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gitler AD, et al. 2008. The Parkinson's disease protein α-synuclein disrupts cellular Rab homeostasis. Proc. Natl Acad. Sci. USA 105, 145–150. ( 10.1073/pnas.0710685105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pianu B, Lefort R, Thuiliere L, Tabourier E, Bartolini F. 2014. The Aβ 1–42 peptide regulates microtubule stability independently of tau. J. Cell Sci. 127, 1117–1127. ( 10.1242/jcs.143750) [DOI] [PubMed] [Google Scholar]
- 81.Scott D, Roy S. 2012. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 32, 10 129–10 135. ( 10.1523/JNEUROSCI.0535-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly BL, Ferreira A. 2007. β-amyloid disrupted synaptic vesicle endocytosis in cultured hippocampal neurons. Neuroscience 147, 60–70. ( 10.1016/j.neuroscience.2007.03.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinas RR. 2009. Synaptic transmission block by presynaptic injection of oligomeric amyloid β. Proc. Natl Acad. Sci. USA 106, 5901–5906. ( 10.1073/pnas.0900944106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cabin DE, et al. 2002. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J. Neurosci. 22, 8797–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. 2000. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. 2010. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65, 66–79. ( 10.1016/j.neuron.2009.12.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yavich L, Tanila H, Vepsalainen S, Jakala P. 2004. Role of α-synuclein in presynaptic dopamine recruitment. J. Neurosci. 24, 11 165–11 170. ( 10.1523/JNEUROSCI.2559-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abeliovich A, et al. 2000. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252. ( 10.1016/S0896-6273(00)80886-7) [DOI] [PubMed] [Google Scholar]
- 89.Larsen KE, et al. 2006. α-Synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J. Neurosci. 26, 11 915–11 922. ( 10.1523/JNEUROSCI.3821-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ripoli C, Piacentini R, Riccardi E, Leone L, Li Puma DD, Bitan G, Grassi C. 2013. Effects of different amyloid β-protein analogues on synaptic function. Neurobiol. Aging 34, 1032–1044. ( 10.1016/j.neurobiolaging.2012.06.027) [DOI] [PubMed] [Google Scholar]
- 91.Park J, Jang M, Chang S. 2013. Deleterious effects of soluble amyloid-β oligomers on multiple steps of synaptic vesicle trafficking. Neurobiol. Dis. 55, 129–139. ( 10.1016/j.nbd.2013.03.004) [DOI] [PubMed] [Google Scholar]
- 92.Russell CL, Semerdjieva S, Empson RM, Austen BM, Beesley PW, Alifragis P. 2012. Amyloid-β acts as a regulator of neurotransmitter release disrupting the interaction between synaptophysin and VAMP2. PLoS ONE 7, e43201 ( 10.1371/journal.pone.0043201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Honer WG. 2003. Pathology of presynaptic proteins in Alzheimer's disease: more than simple loss of terminals. Neurobiol. Aging 24, 1047–1062. ( 10.1016/j.neurobiolaging.2003.04.005) [DOI] [PubMed] [Google Scholar]
- 94.Kim S, Kim H, Chang B, Ahn N, Hwang S, Di Paolo G, Chang S. 2006. Regulation of transferrin recycling kinetics by PtdIns[4,5]P2 availability. FASEB J. 20, 2399–2401. ( 10.1096/fj.05-4621fje) [DOI] [PubMed] [Google Scholar]
- 95.Di Paolo G, De Camilli P. 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657. ( 10.1038/nature05185) [DOI] [PubMed] [Google Scholar]
- 96.Yin HL, Janmey PA. 2003. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65, 761–789. ( 10.1146/annurev.physiol.65.092101.142517) [DOI] [PubMed] [Google Scholar]
- 97.Suh BC, Hille B. 2005. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15, 370–378. ( 10.1016/j.conb.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 98.Hilgemann DW, Feng S, Nasuhoglu C. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Science STKE 2001, re19 ( 10.1126/stke.2001.111.re19) [DOI] [PubMed] [Google Scholar]
- 99.Berman DE, et al. 2008. Oligomeric amyloid-β peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat. Neurosci. 11, 547–554. ( 10.1038/nn.2100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yavich L, et al. 2005. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human α-synuclein. Neurobiol. Dis. 20, 303–313. ( 10.1016/j.nbd.2005.03.010) [DOI] [PubMed] [Google Scholar]
- 101.Lee FJ, Liu F, Pristupa ZB, Niznik HB. 2001. Direct binding and functional coupling of α-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 15, 916–926. ( 10.1096/fj.00-0334com) [DOI] [PubMed] [Google Scholar]
- 102.Maiya R, Mayfield RD. 2004. Dopamine transporter network and pathways. Int. Rev. Neurobiol. 61, 79–96. ( 10.1016/S0074-7742(04)61004-X) [DOI] [PubMed] [Google Scholar]
- 103.Wersinger C, Sidhu A. 2005. Disruption of the interaction of α-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry 44, 13 612–13 624. ( 10.1021/bi050402p) [DOI] [PubMed] [Google Scholar]
- 104.Wersinger C, Sidhu A. 2003. Attenuation of dopamine transporter activity by α-synuclein. Neurosci. Lett. 340, 189–192. ( 10.1016/S0304-3940(03)00097-1) [DOI] [PubMed] [Google Scholar]
- 105.Wersinger C, Rusnak M, Sidhu A. 2006. Modulation of the trafficking of the human serotonin transporter by human α-synuclein. Eur. J. Neurosci. 24, 55–64. ( 10.1111/j.1460-9568.2006.04900.x) [DOI] [PubMed] [Google Scholar]
- 106.Oaks AW, Sidhu A. 2011. Synuclein modulation of monoamine transporters. FEBS Lett. 585, 1001–1006. ( 10.1016/j.febslet.2011.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lundblad M, Decressac M, Mattsson B, Bjorklund A. 2012. Impaired neurotransmission caused by overexpression of α-synuclein in nigral dopamine neurons. Proc. Natl Acad. Sci. USA 109, 3213–3219. ( 10.1073/pnas.1200575109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fischer-Parton S, Parton RM, Hickey PC, Dijksterhuis J, Atkinson HA, Read ND. 2000. Confocal microscopy of FM4–64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 198, 246–259. ( 10.1046/j.1365-2818.2000.00708.x) [DOI] [PubMed] [Google Scholar]
- 109.Betz WJ, Bewick GS, Ridge RM. 1992. Intracellular movements of fluorescently labeled synaptic vesicles in frog motor nerve terminals during nerve stimulation. Neuron 9, 805–813. ( 10.1016/0896-6273(92)90235-6) [DOI] [PubMed] [Google Scholar]
- 110.Balaji J, Desai R, Kaushalya SK, Eaton MJ, Maiti S. 2005. Quantitative measurement of serotonin synthesis and sequestration in individual live neuronal cells. J. Neurochem. 95, 1217–1226. ( 10.1111/j.1471-4159.2005.03489.x) [DOI] [PubMed] [Google Scholar]
- 111.Nag S, Sarkar B, Bandyopadhyay A, Sahoo B, Sreenivasan VKA, Kombrabail M, Muralidharan C, Maiti S. 2011. Nature of the amyloid-β monomer and the monomer-oligomer equilibrium. J. Biol. Chem. 286, 13 827–13 833. ( 10.1074/jbc.M110.199885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sengupta P, Garai K, Balaji J, Periasamy N, Maiti S. 2003. Measuring size distribution in highly heterogeneous systems with fluorescence correlation spectroscopy. Biophys. J. 84, 1977–1984. ( 10.1016/S0006-3495(03)75006-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nag S, et al. 2013. A folding transition underlies the emergence of membrane affinity in amyloid-β. Phys. Chem. Chem. Phys. 15, 19 129–19 133. ( 10.1039/c3cp52732h) [DOI] [PubMed] [Google Scholar]
- 114.Sarkar B, Das AK, Maiti S. 2013. Thermodynamically stable amyloid-β monomers have much lower membrane affinity than the small oligomers. Front. Physiol. 4, 84 ( 10.3389/fphys.2013.00084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarkar B, Mithu VS, Chandra B, Mandal A, Chandrakesan M, Bhowmik D, Madhu PK, Maiti S. 2014. Significant structural differences between transient amyloid-β oligomers and less-toxic fibrils in regions known to harbor familial Alzheimer's mutations. Angew. Chem. Int. Ed. Engl. 53, 6888–6892. ( 10.1002/anie.201402636) [DOI] [PubMed] [Google Scholar]
- 116.Bhowmik D, Das AK, Maiti S. In press. Rapid, cell-free assay for membrane-active forms of amyloid-β. Langmuir. ( 10.1021/la502679t) [DOI] [PubMed] [Google Scholar]
- 117.Maiti S, Shear JB, Williams RM, Zipfel WR, Webb WW. 1997. Measuring serotonin distribution in live cells with three-photon excitation. Science 275, 530–532. ( 10.1126/science.275.5299.530) [DOI] [PubMed] [Google Scholar]