Abstract
Inflammatory mechanisms are proposed to play a role in l-DOPA-induced dyskinesia. Cyclooxygenase-2 (COX2) contributes to inflammation pathways in the periphery and is constitutively expressed in the central nervous system. Considering that inhibition of nitric oxide (NO) formation attenuates l-DOPA-induced dyskinesia, this study aimed at investigating if a NO synthase (NOS) inhibitor would change COX2 brain expression in animals with l-DOPA-induced dyskinesia. To this aim, male Wistar rats received unilateral 6-hydroxydopamine microinjection into the medial forebrain bundle were treated daily with l-DOPA (21 days) combined with 7-nitroindazole or vehicle. All hemi-Parkinsonian rats receiving l-DOPA showed dyskinesia. They also presented increased neuronal COX2 immunoreactivity in the dopamine-depleted dorsal striatum that was directly correlated with dyskinesia severity. Striatal COX2 co-localized with choline-acetyltransferase, calbindin and DARPP-32 (dopamine-cAMP-regulated phosphoprotein-32), neuronal markers of GABAergic neurons. NOS inhibition prevented l-DOPA-induced dyskinesia and COX2 increased expression in the dorsal striatum. These results suggest that increased COX2 expression after l-DOPA long-term treatment in Parkinsonian-like rats could contribute to the development of dyskinesia.
Keywords: COX2, prostaglandin H-synthase, neuroinflammation, striatum interneurons, volume transmission, extrasynaptic signalling
1. Introduction
Parkinson's disease is an incurable neurodegenerative disease. The incapacitating motor symptoms are mainly due to the degeneration of dopaminergic neurons in substantia nigra compacta, leading to a loss of striatal dopaminergic fibres [1]. Dopamine is a key transmitter in the basal ganglia, yet dopamine transmission does not conform to several aspects of the classic synaptic doctrine [2]. In the striatum, axonal release sites are controversial, with evidence for dopamine varicosities that lack postsynaptic specializations. Instead, dopamine acts on extrasynaptic receptors and transporters. The dopamine precursor l-DOPA (l-3,4-dihydroxyphenylalanine) represents the most effective and best-tolerated compound for therapy for Parkinson's disease. Unfortunately, in many cases, l-DOPA treatment has to be suspended because of its side effects, which include motor fluctuations, dyskinesia (abnormal involuntary movements) and psychiatric problems [3]. Currently, the molecular events that underlie these adverse effects are poorly understood.
In vivo brain imaging of Parkinson's disease patients reveals an association between widespread microglial activation and the pathological process [4–6] (see also [7]).In addition, both cellular and molecular studies of post-mortem human brain tissue show neuroinflammatory processes in the affected brain regions of these patients (for review, see [8–10]). Nonetheless, these studies do not indicate whether neuroinflammation is involved in the pathological process or is secondary to the neuronal degeneration. Moreover, because Parkinsonian patients are usually receiving l-DOPA or other anti-Parkinsonian medication at the time of death, it cannot be excluded that these treatments are also involved in the neuroinflammatory reaction [4,11–16]. According to this hypothesis, the excessive levels of dopamine in the striatal extracellular fluid following the administration of the dopamine precursor l-DOPA [17–20] would favour the development of a pro-inflammatory environment in the striatum [21].
We discovered that inhibition of the nitric oxide (NO) signalling pathway reduces l-DOPA-induced dyskinesia and the levels of associated molecular markers in hemi-Parkinsonian rodents [22–27]. This finding has been corroborated by studies from other laboratories in rats [28] and monkeys [29] and in the Pitx3-deficient aphakia mouse [30]. From a clinical standpoint, our behavioural analysis suggests that NO synthase (NOS) inhibitors could at least alleviate l-DOPA-induced dyskinesia in Parkinson's disease patients under chronic l-DOPA therapy without compromising its beneficial effect on akinesia [24,27]. NO is an interneuronal signalling molecule that is synthesized on demand from its precursor l-arginine by the NOS enzymes and freely diffuses out from the source cell [31,32]. Interestingly, NO also seems to participate in inflammatory processes observed in Parkinson's disease [33–35]. Hemi-Parkinsonian rats presenting l-DOPA-induced dyskinesia show increased expression in the striatum of neuronal NOS (nNOS) mRNA [24], nNOS and inducible NOS (iNOS) protein [27,36], FosB/ΔFosB [25,27] and inflammatory markers (astrocytes, microglia [36]). These changes are decreased by administration of nNOS preferential inhibitor, raising the possibility that the anti-dyskinetic effects of these drugs would include interference in NO-mediated processes [37–39].
Evidence points to a positive effect of NO on cyclooxygenase-2 [40] (COX2-prostaglandin H-synthase) activity and/or expression. COX2 is a component of the inflammatory cascade in the periphery [41,42]. In the brain, COX2 is constitutive, expressed and regulated in neurons by synaptic activity [43]. NO and prostaglandin (the products COX2) have been proposed to function as retrograde messengers and to facilitate neurotransmitter release in the central nervous system. The beneficial or damaging role played by COX2 in brain pathologies is controversial [44,45].
Recently, non-neuronal factor such as inflammation have been suggested to be involved in l-DOPA-induced dyskinesia [36,46]. The anti-dyskinetic effects of anti-inflammatory treatments with either corticosterone [46] or IRC-82451 (a multitargeting molecule [47]) support the hypothesis. Moreover, there is evidence of an increased expression of inflammatory markers in vivo in human Parkinson's disease patients [4] and in animal models [36,48,49].
To further understand the potential role of the nitrergic system in l-DOPA-induced dyskinesia, this study is aimed at investigating the effect of l-DOPA-induced dyskinesia on the expression of COX2 in brain regions of hemi-Parkinsonian rats. We also analysed the impact of nNOS inhibition on COX2 expression.
2. Experimental procedures
(a). Subjects
Male Wistar rats (FMRP-USP, Ribeirão Preto, Brazil; 200–250 g body weight) were housed under 12 L : 12 D cycle with free access to food and water.
(b). 6-Hydroxydopamine lesion
All chemicals, if not specified, were purchased from Sigma-Aldrich, St Louis, MO, USA.
6-Hydroxydopamine (6-OHDA) was microinjected into the medial forebrain bundle as we previously described [27]. The rationale for our approach and the procedure used were provided in our previous papers [24,27,50]. In order to determine the degree of dopaminergic 6-OHDA lesion all rats were tested for apomorphine (0.5 mg kg−1 subcutaneous (s.c.))-induced rotation 21 days after surgery. Only rats showing more than 90 full turns per 45 min (contralateral to the lesion) were selected for the study. The dopamine lesion was confirmed by analysis of tyrosine hydroxylase immunohistochemistry as described before [27] in the striatum and in the substantia nigra compacta (results not presented).
(c). Drug treatment and experimental groups
The dose regimen and route of administration were based on previously published studies [24,27] (electronic supplementary material, figure S1). Chronic treatment consisted of single daily injections for 21 days of: (i) 7-nitroindazole (7NI, a preferential nNOS inhibitor, 30 mg kg−1) followed by l-DOPA (30 mg kg−1 + benserazide 7.5 mg kg−1 (Prolopa dispersive, Hoffman-LaRoche, Brazil; 7NI + l-DOPA, n = 7), (ii) vehicle (50% polyethyleneglycol–saline solution) followed by l-DOPA (VEH + l-DOPA, n = 7; electronic supplementary material, figure S1), (iii) 7NI followed by saline (7NI + SAL, n = 3), or (iv) VEH + SAL (n = 6), corresponding to daily injections of vehicle followed by saline. The dose of l-DOPA (30 mg kg−1) was chosen for its ability to induce consistent abnormal involuntary movements throughout the chronic l-DOPA treatment [51–53].
(d). l-DOPA-induced abnormal involuntary movements
Rats were monitored for abnormal involuntary movements using a rat dyskinesia scale [54–56]. Experimental details were previously described by Bortolanza et al. [36] and Padovan-Neto et al. [24,26,27].
3. COX2 analysis
(a). Immunohistochemistry
Rats were deeply anaesthetized with urethane (25 mg kg−1, i.p.) and sacrificed by transcardiac perfusion with physiological saline, followed by phosphate-buffered 4% paraformaldehyde (pH 7.4). The brains were removed and treated as described elsewhere [24]. Coronal sections of 25 µm thickness were cut using a freezing microtome (LeicaR, model CM1850) throughout the striatum using the Paxinos & Watson [57] atlas.
The sections were processed using the procedure described previously [27,36]. Free-floating sections were incubated at 4°C with the primary antibodies (table 1) diluted in 0.1 M phosphate-buffered saline (pH 7.4), containing 0.15% Triton X-100. Biotinylated secondary antibodies (Vector Labs, Burlingame, CA, USA; diluted 1 : 400), followed by avidin–biotin–peroxidase complex (Vectastain ABC-kit, Vector Labs), were used for detection of the immunocomplexes (90 min for each step). To visualize the reaction, sections were incubated in 0.25 M Trizma base containing 3,3-diaminobenzidine (DAB).
Table 1.
Primary and secondary antibodies used in this studya.
| primary antibodies |
secondary antibodies |
|||||||
|---|---|---|---|---|---|---|---|---|
| antigen | host | dilution | manufacturer | antigen | host | dilution | type | manufacturer |
| COX2 | rabbit | 1 : 300 | Cayman Chemical | (H + L) rabbit | donkey | 1 : 250 | Alexa Fluor 488 | Invitrogen |
| Cy3-GFAP | mouse | 1 : 500 | Sigma-Aldrich | — | — | — | — | — |
| OX-42 | mouse | 1 : 300 | Abcam | (H + L) mouse | donkey | 1 : 250 | Cyanine (Cy3) | JIR |
| NeuN | mouse | 1 : 60000 | Chemicon | (H + L) mouse | donkey | 1 : 250 | Cy3 | JIR |
| DARPP-32 | mouse | 1 : 500 | Dr H. C. Hemmings | (H + L) mouse | donkey | 1 : 250 | Cy3 | JIR |
| Calbindin | mouse | 1 : 500 | Sigma-Aldrich | (H + L) mouse | donkey | 1 : 250 | Cy3 | JIR |
| nNOS | sheep | 1 : 1000 | Dr P. Emson | (H + L) sheep | donkey | 1 : 250 | Cy3 | JIR |
| Calretinin | goat | 1 : 500 | Chemicon | (H + L) goat | donkey | 1 : 250 | Cy3 | JIR |
| ChAT | goat | 1 : 500 | Chemicon | (H + L) goat | donkey | 1 : 250 | Cy3 | JIR |
| Parvalbumin | mouse | 1 : 500 | Sigma-Aldrich | (H + L) mouse | donkey | 1 : 250 | Cy3 | JIR |
aAntibodies used in immunohistochemistry and double fluorescence immunostaining procedure. COX2, cyclooxygenase-2; ChAT, choline-acetyltransferase; Cy3, cyanine-3; DARPP-32, dopamine and cAMP-regulated phosphoprotein of Mr 32 kDa; GFAP, glial fibrillary acidic protein; (H + L), heavy plus light chains; JIR, Jackson Immuno Research; NeuN, neuronal nuclei; nNOS, neuronal nitric oxide synthase; OX-42, CD11b/c equivalent protein.
Sections that had been processed for single-antigen with DAB colour reaction were examined to evaluate the overall pattern of COX2 immunoreactivity. All analyses were performed on both sides of the brain. Three rostrocaudal levels (rostral: 1.7 mm; medial: 0.7 mm; and caudal: −0.8 mm from bregma [57]) were examined within the striatum. An observer blinded to the treatment conditions acquired measurements from the dorsomedial, dorsolateral and ventrolateral striatal parts.
Adjacent or nearly adjacent sections were processed immunohistochemically by double immunolabelling. Distinct fluorescently tagged COX2-neuron-specific secondary antibody and antibodies were used against: NeuN (a neuronal nuclear protein), GFAP (glial fibrillary acidic protein of astrocyte), OX-42 (CD11b/c equivalent protein of microglia) and DARPP-32 (dopamine and cAMP-regulated phosphoprotein 32 kDa-striatum projection neurons) in order to analyse the cellular COX2 location in neurons or glial cells (table 1). The sections were processed using the procedure described previously [36,58].
Other series of striatal adjacent sections were double immunostained with COX2 and antigens to label striatal interneurons as described by Kawaguchi et al. [59] and Bennett & Bolam [60] as follows: (1) the large cholinergic interneurons identified by the presence of choline acetyltransferase (ChAT); (2) GABA (gamma-aminobutyric acid) interneurons identified individually by the presence of antigen against calcium-binding proteins (parvalbumin, calretinin and calbindin) or nNOS (table 1).
High-resolution optical sections were obtained by laser scanning microscopy. Overlapping neighbouring fields of view were acquired and merged with a Leica SP5 confocal laser scanning microscope (Leica Microsystems, Mannheim, Germany) equipped with a motorized stage and hybrid detectors. The images were processed and reconstructed with FIJI [61] and Imaris v. 7.5 software packages (Bitplane, Zurich, Switzerland).
(b). Western immunoblotting
To analyse the antibody specificity by Western immunoblotting, and to quantify striatal COX2-related protein expression, independent experimental groups were composed of rats receiving the following treatments: VEH + SAL, VEH + l-DOPA, 7NI + SAL and 7NI + l-DOPA (n = 4 rats in each group). The animals were decapitated, and the lesion-reactive (right) and the contralateral (left, control) striatum were microdissected on an ice-cooled dissection cover, with the help of a magnifying lens (Leica Zoom 2000), and immediately frozen in liquid nitrogen (−196°C). Tissue samples were stored at −80°C until use.
Left and right striata were processed separately. The homogenates were centrifuged at 10 000 r.p.m. for 25 min at 4°C. The supernatants were recovered for protein concentration measurements using Bradford assay (Bio-Rad Protein assay, Bio-Rad, Germany). Proteins (30 µg) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% SDS-PAGE) and semi-dry transferred to a nitrocellulose membrane. Nitrocellulose membranes were incubated at 4°C overnight using the anti-COX2 antibody (1 : 500, table 1), with anti-α-tubulin as control (1 : 4000, table 1). Bound antibodies were detected with HRP-conjugated secondary anti-rabbit antibody (1 : 4000). Bands were visualized by enhanced chemiluminescence (ECL, Amersham, UK) and quantified with the software ImageJ [25]. Arbitrary units (arb. units) represent integrated density analysis extracted from band intensities of COX2 normalized by their respective tubulin band expression.
(c). Statistical analysis
Data were evaluated by repeated analysis of variance (rANOVA). Comparisons were done for treatment (VEH + SAL, VEH + l-DOPA, 7NI + SAL and 7NI + l-DOPA) and brain side (ipsilateral and contralateral to the lesion). Tukey's test was used for post-hoc comparisons in cases of significant interactions. Data are presented as mean ± s.e.m. and statistical significance level was set at p < 0.05. All statistical analysis was performed using SPSS for Windows (v. 8.0).
4. Results
(a). Behavioural observations and effect of 7NI on l-DOPA-induced dyskinesia
The total abnormal involuntary movements were scored every 60 min over a period of 180 min following l-DOPA administration.
Application of VEH + l-DOPA to 6-OHDA-lesioned rats induced contralateral rotation (day 1, locomotor: 4.85 ± 0.89) and abnormal, purposeless movements affecting cranial, trunk and limb muscles (day 1, axial, limb and orofacial: 22.14 ± 2.42) on the side of the body contralateral to the lesion. This score was maintained during the 21 days of treatment period (day 21, locomotor: 3.78 ± 0.76; axial, limb and orofacial: 20.85 ± 1.28).
7NI + l-DOPA-treated rats expressed less severe dyskinesia compared with l-DOPA alone over time (day 1, axial, limb, orofacial: 10.2 ± 4.03; locomotor: 1.71 ± 0.78). The scores progressively decreased on the subsequent days and at the end of the experiment (day 21) the abnormal involuntary movements scores were similar to control groups VEH + SAL and 7NI + SAL.
These results provide further evidence for the combination of chronic l-DOPA and 7NI ameliorating l-DOPA-induced dyskinesia.
(b). Effect of 6-hydroxydopamine lesion and l-DOPA treatment on COX2 expression
In accordance with the literature [43,62–64], analysis of constitutive COX2 immunoreactivity in the brain of control animals revealed positive neurons in the hippocampus (dentate gyrus granule cells, pyramidal cell neurons), piriform superficial cell layers of neocortex, the amygdala and, at a very low number, in the striatum, thalamus and hypothalamus (electronic supplementary material, figures S2 and S3). There were no positive cells in the substantia nigra, globus pallidus, entopeduncular and subthalamic nuclei.
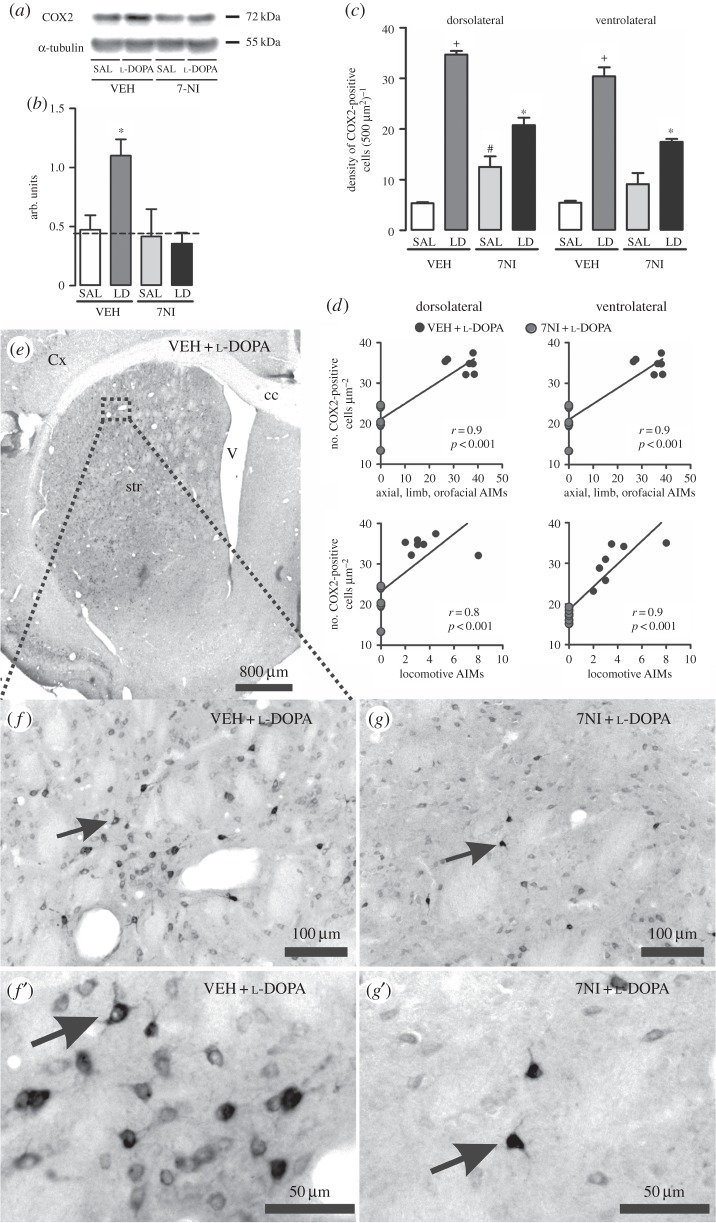
Analysis of the dopamine-depleted (ipsilateral) striatum of the 6-OHDA-hemi-Parkinsonian rats receiving chronic l-DOPA treatment revealed depletion of tyrosine hydroxylase immunoreactivity (more than 80%—results not presented). Simultaneously, there was a remarkable immunopositive reaction for COX2 in the dorsal striatum. COX2 immunoreactivity presented a cytoplasmatic distribution with clean nuclei, at structures resembling neuronal cell bodies with ramifications (figures 1 and 3f’,g’).
Figure 1.
Striatal COX2 immunoreactivity in 6-OHDA-hemiparkinsonian rats with l-DOPA-induced dyskinesia. (a–b) Low-magnification photomicrograph showing NeuN (red) and COX2 immunoreactivity (green) in the striatum. There was COX2 immunoreactivity in the lesioned striatum (b, right) and occasional COX2 immunoreactivity in the contralateral one (a, left). (c–n) Photomicrographs of striatal sections showing immunofluorescence staining for COX2 antibody (green; c,f,i and l) in combination with antibodies (red) for neuronal protein (NeuN; d), dopamine-cAMP-regulated phosphoprotein-32 (DARPP-32; g); glial fibrillary acidic protein (GFAP; j), CD11b/c equivalent protein (OX-42; m). Co-localization with COX2 was observed for NeuN and DARPP-32. (e, h, k and n) correspond to merged images. Arrows indicate cells with co-localization. Arrowheads indicate cells with no co-localization. cc, corpus callosum; Cx, cortex; Str, striatum; V, ventricle. Fluorescent images were taken with the same setting. Scale bars: (a,b) = 100 µm; (c,f,i,l) = 50 µm.
Figure 3.
Analysis of l-DOPA and 7NI effect on COX2 expression in the 6-OHDA-lesioned striatum of rats. (a) Representative Western blots of striatal COX2 (72 kDa) and α-tubulin (50 kDa, control). (b) Western blotting quantification of striatal protein extracts obtained from 6-OHDA-lesioned rats chronically treated with either VEH + SAL, 7NI + SAL, VEH + l-DOPA or 7NI + l-DOPA. Dashed line indicates the mean of COX2 expression in the contralateral side to the lesion. (c) Quantification of COX2-immunostaining cells in the 6-OHDA-lesioned striatum. VEH + l-DOPA treatment significantly increased COX2 protein expression in the dopamine-denervated striatum (p < 0.05 if compared with (*) all other groups, with (+) VEH + SAL and 7NI + SAL, with (#) VEH + SAL). (d) Graphics showing positive correlation between COX2 expression and abnormal involuntary movements induced by l-DOPA treatment in the hemi-Parkinsonian rats. (e,f and g) Photomicrographs showing COX2-immunopositive neurons in the dopamine-depleted striatum of VEH + l-DOPA (e,f and f’) and 7NI + l-DOPA (g and g’)-treated rats. (f’ and g’) show high magnifications of a COX2 neuron. Arrows indicate an example of a COX2-positive cell. AIMs, abnormal involuntary movements; LD, l-DOPA; 7NI, 7-nitroindazole; SAL, saline; VEH, vehicle; cc, corpus callosum; Cx, cortex; str, striatum; V, ventricle.
COX2-positive labelling co-localized with immunoreactivity for NeuN (figure 1a–e), a 46/48 kD neuronal nuclear protein antigen widely used to identify neurons [65,66].
By contrast, COX2-positive immunoreactivity did not overlap either with GFAP-labelled astrocytes (figure 1i–k; electronic supplementary material, figure S4) or OX-42-labelled microglia (figure 1l–n). However, COX2 neurons and GFAP-labelled astrocytes are in intimate proximity (electronic supplementary material, figure S4).
There was either very low or absent COX2 expression in the striatum contralateral to the lesion (figure 1a) or in the striatum of rats that received chronic 7NI + SAL or VEH + SAL, suggesting that COX2 induction does not reflect a general reaction of the neuronal cells within this brain region.
These results confirmed that the COX2 protein was found in neurons of lesioned striatum, which presented more than 80% absence of tyrosine hydroxylase-positive reaction and only after l-DOPA treatment.
(c). Characteristics of COX2 in striatal neurons of dyskinetic rats
In order to characterize COX2-positive neurons in the denervated striatum, we carried out double immunofluorescence staining reactions.
Confocal microscopy evaluation revealed that in the lesioned striatum of dyskinetic rats, striatal COX2 co-localizes with DARPP-32 (approx. 60% of the total neurons examined; figure 1f–h). DARPP-32-stained neurons are GABAergic medium-sized spiny striatal projection neurons and postsynaptic targets of convergent inputs from cortical glutamatergic neurons, cholinergic striatal interneurons and midbrain dopaminergic neurons [67].
Likewise, 100% of the acetylcholine-synthesizing enzyme ChAT-positive neurons analysed in the dorsal striatum co-expressed COX2 (figure 2a–c). ChAT stains cholinergic giant interneurons that can be recognized owing to their somatic size [59,60] (20–50 µm). ChAT interneurons may be protected in several neurological conditions by their lack of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor subunits [68,69], and enrichment in both forms of the superoxide dismutase free radical scavengers [70,71].
Figure 2.
Expression of markers for striatal neurons co-localized with COX2-immunoreactive cells within the dopamine-denervated striatum following l-DOPA treatment. (a–l) Photomicrographs of striatal sections showing immunofluorescence staining with COX2 antibody (green; a, d, g and j) in combination with antibodies (red) for choline-acetyltransferase (ChAT; b), calbindin (CB; e), neuronal nitric oxide synthase (nNOS; h) and parvalbumin (PV; k). Co-localization with COX2 was observed for ChAT and CB. Arrows indicate cells with co-localization. (c,f,i,l) correspond to the merged images. Scale bars, 25 µm.
Approximately 60% of the calbindin-positive neurons in the dorsal striatum (figure 2d–f) co-expressed COX2. Because calbindin–COX2 double-immunostained neurons presented nuclear indentation, a well-established characteristic of striatal interneurons [60,72], they were identified as interneurons (see the electronic supplementary material, movie). However, because calbindin may also be detected in projection neurons, a more detailed analysis is required. Calbindin expression may offer to striatal neurons some protection against excitotoxicity [73]. Immunohistochemistry for calbindin heavily stained neurons in the matrix compartment of the striatum [74]. Cholinergic and DARPP-32-positive neurons at best play a key and selective role in l-DOPA-induced dyskinesia.
No double labelling between COX2 and nNOS or the two other calcium-binding proteins parvalbumin and calretinin was observed within the striatum (figure 2g–l; electronic supplementary material, figure S3).
(d). Analysis of COX2 expression in the striatum by Western blot and by immunohistochemistry
In agreement with a previous report [75], the COX2 antibody revealed a single protein band with a molecular weight of approximately 72 kDa corresponding to the non-glycosylated form of COX2 (figure 3a).
The Western blot analysis confirmed an increased COX2 protein content in the dopamine-depleted striatum of rats presenting l-DOPA-induced dyskinesia when compared with the contralateral side (p < 0.05) and to the lesioned striatum of rats receiving either VEH + SAL or 7NI + SAL treatment (treatment: F3,23 = 3.132, p < 0.05; figure 3b).
Analysis by immunohistochemistry supported the induction of COX2 in the striatum of Parkinsonian rats presenting abnormal involuntary movements elicited by l-DOPA treatment. It revealed an increase in the COX2 immunoreactivity (five- to sevenfold) in the dorsolateral, dorsomedial and ventrolateral striatum only after l-DOPA treatment (figure 3c,e,f and f’) when compared with 6-OHDA-lesioned rats receiving either VEH + SAL or 7NI + SAL (interaction; dorsomedial: F3,22 = 29.134; dorsolateral: F3,22 = 130.510; ventrolateral: F3.22 = 77.878; p < 0.05; figure 3c).
Pre-treatment with 7NI reduced l-DOPA-induced dyskinesia and COX2 immunoreactivity in the dorsolateral and ventrolateral striatum when compared with VEH + l-DOPA (p < 0.05 figure 3c,g and g’). There was also a trend (p < 0.08) for reduction in COX2 immunoreactivity in the dorsomedial striatum.
Finally, there was a significant correlation between the number of COX2-positive cells and abnormal involuntary movements (figure 3d; axial, limb, orofacial: dorsolateral and ventrolateral striatum r = 0.9, p < 0.001; locomotive: dorsolateral striatum r = 0.7, p < 0.05 and ventrolateral striatum r = 0.9, p < 0.001).
Taken together, these results show that during l-DOPA-induced dyskinesia, COX2 immunostaining is preferentially upregulated in neurons located in the dopamine-depleted striatum, which might influence l-DOPA-induced dyskinesia. The nNOS inhibitor 7NI mitigates dyskinesia and also COX2 neuronal expression.
5. Discussion
Here we show, we believe for the first time, a link between COX2 neuronal expression in the dopamine-depleted striatum that correlates with the severity of l-DOPA-induced dyskinesia. COX2 expression was prevented by co-treatment with the preferential nNOS inhibitor 7NI concurrently with l-DOPA-induced dyskinesia. l-DOPA administration did not induce COX2 expression in the non-lesioned striatum, suggesting that dopamine denervation is at the core of the mechanism leading to COX2 presence. We verified, using double-immunostaining that the COX2-positive immunoreactivity occurred in striatal neurons positive for DARPP-32, ChAT and calbindin. Cholinergic interneurons and DARP-32 projection neurons play a key and selective role in l-DOPA-induced dyskinesia.
In Parkinson's disease, COX2 expression occurs mostly in glial cells in the substantia nigra compacta, being potentially involved with neurodegeneration [76–80]. In agreement with this, COX2 knockout mice are more resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated intoxication [77,80,81]. On the other hand, evidence has been accumulated indicating that COX2 expression in Parkinson's disease does seem to derive from a mechanism distinct from the inflammatory properties of prostanoids [77]. In accordance, the inflammatory response associated with dopaminergic neurodegeneration in COX2 knockout mice did not differ from that observed in their wild-type counterparts [77]. Finally, non-steroidal anti-inflammatory drug treatment targeting COX2 has been studied in Parkinson's disease patients but with variable results [82].
(a). Mechanisms linking NO with COX2 expression
Compelling data have been generated supporting the concept that NO signalling is tightly coupled to the COX2-prostaglandin pathway and vice versa [83,84]. In the central nervous system, nNOS-derived NO stimulates COX2 activity in vivo and in vitro.
7NI chronic treatment per se increases COX2-positive neurons in the dorsolateral striatum of 6-OHDA-lesioned rats. This may be interpreted as a NOS compensatory mechanism. In agreement, chronic inhibition of NO synthesis has been reported to lead to an enhanced expression of COX2 in rat mesenteric arteries [85]. Therefore, another system in addition to the brain the induced absence of NO simulated by NOS inhibitor chronic treatment exhibit increase the expression of COX2 with less amounts of NO [85].
Overexpression of nNOS elicits S-nitrosylation of COX2 and activation of prostaglandin formation [86]. On the contrary, a large number of reports support the idea that NO can inactivate COX2 [87,88]. In several experimental models, COX2 has been linked to anti-inflammatory and neuroprotective properties [89,90]. In fact, prolonged overexpression of COX2 in the brain has been associated with the delayed cell death that occurs after many types of brain injuries [91]. These apparently contradictory results of NO influence on COX2 expression may be related to the levels of NO, the cell type and/or the state of activation of the cells [83,92].
Another possibility would be COX2 expression regulation of transcription, since COX2 end products can modulate gene expression [93]. Berke et al. [94] and Gerfen [95] proposed that COX2 mRNA induction in the striatum of hemi-Parkinsonian rat involves genetic adaptation mechanisms triggered by dopamine depletion. In general, COX2 expression under inductive stimuli follows the pattern of the so-called early genes that can directly modify cellular function [44,96]. Regulation of cox2 gene transcription is controlled by consensus elements present in the cox2 gene promoter. It contains a TATA box motif and a number of cis-acting elements, including a CREB-response element (cyclic AMP response element) nuclear factor interleukin-6, AP-1 (activator protein-1 transcription factor), and nuclear factor-kappa B (NF-κB). Interestingly, transcription factors that are activated in the striatum in l-DOPA-induced dyskinesia [97] may bind to these consensus elements of the cox2 gene [98]. For example, the mitogen-activated protein kinase extracellular signal-regulated kinase-1 (ERK1) and ERK2 in the cell nucleus, together with cAMP, trigger the activation of CREB, which drives the expression of COX2 [99] and of l-DOPA-induced dyskinesia proteins [100].
Finally, the capacity of NO and its effector cyclic GMP to modulate the function of several target proteins, including transcription factors such as NF-κB and AP1, appears as the key pathway by which NO may regulate COX2 expression [101].
Whether or not these factors contribute to regulation of COX2 expression in the denervated striatum during l-DOPA action should be determined.
(b). COX2 expression in the DARP-32, calbindin and cholinergic neurons
The main candidates for the mechanism underlying l-DOPA-induced dyskinesia are functional and structural alterations induced in the dopamine-depleted striatum [97,102–105]. Herein, besides an increase in COX2 expression in the dopamine-depleted striatum, we showed, we believe for the first time, that l-DOPA administration positively regulates COX2 expression in DARPP-32, acetylcholine and calbindin neurons.
There are higher levels of DARPP-32 in the striatum of dyskinetic rats compared with 6-OHDA rats that have not developed dyskinesia under l-DOPA treatment [106]. Most of the abnormal involuntary movements developed following chronic l-DOPA are associated with hyperactivation in striatal medium spiny neurons of a signalling pathway comprising sequential phosphorylation of DARPP-32 [107]. l-DOPA-induced dyskinesia is associated with changes in dopamine-D1 receptor signalling in the dopamine-depleted striatum. The dopamine-D1 and the prostaglandin type 1 (EP1) receptors are co-expressed in striatal neurons [108]. In addition, EP1 prostaglandin signalling augments dopamine-D1-induced phosphorylation of DARPP-32 and facilitates hyperlocomotion induced by dopamine-D1 agonists [108].
Calbindin, a major calcium-binding protein in the cytosol, plays a critical role during intracellular Ca2+ homeostasis and is implicated in neuroprotection (when expressed at high levels) owing to its ability to buffer free intracellular Ca2+ [109–111]. Dopamine-D1 receptor signalling is modulated by calcium level and NO [2,67]. In many neurodegenerative diseases, intracellular Ca2+ homeostasis appears to be disrupted and might be a serious risk factor [112]. l-DOPA-induced toxicity in cultured midbrain neurons is also dependent on calcium regulation [113]. Considering that cellular degeneration is often accompanied by impaired calcium homeostasis, a protective role for calcium-binding proteins has been postulated [114].
Elimination of striatal cholinergic interneurons in the lesioned striatum of hemi-Parkinsonian mice attenuates the development of l-DOPA-induced dyskinesia without affecting the beneficial anti-Parkinsonian action of l-DOPA [100,115]. Cholinergic interneurons in the striatum establish intricate axonal projections that represent a widespread neurotransmission system [116–118]. Mechanistic studies revealed local regulation of dopamine release by acetylcholine as well as by proteins known to be disrupted in Parkinson's disease and other movement disorders [2,119]. Similarly, dopamine-D1 and -D2 receptor signalling is modulated by acetylcholine and NO [2,67]. Strategies reported to reduce striatal cholinergic tone after dopamine deregulation may represent a promising approach to decreasing l-DOPA-induced motor complications in Parkinson's disease.
We propose that l-DOPA induction of COX2 transcription in striatum of Parkinsonian-dyskinetic rats directly influences discrete striatal neurons containing DARPP-32, acetylcholine and calbindin, which play a significant role in l-DOPA-induced dyskinesia. Further studies are needed to analyse the role of COX2 in these neurons in l-DOPA-induced dyskinesia.
6. Extrasynaptic signalling/volume signalling
Because extrasynaptic transmission is modulated by NO, COX2 product prostaglandin, dopamine and acetylcholine modulate extrasynaptic transmission, our observation reveals a new mode of neurotransmitter action upon dopamine-depleted striatum submitted to l-DOPA chronic treatment. The role of extrasynaptic signalling in neurological diseases only recently has been described in cases of neurodegenerative disease. That there is evidence of cell death associated with neurodegenerative diseases may be partly due to an imbalance of synaptic and extrasynaptic signalling [120]. Also, functional recovery might be facilitated by non-synaptic neurotransmission [121].
The dense dopaminergic nerve terminal plexus in the striatal cellular networks mainly operates via extrasynaptic/volume transmission [122]. Various subtypes of extrasynaptic dopamine receptors [123–125] are located in projection neurons and interneurons as well as on the afferent nerve terminal networks.
The most extreme case of global volume signalling is that of gaseous NO. In the striatum, a single source of the NO signal can tune the activity of a set of nerve and glial cells by non-synaptic diffuse neurotransmission [126]. A close anatomical link between NO and dopamine-releasing neurons is proposed via the localization of NOS- and tyrosine hydroxylase-containing neuronal fibres and cell bodies in the nigrostriatal pathway [58].
The actions of acetylcholine on dopamine release do not conform to the synaptic doctrine, owing to the absence of direct synaptic contacts of cholinergic terminals on striatal dopaminergic axons. A widespread plexus of cholinergic nerve terminals exist especially in the striatum and thus mainly operate via extrasynaptic release and volume transmission [118] and may act by modulating the transmission of GABA-projection neurons.
COX2 and its products, especially prostaglandin E2 (PGE2), in addition to being involved in the inflammatory responses, have been reported to be important in modulating synaptic activity [127–130]. Teismann et al. [77] (for review, see [10]) suggested that the beneficial role of COX2 inhibition in the mouse model of Parkinson's disease is mediated by a mechanism that could involve facilitation of dopamine-mediated neurotransmission. For example, the COX2 products PGE2 are formed in response to dopamine receptor stimulation in the striatum and regulate signalling and function of dopamine-D1 and -D2 receptors [108].
COX2 is expressed predominantly in dendrites of glutamatergic neurons [76], localization consistent with its role in synaptic and extrasynaptic function [131] and neurovascular regulation [132]. Under pathological conditions, the most active NMDA-type glutamate receptor (NMDAR) subpopulation appears to be composed of extrasynaptic NMDAR activity and synaptic NMDAR activity [133]. Extrasynaptic-NMDARs may regulate COX2 expression or activity [134,135].
Given that COX2 expression in l-DOPA-induced dyskinesia may last for days [136], PGE2 might function as a long-term adaptive, paracrine-like mechanism to amplify dopamine signalling [108] and/or modify glutamatergic and cholinergic striatal neurotransmission.
7. Conclusion
Although additional studies are clearly needed to establish a causal relationship, our results suggest that NO-dependent changes in COX2 expression are correlated with the development of motor side effects after l-DOPA long-term treatment in Parkinson's disease. Because COX2-prostaglandin, NO, dopamine and acetylcholine modulate synaptic as well as extrasynaptic/volume transmission we postulate the intriguing possibility of extrasynaptic/volume transmission-mediated actions of these neurotransmitters in l-DOPA-induced dyskinesia [124].
Supplementary Material
Acknowledgements
E.D.-B. is a CNPq research fellow. The authors wish to thank Prof. Francisco S. Guimarães for helpful comments and suggestions.
Ethics statement
The experiments were conducted according to the principles and procedures described by the guidelines for the care and use of mammals in neuroscience and behavioural research (ILAR, USA). The local Ethical Committee approved the protocol.
Funding statement
The authors are grateful for the financial support and grants provided by the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), CAPES-COFECUB program (France/Brazil; 681/2010), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq)—Programa Ciências sem Fronteiras (Pesquisador Visitante Especial) and the Cátedra França-Universidade de São Paulo.
Conflict of interests
The authors have no financial or personal conflicts of interest related to this study.
References
- 1.Obeso JA, Rodriguez-Oroz MC, Lanciego JL, Rodriguez Diaz M. 2004. How does Parkinson's disease begin? The role of compensatory mechanisms. Trends Neurosci. 27, 125–127. ( 10.1016/j.tins.2003.12.006) [DOI] [PubMed] [Google Scholar]
- 2.Rice ME, Patel JC, Cragg SJ. 2011. Dopamine release in the basal ganglia. Neuroscience 198, 112–137. ( 10.1016/j.neuroscience.2011.08.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocchi F. 2005. Optimising levodopa therapy for the management of Parkinson's disease. J. Neurol. 252, IV43–IV48. ( 10.1007/s00415-005-4009-4) [DOI] [PubMed] [Google Scholar]
- 4.Bartels AL, Leenders KL. 2007. Neuroinflammation in the pathophysiology of Parkinson's disease: evidence from animal models to human in vivo studies with [11c]-PK11195 PET. Mov. Disord. 22, 1852–1856. ( 10.1002/mds.21552) [DOI] [PubMed] [Google Scholar]
- 5.Gerhard A, et al. 2006. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol. Dis. 21, 404–412. ( 10.1016/j.nbd.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 6.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. 2005. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann. Neurol. 57, 168–175. ( 10.1002/ana.20338) [DOI] [PubMed] [Google Scholar]
- 7.Collins LM, Toulouse A, Connor TJ, Nolan YM. 2012. Contributions of central and systemic inflammation to the pathophysiology of Parkinson's disease. Neuropharmacology 62, 2154–2168. ( 10.1016/j.neuropharm.2012.01.028) [DOI] [PubMed] [Google Scholar]
- 8.Hirsch EC, Hunot S. 2009. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 8, 382–397. ( 10.1016/S1474-4422(09)70062-6) [DOI] [PubMed] [Google Scholar]
- 9.Tansey MG, Goldberg MS. 2010. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 37, 510–518. ( 10.1016/j.nbd.2009.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przedborski S. 2010. Inflammation and Parkinson's disease pathogenesis. Mov. Disord. 25, S55–S57. ( 10.1002/mds.22638) [DOI] [PubMed] [Google Scholar]
- 11.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. 1994. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci. Lett. 172, 151–154. ( 10.1016/0304-3940(94)90684-X) [DOI] [PubMed] [Google Scholar]
- 12.Mogi M, Harada M, Kondo K, Riederer P, Inagaki H, Minami M, Nagatsu T. 1994. Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from Parkinsonian patients. Neurosci. Lett. 180, 147–150. ( 10.1016/0304-3940(94)90508-8) [DOI] [PubMed] [Google Scholar]
- 13.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. 1994. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from Parkinsonian patients. Neurosci. Lett. 165, 208–210. ( 10.1016/0304-3940(94)90746-3) [DOI] [PubMed] [Google Scholar]
- 14.Mogi M, Harada M, Kondo K, Narabayashi H, Riederer P, Nagatsu T. 1995. Transforming growth factor-β1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson's disease. Neurosci. Lett. 193, 129–132. ( 10.1016/0304-3940(95)11686-Q) [DOI] [PubMed] [Google Scholar]
- 15.Mogi M, Kondo T, Mizuno Y, Nagatsu T. 2007. P53 protein, interferon-γ, and NF-κB levels are elevated in the Parkinsonian brain. Neurosci. Lett. 414, 94–97. ( 10.1016/j.neulet.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 16.Shimoji M, Pagan F, Healton EB, Mocchetti I. 2009. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson's disease. Neurotox. Res. 16, 318–328. ( 10.1007/s12640-009-9076-3) [DOI] [PubMed] [Google Scholar]
- 17.Buck K, Ferger B. 2008. Intrastriatal inhibition of aromatic amino acid decarboxylase prevents l-DOPA-induced dyskinesia: a bilateral reverse in vivo microdialysis study in 6-hydroxydopamine lesioned rats. Neurobiol. Dis. 29, 210–220. ( 10.1016/j.nbd.2007.08.010) [DOI] [PubMed] [Google Scholar]
- 18.Meissner W, et al. 2006. Increased slow oscillatory activity in substantia nigra pars reticulata triggers abnormal involuntary movements in the 6-OHDA-lesioned rat in the presence of excessive extracellular striatal dopamine. Neurobiol. Dis. 22, 586–598. ( 10.1016/j.nbd.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 19.Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P. 2002. Striatal metabotropic glutamate receptor function following experimental Parkinsonism and chronic levodopa treatment. Brain 125, 2635–2645. ( 10.1093/brain/awf269) [DOI] [PubMed] [Google Scholar]
- 20.Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L. 2004. Chronic l-DOPA treatment increases extracellular glutamate levels and Glt1 expression in the basal ganglia in a rat model of Parkinson's disease. Eur. J. Neurosci. 20, 1255–1266. ( 10.1111/j.1460-9568.2004.03591.x) [DOI] [PubMed] [Google Scholar]
- 21.Färber K, Pannasch U, Kettenmann H. 2005. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell Neurosci. 29, 128–138. ( 10.1016/j.mcn.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 22.Del-Bel E, Padovan-Neto FE, Raisman-Vozari R, Lazzarini M. 2011. Role of nitric oxide in motor control: implications for Parkinson's disease pathophysiology and treatment. Curr. Pharm. Des. 17, 471–488. ( 10.2174/138161211795164176) [DOI] [PubMed] [Google Scholar]
- 23.Novaretti N, Padovan-Neto FE, Tumas V, da-Silva CA, Del Bel EA. 2010. Lack of tolerance for the anti-dyskinetic effects of 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, in rats. Braz. J. Med. Biol. Res. 43, 1047–1053. ( 10.1590/S0100-879X2010007500111) [DOI] [PubMed] [Google Scholar]
- 24.Padovan-Neto FE, Echeverry MB, Tumas V, Del-Bel EA. 2009. Nitric oxide synthase inhibition attenuates l-DOPA-induced dyskinesias in a rodent model of Parkinson's disease. Neuroscience 159, 927–935. ( 10.1016/j.neuroscience.2009.01.034) [DOI] [PubMed] [Google Scholar]
- 25.Padovan-Neto FE, Echeverry MB, Chiavegatto S, Del-Bel E. 2011. Nitric oxide synthase inhibitor improves de novo and long-term l-DOPA-induced dyskinesia in hemiparkinsonian rats. Front. Syst. Neurosci. 5, 40 ( 10.3389/fnsys.2011.00040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padovan-Neto FE, Ferreira NR, de Oliveira-Tavares D, de Aguiar D, da Silva CA, Raisman-Vozari R, Del Bel E. 2013. Anti-dyskinetic effect of the neuronal nitric oxide synthase inhibitor is linked to decrease of FosB/deltaFosB expression. Neurosci. Lett. 541, 126–131. ( 10.1016/j.neulet.2013.02.015) [DOI] [PubMed] [Google Scholar]
- 27.Padovan-Neto FE, Cavalcanti-Kiwiatkoviski R, Carolino RO, Anselmo-Franci J, Del-Bel EA. 2014. Effects of prolonged neuronal nitric oxide synthase inhibition on the development and expression of l-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Neuropharmacology 89C, 87–99. ( 10.1016/j.neuropharm.2014.08.019) [DOI] [PubMed] [Google Scholar]
- 28.Takuma K, Tanaka T, Takahashi T, Hiramatsu N, Ota Y, Ago Y, Matsuda T. 2012. Neuronal nitric oxide synthase inhibition attenuates the development of l-DOPA-induced dyskinesia in hemi-Parkinsonian rats. Eur. J. Pharmacol. 683, 166–173. ( 10.1016/j.ejphar.2012.03.008) [DOI] [PubMed] [Google Scholar]
- 29.Yuste JE, Echeverry MB, Ros-Bernal F, Gomez A, Ros CM, Campuzano CM, Fernandez-Villalba E, Herrero MT. 2012. 7-Nitroindazole down-regulates dopamine/DARPP-32 signaling in neostriatal neurons in a rat model of Parkinson's disease. Neuropharmacology 63, 1258–1267. ( 10.1016/j.neuropharm.2012.07.031) [DOI] [PubMed] [Google Scholar]
- 30.Solís O, Espadas I, Del-Bel EA, Moratalla R. 2015. Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3−/− aphakia mice. Neurobiol. Dis. 73, 49–59. ( 10.1016/j.nbd.2014.09.010) [DOI] [PubMed] [Google Scholar]
- 31.Garthwaite J, Garthwaite G, Palmer RM, Moncada S. 1989. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur. J. Pharmacol. 172, 413–416. ( 10.1016/0922-4106(89)90023-0) [DOI] [PubMed] [Google Scholar]
- 32.Gally JA, Montague PR, Reeke GN, Edelman GM. 1990. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc. Natl Acad. Sci. USA 87, 3547–3551. ( 10.1073/pnas.87.9.3547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunot S, Boissière F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC. 1996. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience 72, 355–363. ( 10.1016/0306-4522(95)00578-1) [DOI] [PubMed] [Google Scholar]
- 34.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. 1997. Nuclear translocation of NF-κB is increased in dopaminergic neurons of patients with Parkinson disease. Proc. Natl Acad. Sci. USA 94, 7531–7536. ( 10.1073/pnas.94.14.7531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L'Episcopo F, Tirolo C, Caniglia S, Testa N, Serra PA, Impagnatiello F, Morale MC, Marchetti B. 2010. Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J. Neuroinflammation 7, 83 ( 10.1186/1742-2094-7-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bortolanza M, Cavalcanti-Kiwiatkoski R, Padovan-Neto FE, da-Silva CA, Mitkovski M, Raisman-Vozari R, Del-Bel EA. 2014. Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson's disease. Neurobiol. Dis. 73C, 377–387. ( 10.1016/j.nbd.2014.10.017) [DOI] [PubMed] [Google Scholar]
- 37.Coleman JW. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1, 1397–1406. ( 10.1016/S1567-5769(01)00086-8) [DOI] [PubMed] [Google Scholar]
- 38.Guzik TJ, Korbut R, Adamek-Guzik T. 2003. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 54, 469–487. [PubMed] [Google Scholar]
- 39.Tripathi P, Kashyap L, Singh V. 2007. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 51, 443–452. ( 10.1111/j.1574-695X.2007.00329.x) [DOI] [PubMed] [Google Scholar]
- 40.Iadecola C, Gorelick PB. 2005. The Janus face of cyclooxygenase-2 in ischemic stroke: shifting toward downstream targets. Stroke 36, 182–185. ( 10.1161/01.STR.0000153797.33611.d8) [DOI] [PubMed] [Google Scholar]
- 41.Laflamme N, Lacroix S, Rivest S. 1999. An essential role of interleukin-1β in mediating NF-κB activity and Cox-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J. Neurosci. 19, 10 923–10 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Banion MK, Winn VD, Young DA. 1992. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl Acad. Sci. USA 89, 4888–4892. ( 10.1073/pnas.89.11.4888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breder CD, Dewitt D, Kraig RP. 1995. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 355, 296–315. ( 10.1002/cne.903550208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi SH, Aid S, Bosetti F. 2009. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 30, 174–181. ( 10.1016/j.tips.2009.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minghetti L. 2004. Cyclooxygenase-2 (Cox-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 63, 901–910. [DOI] [PubMed] [Google Scholar]
- 46.Barnum CJ, Eskow KL, Dupre K, Blandino P, Jr, Deak T, Bishop C. 2008. Exogenous corticosterone reduces l-DOPA-induced dyskinesia in the hemi-Parkinsonian rat: role for interleukin-1β. Neuroscience 156, 30–41. ( 10.1016/j.neuroscience.2008.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aron Badin R, et al. 2013. IRC-082451, a novel multitargeting molecule, reduces L-DOPA-induced dyskinesias in MPTP Parkinsonian primates. PLoS ONE 8, e52680 ( 10.1371/journal.pone.0052680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muñoz A, Garrido-Gil P, Dominguez-Meijide A, Labandeira-Garcia JL. 2014. Angiotensin type 1 receptor blockage reduces l-dopa-induced dyskinesia in the 6-OHDA model of Parkinson's disease. Involvement of vascular endothelial growth factor and interleukin-1β. Exp. Neurol. 261, 720–732. ( 10.1016/j.expneurol.2014.08.019) [DOI] [PubMed] [Google Scholar]
- 49.Spinnewyn B, Mautino G, Marin JG, Rocher MN, Grandoulier AS, Ferrandis E, Auguet M, Chabrier PE. 2011. BN82451 attenuates l-dopa-induced dyskinesia in 6-OHDA-lesioned rat model of Parkinson's disease. Neuropharmacology 60, 692–700. ( 10.1016/j.neuropharm.2010.11.019) [DOI] [PubMed] [Google Scholar]
- 50.Del-Bel E, Padovan-Neto FE, Szawka RE, da-Silva CA, Raisman-Vozari R, Anselmo-Franci J, Romano-Dutra AC, Guimaraes FS. 2014. Counteraction by nitric oxide synthase inhibitor of neurochemical alterations of dopaminergic system in 6-OHDA-lesioned rats under l-DOPA treatment. Neurotox. Res. 25, 33–44. ( 10.1007/s12640-013-9406-3) [DOI] [PubMed] [Google Scholar]
- 51.Cao X, Yasuda T, Uthayathas S, Watts RL, Mouradian MM, Mochizuki H, Papa SM. 2010. Striatal overexpression of FosB reproduces chronic levodopa-induced involuntary movements. J. Neurosci. 30, 7335–7343. ( 10.1523/JNEUROSCI.0252-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CS, Cenci MA, Schulzer M, Björklund A. 2000. Embryonic ventral mesencephalic grafts improve levodopa-induced dyskinesia in a rat model of Parkinson's disease. Brain 123, 1365–1379. ( 10.1093/brain/123.7.1365) [DOI] [PubMed] [Google Scholar]
- 53.Marin C, Aguilar E, Obeso JA. 2006. Coadministration of entacapone with levodopa attenuates the severity of dyskinesias in hemiparkinsonian rats. Mov. Disord. 21, 646–653. ( 10.1002/mds.20780) [DOI] [PubMed] [Google Scholar]
- 54.Cenci MA, Lee CS, Björklund A. 1998. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 10, 2694–2706. ( 10.1046/j.1460-9568.1998.00285.x) [DOI] [PubMed] [Google Scholar]
- 55.Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. 2002. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur. J. Neurosci. 15, 120–132. ( 10.1046/j.0953-816x.2001.01843.x) [DOI] [PubMed] [Google Scholar]
- 56.Winkler C, Kirik D, Björklund A, Cenci MA. 2002. l-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of Parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 10, 165–186. ( 10.1006/nbdi.2002.0499) [DOI] [PubMed] [Google Scholar]
- 57.Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- 58.Mitkovski M, Padovan-Neto FE, Raisman-Vozari R, Ginestet L, da-Silva CA, Del-Bel EA. 2012. Investigations into potential extrasynaptic communication between the dopaminergic and nitrergic systems. Front. Physiol. 3, 372 ( 10.3389/fphys.2012.00372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. 1995. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 18, 527–535. ( 10.1016/0166-2236(95)98374-8) [DOI] [PubMed] [Google Scholar]
- 60.Bennett BD, Bolam JP. 1993. Two populations of calbindin D28k-immunoreactive neurones in the striatum of the rat. Brain Res. 610, 305–310. ( 10.1016/0006-8993(93)91414-N) [DOI] [PubMed] [Google Scholar]
- 61.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. 1993. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11, 371–386. ( 10.1016/0896-6273(93)90192-T) [DOI] [PubMed] [Google Scholar]
- 63.Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper CB. 1992. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J. Comp. Neurol. 322, 409–438. ( 10.1002/cne.903220309) [DOI] [PubMed] [Google Scholar]
- 64.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. 1996. Cox-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl Acad. Sci. USA 93, 2317–2321. ( 10.1073/pnas.93.6.2317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weyer A, Schilling K. 2003. Developmental and cell type-specific expression of the neuronal marker neun in the murine cerebellum. J. Neurosci. Res. 73, 400–409. ( 10.1002/jnr.10655) [DOI] [PubMed] [Google Scholar]
- 66.Mullen RJ, Buck CR, Smith AM. 1992. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211. [DOI] [PubMed] [Google Scholar]
- 67.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. 2004. DARPP-32: an integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 44, 269–296. ( 10.1146/annurev.pharmtox.44.101802.121415) [DOI] [PubMed] [Google Scholar]
- 68.Chen SY, Harding JW, Barnes CD. 1996. Neuropathology of synthetic beta-amyloid peptide analogs in vivo. Brain Res. 715, 44–50. ( 10.1016/0006-8993(96)84503-3) [DOI] [PubMed] [Google Scholar]
- 69.Tallaksen-Greene SJ, Albin RL. 1994. Localization of ampa-selective excitatory amino acid receptor subunits in identified populations of striatal neurons. Neuroscience 61, 509–519. ( 10.1016/0306-4522(94)90430-8) [DOI] [PubMed] [Google Scholar]
- 70.Inagaki S, Takagi H, Suzuki K, Akai F, Taniguchi N. 1991. Intense immunoreactivity for Mn-superoxide dismutase (Mn-Sod) in cholinergic and non-cholinergic neurons in the rat basal forebrain. Brain Res. 541, 354–357. ( 10.1016/0006-8993(91)91038-3) [DOI] [PubMed] [Google Scholar]
- 71.Medina L, Reiner A. 1995. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implications for the evolution of basal ganglia. Brain Behav. Evol. 46, 35–58. ( 10.1159/000113277) [DOI] [PubMed] [Google Scholar]
- 72.Difiglia M, Pasik T, Pasik P. 1980. Ultrastructure of Golgi-impregnated and gold-toned spiny and aspiny neurons in the monkey neostriatum. J. Neurocytol. 9, 471–492. ( 10.1007/BF01204837) [DOI] [PubMed] [Google Scholar]
- 73.Figueredo-Cardenas G, Harris CL, Anderson KD, Reiner A. 1998. Relative resistance of striatal neurons containing calbindin or parvalbumin to quinolinic acid-mediated excitotoxicity compared to other striatal neuron types. Exp. Neurol. 149, 356–372. ( 10.1006/exnr.1997.6724) [DOI] [PubMed] [Google Scholar]
- 74.Gerfen CR, Baimbridge KG, Miller JJ. 1985. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc. Natl Acad. Sci. USA 24, 8780–8784. ( 10.1073/pnas.82.24.8780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. 1998. Cyclooxygenase-2 inhibition prevents delayed death of Ca1 hippocampal neurons following global ischemia. Proc. Natl Acad. Sci. USA 95, 10 954–10 959. ( 10.1073/pnas.95.18.10954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Hitron IM, Iadecola C, Pickel VM. 2005. Synaptic and vascular associations of neurons containing cyclooxygenase-2 and nitric oxide synthase in rat somatosensory cortex. Cereb. Cortex 15, 1250–1260. ( 10.1093/cercor/bhi008) [DOI] [PubMed] [Google Scholar]
- 77.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. 2003. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc. Natl Acad. Sci. USA 100, 5473–5478. ( 10.1073/pnas.0837397100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sánchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. 2004. Selective Cox-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J. Neuroinflammation 1, 6 ( 10.1186/1742-2094-1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teismann P, Ferger B. 2001. Inhibition of the cyclooxygenase isoenzymes Cox-1 and Cox-2 provide neuroprotection in the MPTP-mouse model of Parkinson's disease. Synapse 39, 167–174. () [DOI] [PubMed] [Google Scholar]
- 80.Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R, Hong JS. 2002. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci. Lett. 329, 354–358. ( 10.1016/S0304-3940(02)00704-8) [DOI] [PubMed] [Google Scholar]
- 81.Feng Z, Li D, Fung PC, Pei Z, Ramsden DB, Ho SL. 2003. Cox-2-deficient mice are less prone to MPTP-neurotoxicity than wild-type mice. Neuroreport 14, 1927–1929. ( 10.1097/00001756-200310270-00009) [DOI] [PubMed] [Google Scholar]
- 82.Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. 2003. The role of glial reaction and inflammation in Parkinson's disease. Ann. NY Acad. Sci. 991, 214–228. ( 10.1111/j.1749-6632.2003.tb07478.x) [DOI] [PubMed] [Google Scholar]
- 83.Salvemini D, Kim SF, Mollace V. 2013. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R473–R487. ( 10.1152/ajpregu.00355.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. 2005. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 57, 217–252. ( 10.1124/pr.57.2.1) [DOI] [PubMed] [Google Scholar]
- 85.Henrion D, Dechaux E, Dowell FJ, Maclour J, Samuel JL, Levy BI, Michel JB. 1997. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br. J. Pharmacol. 121, 83–90. ( 10.1038/sj.bjp.0701109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian J, Kim SF, Hester L, Snyder SH. 2008. S-nitrosylation/activation of COX-2 mediates NMDA neurotoxicity. Proc. Natl Acad. Sci. USA 105, 10 537–10 540. ( 10.1073/pnas.0804852105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JM. 1995. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br. J. Pharmacol. 114, 1335–1342. ( 10.1111/j.1476-5381.1995.tb13353.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stadler J, Harbrecht BG, Di SM, Curran RD, Jordan ML, Simmons RL, Billiar TR. 1993. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J. Leukoc. Biol. 53, 165–172. [DOI] [PubMed] [Google Scholar]
- 89.Aid S, Langenbach R, Bosetti F. 2008. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J. Neuroinflammation 5, 17 ( 10.1186/1742-2094-5-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toscano CD, Ueda Y, Tomita YA, Vicini S, Bosetti F. 2008. Altered GABAergic neurotransmission is associated with increased kainate-induced seizure in prostaglandin-endoperoxide synthase-2 deficient mice. Brain Res. Bull. 75, 598–609. ( 10.1016/j.brainresbull.2007.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallace C, Lyford G, Worley P, Steward O. 1998. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J. Neurosci. Res. 18, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim SF. 2011. The role of nitric oxide in prostaglandin biology. Nitric Oxide 25, 255–264. ( 10.1016/j.niox.2011.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Toscano CD, Prabhu VV, Langenbach R, Becker KG, Bosetti F. 2007. Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain. Genome Biol. 8, R14 ( 10.1186/gb-2007-8-1-r14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. 1998. A complex program of striatal gene expression induced by dopaminergic stimulation. J. Neurosci. 18, 5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerfen CR. 2003. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum animal model of Parkinson's disease. Neuroscientist 9, 455–462. ( 10.1177/1073858403255839) [DOI] [PubMed] [Google Scholar]
- 96.Herdegen T, Leah JD. 1998. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 28, 370–490. ( 10.1016/S0165-0173(98)00018-6) [DOI] [PubMed] [Google Scholar]
- 97.Cenci MA, Konradi C. 2010. Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Prog. Brain Res. 183, 209–233. ( 10.1016/S0079-6123(10)83011-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chun KS, Surh YJ. 2004. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem. Pharmacol. 68, 1089–1100. ( 10.1016/j.bcp.2004.05.03) [DOI] [PubMed] [Google Scholar]
- 99.Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT, Chen YC. 2010. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE2 activation. Neurobiol. Dis. 37, 118–129. ( 10.1016/j.nbd.2009.09.015) [DOI] [PubMed] [Google Scholar]
- 100.Won L, Ding Y, Singh P, Kang UJ. 2014. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. J. Neurosci. 34, 3090–3094. ( 10.1523/JNEUROSCI.2888-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park SW, Sung MW, Heo DS, Inoue H, Shim SH, Kim KH. 2005. Nitric oxide upregulates the cyclooxygenase-2 expression through the cAMP response element in its promoter in several cancer cell lines. Oncogene 24, 6689–6698. ( 10.1038/sj.onc.1208816) [DOI] [PubMed] [Google Scholar]
- 102.Jenner P. 2008. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 9, 665–677. ( 10.1038/nrn2471) [DOI] [PubMed] [Google Scholar]
- 103.Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E. 2009. Chronic dopaminergic stimulation in Parkinson's disease: from dyskinesias to impulse control disorders. Lancet Neurol. 8, 1140–1149. ( 10.1016/S1474-4422(09)70287-X) [DOI] [PubMed] [Google Scholar]
- 104.Cenci MA. 2014. Presynaptic mechanisms of L-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front. Neurol. 5, 242 ( 10.3389/fneur.2014.00242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murer MG, Moratalla R. 2011. Striatal signaling in L-DOPA-induced dyskinesia: common mechanisms with drug abuse and long term memory involving D1 dopamine receptor stimulation. Front. Neuroanat. 5, 51 ( 10.3389/fnana.2011.00051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. 2003. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci. 6, 501–506. ( 10.1038/nn1040) [DOI] [PubMed] [Google Scholar]
- 107.Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Hervé D, Greengard P, Fisone G. 2007. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in l-DOPA-induced dyskinesia. J. Neurosci. 27, 6995–7005. ( 10.1523/JNEUROSCI.0852-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kitaoka S, Furuyashiki T, Nishi A, Shuto T, Koyasu S, Matsuoka T, Miyasaka M, Greengard P, Narumiya S. 2007. Prostaglandin E2 acts on Ep1 receptor and amplifies both dopamine D1 and D2 receptor signaling in the striatum. J. Neurosci. 27, 12 900–12 907. ( 10.1523/JNEUROSCI.3257-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hilton GD, Ndubuizu A, Nunez JL, McCarthy MM. 2005. Simultaneous glutamate and GABA(A) receptor agonist administration increases calbindin levels and prevents hippocampal damage induced by either agent alone in a model of perinatal brain injury. Dev. Brain Res. 159, 99–111. ( 10.1016/j.devbrainres.2005.07.007) [DOI] [PubMed] [Google Scholar]
- 110.Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. 2006. Estradiol modulation of kainic acid-induced calcium elevation in neonatal hippocampal neurons. Endocrinology 147, 1246–1255. ( 10.1210/en.2005-1258) [DOI] [PubMed] [Google Scholar]
- 111.Fan Y, Shi L, Gu Y, Zhao Y, Xie J, Qiao J, Yang GY, Wang Y, Lu CZ. 2007. Pretreatment with PTD-calbindin D 28k alleviates rat brain injury induced by ischemia and reperfusion. J. Cereb. Blood Flow Metab. 27, 719–728. ( 10.1038/sj.jcbfm.9600373) [DOI] [PubMed] [Google Scholar]
- 112.Chan CS, Gertler TS, Surmeier DJ. 2009. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 32, 249–256. ( 10.1016/j.tins.2009.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Soto-Ortolaza AI, et al. 2010. Calbindin-1 association and Parkinson's disease. Eur. J. Neurol. 17, 208–211. ( 10.1111/j.1468-1331.2009.02769) [DOI] [PubMed] [Google Scholar]
- 114.Heizmann CW, Braun K. 1992. Changes in Ca2+-binding proteins in human neurodegenerative disorders. Trends Neurosci. 15, 259–264. ( 10.1016/0166-2236(92)90067-I) [DOI] [PubMed] [Google Scholar]
- 115.Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. 2011. Enhanced striatal cholinergic neuronal activity mediates l-DOPA-induced dyskinesia in Parkinsonian mice. Proc. Natl Acad. Sci. USA 108, 840–845. ( 10.1073/pnas.1006511108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cachope R, Cheer JF. 2014. Local control of striatal dopamine release. Front. Behav. Neurosci. 8, 188 ( 10.3389/fnbeh.2014.00188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Descarries L, Gisiger V, Steriade M. 1997. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 53, 603–625. ( 10.1016/S0301-0082(97)00050-6) [DOI] [PubMed] [Google Scholar]
- 118.Descarries L, Mechawar N. 2000. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 125, 27–47. ( 10.1016/S0079-6123(00)25005-X) [DOI] [PubMed] [Google Scholar]
- 119.Carta M, Bezard E. 2011. Contribution of pre-synaptic mechanisms to l-DOPA-induced dyskinesia. Neuroscience 198, 245–251. ( 10.1016/j.neuroscience.2011.07.070) [DOI] [PubMed] [Google Scholar]
- 120.Molokanova E, Akhtar MW, Sanz-Blasco S, Tu S, Piña-Crespo JC, McKercher SR, Lipton SA. 2014. Differential effects of synaptic and extrasynaptic NMDA receptors on Aβ-induced nitric oxide production in cerebrocortical neurons. J. Neurosci. 34, 5023–5028. ( 10.1523/JNEUROSCI.2907-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zoli M, Jansson A, Syková E, Agnati LF, Fuxe K. 1999. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 20, 142–150. ( 10.1016/S0165-6147(99)01343-7) [DOI] [PubMed] [Google Scholar]
- 122.Vizi ES, Fekete A, Karoly R, Mike A. 2010. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 160, 785–809. ( 10.1111/j.1476-5381.2009.00624.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuxe K, et al. 2008. Receptor–receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Rev. 58, 415–452. ( 10.1016/j.brainresrev.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 124.Fuxe K, et al. 2012. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal–glial networks. Front. Physiol. 3, 136 ( 10.3389/fphys.2012.00136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rice ME, Cragg SJ. 2008. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res. Rev. 58, 303–313. ( 10.1016/j.brainresrev.2008.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Philippides A, Ott SR, Husbands P, Lovick TA, O'Shea M. 2005. Modeling cooperative volume signaling in a plexus of nitric-oxide-synthase-expressing neurons. J. Neurosci. 25, 6520–6532. ( 10.1523/JNEUROSCI.1264-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawano T, et al. 2006. Prostaglandin E2 Ep1 receptors: downstream effectors of Cox-2 neurotoxicity. Nat. Med. 12, 225–229. ( 10.1038/nm1362) [DOI] [PubMed] [Google Scholar]
- 128.Katori M, Majima M. 2000. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm. Res. 49, 367–392. ( 10.1007/s000110050605) [DOI] [PubMed] [Google Scholar]
- 129.O'Banion MK. 1999. Cox-2 and Alzheimer's disease: potential roles in inflammation and neurodegeneration. Expert Opin. Investig. Drug 8, 1521–1536. ( 10.1517/13543784.8.10.1521) [DOI] [PubMed] [Google Scholar]
- 130.Warner TD, Mitchell JA. 2004. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 18, 790–804. ( 10.1096/fj.03-0645rev) [DOI] [PubMed] [Google Scholar]
- 131.Chen C, Magee JC, Bazan NG. 2002. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 87, 2851–2857. [DOI] [PubMed] [Google Scholar]
- 132.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. 2000. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J. Neurosci. 20, 763–770. ( 10.1152/jn.00820.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hardingham GE, Bading H. 2010. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696. ( 10.1038/nrn2911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. 2004. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J. Neurosci. 24, 257–268. ( 10.1523/JNEUROSCI.4485-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stark DT, Bazan NG. 2011. Synaptic and extrasynaptic NMDA receptors differentially modulate neuronal cyclooxygenase-2 function, lipid peroxidation, and neuroprotection. J. Neurosci. 31, 13 710–13 721. ( 10.1523/JNEUROSCI.3544-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith WL, DeWitt DL, Garavito RM. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69, 145–182. ( 10.1146/annurev.biochem.69.1.145) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.