Abstract
Effects of glial cells on electrical isolation and shaping of synaptic transmission between neurons have been extensively studied. Here we present evidence that the release of proteins from astrocytes as well as microglia may regulate voltage-activated Na+ currents in neurons, thereby increasing excitability and speed of transmission in neurons kept at distance from each other by specialized glial cells. As a first example, we show that basic fibroblast growth factor and neurotrophin-3, which are released from astrocytes by exposure to thyroid hormone, influence each other to enhance Na+ current density in cultured hippocampal neurons. As a second example, we show that the presence of microglia in hippocampal cultures can upregulate Na+ current density. The effect can be boosted by lipopolysaccharides, bacterial membrane-derived stimulators of microglial activation. Comparable effects are induced by the exposure of neuron-enriched hippocampal cultures to tumour necrosis factor-α, which is released from stimulated microglia. Taken together, our findings suggest that release of proteins from various types of glial cells can alter neuronal excitability over a time course of several days. This explains changes in neuronal excitability occurring in states of thyroid hormone imbalance and possibly also in seizures triggered by infectious diseases.
Keywords: thyroid hormone, voltage-activated sodium currents, fibroblast growth factor-2, neurotrophin-3, microglia, tumour necrosis factor-α
1. Introduction
Glial cells can influence neuronal function in various ways. As electrical insulators, oligodendrocytes and Schwann cells ensure fast transmission of action potentials. As siphons for K+ and by removal of excitatory amino acids out of the extracellular space, astrocytes prevent mutual excitation of closely opposed neurons. Also glial cells influence synaptic transmission by releasing gliotransmitters as well as by transmitter uptake in membrane structures engulfing synaptic endings, which has led to the term ‘tripartite synapse’ [1]. Release of steroids and even cholesterol from astrocytes has additionally attracted attention that glial cells might exert endocrine functions [2,3], leading to regulation of, for instance, glutamate transporters in neurons [4]. Likewise, Müller cells in the retina control photoreceptor function and blood flow by the release of various vasoactive agents [5]. In addition, they may act as source of polyamines [6], which may modulate neuronal function at a fast time scale by modulating the activity of a variety of ion channels, including inactivation of Na+ channels [7].
Secreted factors have also been shown to influence the structure and function of astrocytes. Hence the thyroid hormone triiodo-l-thyronine (T3) induces the secretion of various protein factors from cerebellar astrocytes [8], resulting in a change in astrocyte morphology as well as neuronal proliferation. Among several candidates, basic fibroblast growth factor (FGF-2) was identified as the most potent factor and its inactivation by neutralizing antibodies in cell cultures reduces the T3-induced increase in astrocyte proliferation by 40% [9].
We have shown that FGF-2 not only increases astrocyte proliferation and induces changes in their shape to a more stellate type [10,11] but also upregulates the Na+ current density in cultured postnatal neurons from rat hippocampus [12]. In this preceding study, we further showed that the upregulation of voltage-gated Na+ currents, which can be induced by incubation of hippocampal neurons for several days with T3 [13,14], was only observed if neurons maintained in NB/B18 medium had been cultured in the presence of a surplus of astrocytes. The effect of T3 could be induced in neuron-enriched cultures by introducing cell culture inserts containing astrocytes into the culture medium and could also be reproduced by exposing neuron-enriched cultures to a conditioned medium from astrocytes that had been exposed for 2 days to T3. The effect evoked by the conditioned medium was heat sensitive, blockable by FGF-2 neutralizing antibodies and could be abolished by adding the antibodies to T3-treated hippocampal cultures containing a surplus of glial cells. We thus concluded that protein factors released from T3-stimulated astrocytes can modulate neuronal excitability by regulating the Na+ current density, and that FGF-2 plays a prominent role in this effect [12].
A further factor, the expression of which is stimulated by T3 in the developing brain, is neurotrophin-3 (NT-3). Its mRNA expression is induced by exposure to T3 in cerebellar granule cells [15]. Addition of NT-3, in turn, increases neurite outgrowth in Purkinje cell-enriched cultures, which express the trkC-receptor [15], suggesting that T3 affects the differentiation of Purkinje cells indirectly by regulating the NT-3 expression in granule cells [15]. NT-3 is also expressed in hippocampal astrocytes [16,17]. As NT-3 can also influence the firing properties of some cell types, including embryonic geniculate neurons [18], we here tested whether exposure of neurons for several days to NT-3 influences Na+ current density in cultured hippocampal neurons. Furthermore, we investigated whether an enhanced release of NT-3 contributed to the regulation of Na+ currents by exposure of brain tissue to T3.
Unlike the stationary astrocytes, microglia are motile cells which are activated during pathological events in the brain and can remove invading and necrotic cells (e.g. [19]). They have been mainly implicated in immune reactions of the brain and release various inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α), interferons and interleukins (e.g. [20,21]). Moreover, they refine synaptic connections by removal of synaptic boutons [22,23]. In the immature brain release of cytokines, such as TNF-α and interferon-gamma, from astrocytes and microglia is involved in oligodendrocyte precursor cell death and arrest of maturation (e.g. [24–27]). Adverse effects of microglia-derived cytokines are also discussed to play a role in the induction of multiple sclerosis (e.g. [28,29]). Furthermore, in inflammatory conditions TNF-α may play a role in causing hyperalgesia or neuropathic pain [30–32] and cytokines are also upregulated in epilepsy [33,34]. As the enhanced excitability in inflammation could be evoked by an upregulation of Na+ current density [35,36], here we also asked whether the release of cytokines from microglia could influence the Na+ current density in cultured hippocampal neurons. To answer this question, we investigated whether the addition of microglia and their activation with lipopolysaccharides (LPS) (e.g. [28,34]) influences the Na+ current density and whether a cytokine supplementation to the cell cultures elicits an effect comparable with the addition of activated microglia.
2. Material and methods
(a). Cell cultures
Experiments were performed on cells obtained from hippocampi dissected from brains of 2- to 4-day-old Wistar rats. Tissue was collected in ice-cold modified phosphate buffered saline (MPBS) composed of 137 mM NaCl, 2.7 mM KCl, 5 mM Na2HPO4, 0.89 mM KH2PO4, 10 mM HEPES, 1 mM pyruvate, 10 mM glucose, 1 mM glutamine (GE Healthcare, formerly PAA, (M11-004) purity ≥ 99%) and 1 mg ml−1 bovine serum albumin, 25 U ml−1 penicillin and 25 µg ml−1 streptomycin (P/S from PAA, Germany). After addition of 10 µg ml−1 deoxyribonuclease I and 5 µl ml−1 of a 2.5% trypsin solution, the tissue was incubated under gentle agitation for 7 min at 37°C and then triturated 15 times with a 1000 µl Eppendorf pipette tip. The dissociated cells were then collected in 10 ml tubes containing MPBS and centrifuged at 1000 r.p.m. at 4°C for 10 min. The pellet was resuspended in RPMI (PAA, Cölbe, Germany), supplemented with 10% fetal calf serum (FCS, Invitrogen, Karlsruhe, Germany), P/S and glutamine and then pre-plated at 37°C and 5% CO2 in humidified atmosphere for 1 h in a B 5060 incubator (Heraeus, Hanau, Germany). Following the pre-plating step, the neuron-enriched supernatant was collected and centrifuged again at 1000 r.p.m. at room temperature for 10 min. The pellet was resuspended in RPMI medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% FCS. A total of 300 000 cells of the suspension were then transferred into 1 cm diameter glass rings positioned into the centres of 3.5 cm plastic Petri dishes that had been coated with poly-d-lysine (5 µg ml−1 in sterile water for 1 h). After culturing for 1 day in RPMI medium for equilibration, cells were transferred to Neurobasal medium in combination with T3-depleted B18 supplement (composed as published in [37] without T3) and treated for 24 h with 4 µM cytosin-β-d-arabinofuranoside to prevent further overgrowth of the cultures with astrocytes. After the third day of culture, cells were incubated for a further 4 days under the following conditions:
control: neurobasal medium supplemented with T3-free B18 supplement, glutamine and P/S;
T3: control medium supplemented with 50 nM T3 (Sigma, cat. no. T2502);
FGF-2: control medium supplemented with FGF-2 (Biomol, Hamburg, Germany, rHuFGF-basic, cat. no. 50361.50), at concentrations of 0.6 or 6 nM, which both have been shown to induce a significant upregulation of the Na+ current density [12];
NT-3: control medium supplemented with 7 nM NT-3 (Biomol, cat. no. 87368);
TNF-α: control medium supplemented with 100 ng ml−1 TNF-α (Peprotech, Hamburg, Germany, cat. no. 400–14); and
K252a: 10 nM K252a (Alomone Labs, cat. no. K150) was dissolved in dimethyl sulfoxide (DMSO).
For neutralization assays, 8 µg ml−1 anti-FGF-2 antibodies (Peprotech cat. no. 500-P18-B) or anti-NT-3 antibodies (Peprotech, cat. no. 500-P82G-B) were added to untreated control and treated sister cultures.
Control sister cultures were treated with identical solvent dilutions as used for the test substances as indicated in the figure legends.
(b). Isolation of microglia
Microglial cells were obtained from whole brain mixed glial cultures by a shaking procedure following the procedures published by McCarthy & de Vellis [38], modified as described in [39]. In brief, mixed glial cultures were obtained from whole brains of postnatal day 0–3 Wistar Hannover rat pups rostral of the cerebellum, dissociated by passing through 125 µm and 36 µm nylon meshes. After centrifugation at 900 r.p.m. for 10 min at room temperature, the cell pellet was resuspended in 5 ml glial mixed medium (GMM) composed of DMEM : Ham's F12 (1 : 1) supplemented with 10% heat inactivated FCS, P/S and glutamine. A yield of cells from 1.5 brains per uncoated T-75 flask (Sarstedt, Nümbrecht, Germany) were pre-cultured for 10–12 days in GMM at 37°C and 5% CO2 in a Haereus B5060 incubator (Hanau, Germany) with medium changes every 3–4 days.
Following a pre-culture period of 10–12 days, in the course of which a confluent astrocyte layer had formed, microglia were isolated from the underlying stronger layer of adherent astrocytes and oligodendrocyte precursors by shaking the flasks for 3 h on an orbital shaker (ES-W, Kühner AG, Birsfelden, Switzerland) in the incubator. The supernatant containing more than 90% microglia was centrifuged for 5 min at 1000 r.p.m. at room temperature; the cells were counted in the supernatant and added to the cultures in quantities of 5 or 10%.
(c). Patch-clamp recordings
Na+ currents were quantified at day 7 in culture using whole cell patch-clamp recordings. Measurements were performed at room temperature using a Patch-Clamp L/M-EPC7 amplifier (List Medical, Darmstadt, Germany). Patch pipettes were fabricated from borosilicate glass capillaries (GB-150TF-8P, Science Products, Hofheim, Germany) using a PP-830 puller (Narishige Europe, London, UK) and had resistances of 4–5 MΩ. Pipette solutions contained, in mM: CaCl2 0.1, EGTA 1.1, MgCl2 5, CsF 100, NaCl 5, HEPES 10; bath solutions contained, in mM: 4-aminopyridine 4, TEA-Cl 10, CaCl2 1, CdCl2 0.5, MgCl2 1, glucose 10, HEPES 10, NaCl 100. Osmolarities of pipette and bath solution were adjusted to the low osmolarity of the NB medium which inhibits the proliferation of oligodendrocyte precursors [39].
Na+ currents were recorded using a series of step depolarizations starting from a holding potential of −77 mV (after correction of a liquid junction potential of −7 mV) in increments of 5 mV. Maximal peak Na+ currents were determined as peak currents at a test potential of −12 mV, which corresponded to the maximum of the current/voltage relationship.
Voltage-dependent inactivation of the Na+ currents was determined by applying a series of pre-pulse steps of 200 ms duration in 5 mV increments, starting at an initial hyperpolarization to −107 mV. The pre-pulses were followed by a depolarization to a test potential of −17 mV to evoke maximal Na+ currents. Peak Na+ currents versus pre-pulse potential were then fitted to a modified Boltzmann equation (I/I0 = 1/(1+exp([Vm − VIn1/2]/S)) with VIn1/2 denoting the pre-pulse potential at which half of the channels are inactivated, Vm the respective pre-pulse potential, I0 the current elicited from the most negative pre-pulse potential and S the slope factor).
Signals were filtered using the EPC7 10 kHz low-pass filter and digitized with PClamp 10 (Molecular Devices, Sunnyvale, CA, USA) at a sampling rate of 20 kHz. Data were digitized with a Digidata 1440A board (Molecular Devices), stored on a personal computer and analysed with clampfit software. Leakage and capacitive artefacts were subtracted using a P/4 protocol. Na+ current densities were calculated by normalizing the peak Na+ current to the cell capacitance calculated from the integral of the charging curve for a test potential of 20 mV after compensation of the electrode capacitance. Cells with leak currents of more than 100 pA, series resistances of more than 20 MΩ and an activation voltage range of less than 20 mV in the I/V curve were discarded to minimize errors of poor membrane voltage control. A liquid junction potential of −7 mV with respect to the bath solution was corrected offline.
For multiple comparisons, one-way ANOVA followed by Tukey's post hoc test was used (Origin 8.5, Origin Lab Corporation, Northampton, MA, USA).
(d). Immunostaining
Astrocytes were identified with antibodies against glial fibrillary acidic protein (GFAP, Sigma G9269 from rabbit, diluted 1 : 500) and activated microglia with OX-42 staining (primary antibody: mouse anti-rat CD11b IgG (anti-OX-42, Millipore, CBL 1512Z) diluted 1 : 200 in PBS). Cells were fixed for 20 min with 4% paraformaldehyde at room temperature, washed three times with PBS and then incubated with blocking buffer containing PBS (with 0.1% Triton X-100) and 3% goat serum. Cells were then incubated with the primary antibodies for 1 h at room temperature, washed with PBS and then incubated with fluorescence conjugated secondary antibodies (AlexaFluor® 488 goat anti-mouse IgG (Invitrogen, A11001) and Alexa Fluor 594 goat anti-rabbit IgG (Life Technologies A11012) both diluted 1 : 500 in PBS), for 1 h under gentle shaking at room temperature in a dark chamber. Nuclei of all cells were visualized by staining with Hoechst-33258 (10 ng ml−1 in PBS, from Sigma Aldrich) for 20 min at room temperature. Fluorescence microphotographs were obtained using a 20× objective on an Olympus IX51 microscope equipped with cellsens software and a ColorView 12 camera.
3. Results
(a). Effects of proteins secreted from astrocytes
(i). Effects of a pre-incubation of hippocampal neurons with NT-3
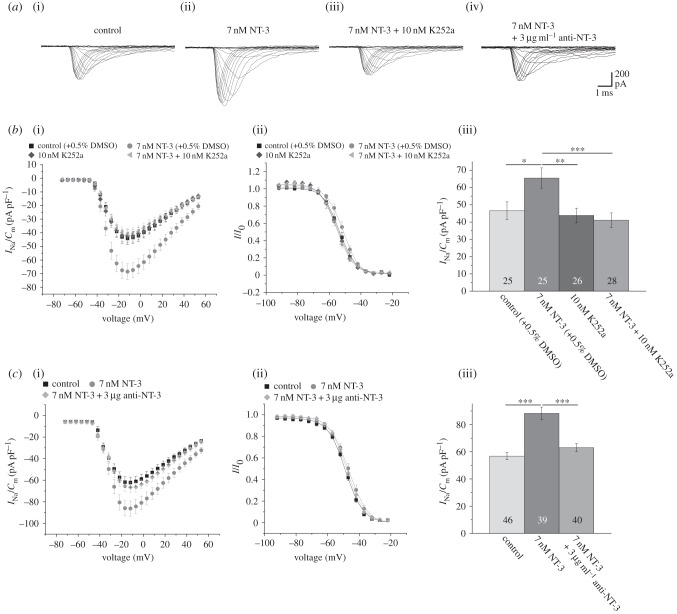
We first investigated whether exposure of postnatal hippocampal neurons to NT-3 for 4 days influences their Na+ current density. To this end, hippocampal cultures obtained from 2- to 3-day-old postnatal rat pups were pre-cultured for 3 days and then exposed to NB medium without NT-3 (control) or supplemented with 7 nM NT-3 for a further 4 days. As shown in figure 1, this treatment induced a significant upregulation of the peak Na+ current density from control levels of approximately 40–60 pA pF−1 to values of 60–90 pA pF−1. This increase occurred for all depolarization steps without changing the voltage dependence of activation and inactivation. As the recordings were performed for both experimental conditions in identical solutions containing no growth factors, these observations suggest a long-lasting upregulation of the Na+ current density by this treatment. When control cultures had been pre-incubated with 10 nM of the trk-receptor blocker K252a [40] for 4 days prior to recording, Na+ currents were unchanged (figure 1b). In the presence of the blocker, the upregulation of the Na+ current density after pre-incubation with 7 nM NT-3 was absent (figure 1b). As K252a is an unspecific blocker of trk-receptors and also blocks protein kinase C and calmodulin with an IC50 of 33 nM [41] and phosphorylase kinase even with an IC50 of 1.7 nM [42], we repeated these experiments in the presence of antibodies against NT-3. Similarly, as observed after a pre-incubation with 10 nM K252a, a pre-incubation with antibodies against NT-3 prevented the upregulation of the Na+ current density after 7 days in culture, suggesting that this antibody at a concentration of 3 µg ml−1 was efficient in neutralizing 7 nM NT-3 in the incubation solution (figure 1c).
Figure 1.
NT-3 exposure for 4 days increases the Na+ current density of cultured hippocampal neurons. (a) Series of original current recordings elicited by step depolarizations in 5 mV increments starting from a holding potential of −77 mV. (b)(i) Mean current–voltage relationships for Na+ currents normalized to capacitance recorded from neurons cultured in the presence or absence of 7 nM NT-3, 10 nM K252a or a combination of both. (ii) Influence of NT-3 or K252a on steady-state inactivation of Na+ currents. Solid lines represent fits to the modified Boltzman equation as detailed in Material and methods. (iii) Peak Na+ current densities of neurons cultured in the presence or absence of NT-3 and/or K252a determined at test potentials of −12 mV starting from holding potentials of −77 mV. (c)(i) Mean current–voltage relationships for Na+ current densities after treatment with 7 nM NT-3 in the presence or absence of 3 µg ml−1 anti-NT-3 antibodies. (ii) Influence of NT-3 and antibodies neutralizing NT-3 on steady-state inactivation of Na+ currents. (iii) Mean peak Na+ current densities measured after pre-incubation of neurons with NT-3 in the presence or absence of anti-NT-3 antibodies at a test potential of −12 mV. All recordings were performed at day 7 in culture after incubation with test substances for 4 days following a pre-culture of 3 days. Numbers in bar charts indicate numbers of cells recorded from. The database for every column is derived from three to five different preparations. Error bars represent means ± s.e., *p < 0.05, **p < 0.01, ***p < 0.005.
(ii). Influence of NT-3 on increases in Na+ current density evoked by thyroid hormone
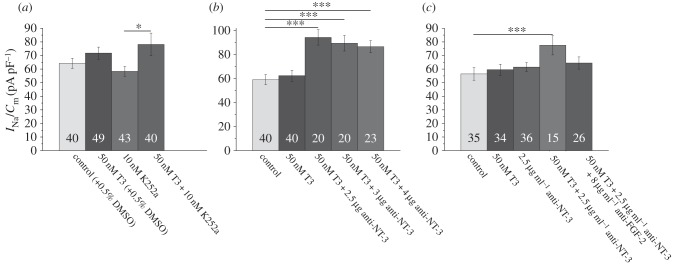
As detailed in Introduction, a prolonged exposure to thyroid hormone increases neuronal Na+ current density in hippocampal neurons. This upregulation requires the presence of glial cells and is induced by the release of soluble proteins, most likely including FGF-2 [12]. In the following experiments, we investigated whether the upregulation of the Na+ current density by T3 includes a component regulated by NT-3. In this case, a co-incubation of T3-treated cultures with NT-3 blockers should at least to some extent reduce the effect of T3 on the Na+ current density. To test this hypothesis, we incubated mixed cultures obtained from 3- to 4-day-old pups, containing neurons as well as glial cells, with either 50 nM T3, 10 nM K252a or a combination of 50 nM T3 plus 10 nM K252a for 4 days and compared the resulting current densities with those of untreated control cultures (figure 2a). These cells had been treated for 24 h with AraC. Therefore, only insignificant increases of the Na+ current density by treatment of the cultures with T3 for 4 days were seen. In contrast to our expectation that the trk-receptor blocker would reduce the effect of the T3 treatment, we clearly observed the opposite: the co-incubation of the cultures with 50 nM T3 plus 10 nM K252a enhanced the T3 action on the neuronal Na+ current density (figure 2a). To investigate whether this effect occurs at the level of the receptor or could have been induced by blocking the effects of NT-3 secreted into the medium by neighbouring cells, we added 2.5 to 4 µg ml−1 of neutralizing anti-NT-3 antibodies to the incubation solution throughout the 4-day-long treatment with 50 nM T3. Similarly, as observed after treatment with K252a, and in contrast to our observations in NT-3-treated cultures, all concentrations of antibodies produced a significant enhancement of the effect of a pre-treatment with 50 nM T3 alone, suggesting that the release of NT-3 from cultured cells reduces the upregulation of the Na+ current density by T3 (figure 2b). Since we had previously observed that the upregulation of the Na+ current density by T3 could be blocked by a co-incubation with antibodies against FGF-2 [12], we now tested whether the maximal Na+ current density obtained by a pre-incubation of the cultures with 50 nM T3 in the presence of antibodies against NT-3 could be influenced by adding additional anti-FGF-2 antibodies to the incubation medium. As shown in figure 2c, indeed, the presence of 8 µg FGF-2 antibodies per millilitre of culture medium, which prevents the increase in Na+ current density by T3 [12], reduced the increase in Na+ current density induced by the combined pre-treatment with 50 nM T3 plus anti-NT-3 antibodies. This confirms our previous finding that the release of FGF-2 plays a dominant role in the effect of thyroid hormone on Na+ current density and further suggests an interaction between the actions of NT-3 and FGF-2. As illustrated in figure 1, none of the treatments influenced the voltage dependence of activation or inactivation of the Na+ currents. To investigate, in addition, potential changes in the inactivation kinetics caused by the treatments, we fitted the time course of inactivation of the peak Na+ currents elicited by step depolarizations to −12 mV to exponentials decaying with two time constants. Na+ currents from control cells decayed with τ1 = 0.93 ± 0.04 ms and τ2 = 7.54 ± 1.33 ms (n = 35); maximal Na+ currents had been observed in cultures treated with 50 nM T3 + 2.5 µg anti-NT3. In these cultures τ1 amounted to 0.77 ± 0.04 ms and τ2 was 5.61 ± 0.69 ms (n = 15). Following treatment with 50 nM T3 + 2.5 µg anti-NT3 +8 µg anti-FGF2, which reversed the effect of the treatment with anti-NT-3 antibodies, τ1 amounted to 0.80 ± 0.05 ms and τ2 was 8.58 ± 2.39 ms (n = 27). Since no statistically significant changes were found, we assume that treatment with these factors does not significantly influence the availability of inactivated Na+channels as observed after polyamine withdrawal [7].
Figure 2.
Blockage of NT-3 augments the upregulation of the Na+ current density by thyroid hormone (T3). (a) Effects of incubation with 50 nM T3, 10 nM K252a and a combination of both for 4 days prior to recording. Note that co-incubation with the trk-receptor blocker K252a increases the upregulation of the Na+ current density by T3. (b) Effects of incubation with 50 nM T3 and 50 nM T3 plus three concentrations of anti-NT-3 antibodies for 4 days prior to recording. Note that the co-incubation with the antibodies increases the upregulation of the Na+ current density by T3. (c) Effects of incubations with 50 nM T3, controls treated with 2.5 µg ml−1 anti-NT3 or a combination of both in the presence or absence of 8 µg ml−1 anti-FGF-2 antibodies. Note that the addition of antibodies against FGF-2 reverses the upregulation of the Na+ current density by co-incubation with the anti-NT-3 antibodies. Bars represent peak Na+ current densities elicited by step depolarizations to −12 mV starting from holding potentials of −77 mV. Numbers in bar charts indicate numbers of cells recorded from. The database for every column is derived from three to five different preparations. Error bars represent means ± s.e., *p < 0.05, ***p < 0.005.
(iii). Interactions between NT-3 and FGF-2
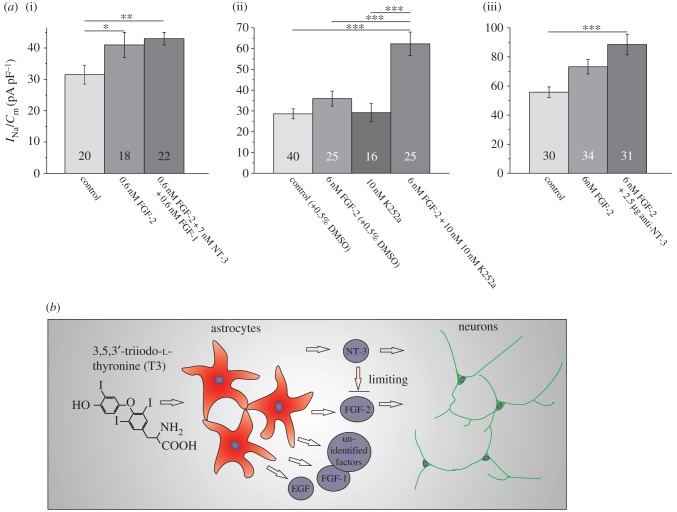
To investigate potential interactions between the action of NT-3 and FGF-2 on Na+ current density, we incubated neuron-enriched cultures prepared from 2-day-old rat pups with FGF-2 and a combination of NT-3, FGF-1 and FGF-2. FGF-1 is another protein, released from cerebellar astrocytes [9], that upregulates Na+ current density [43]. The combined incubation with the three factors for 4 days did not further increase the Na+ current density, suggesting that the effects of the different factors are not additive and thus could converge on a common signal pathway (figure 3a). We then tested potential effects of the trk-receptor blocker K252a on the FGF-2-induced upregulation of the Na+ current density. Most interestingly, the co-incubation of neuron-enriched hippocampal cultures with FGF-2 as well as 10 nM K252a led to maximal Na+ current densities (figure 3b). As K252a even at a concentration of 200 nM did not block the FGF-2-induced neurite outgrowth in PC12 cells [44], it can be assumed that K252a does not interact with the FGF-receptors. Our present findings can thus be interpreted in the sense that some basal release of NT-3 or a different trk-receptor activating agent might inhibit the effect of FGF-2. To further test whether NT-3 might be involved, we investigated the effects of NT-3 neutralizing antibodies on the regulation of the Na+ current density by FGF-2 in neuron-enriched hippocampal cultures. In a manner analogous to the effect elicited by the co-incubation with K252a, a co-incubation with antibodies against NT-3 boosted the upregulation of the Na+ current density evoked by the FGF-2 treatment alone (figure 3c). Our results can be interpreted by assuming that some basal release of NT-3 inhibits the action of FGF-2 on voltage-gated Na+ current density. The stoichiometric ratio of several growth factors secreted from satellite cells in the neuronal microenvironment may thus fine-tune Na+ current density and hence neuronal excitability.
Figure 3.
Interactions of FGF-2 and NT-3 in the regulation of voltage-gated Na+ current density. (a)(i) Comparison of Na+ current density after pre-incubation of a neuron-enriched culture with FGF-2 alone or a combination of FGF-2, NT-3 and FGF-1 shows that the effects of the three growth factors are not additive. (ii) Co-incubation of FGF-2-treated cultures with 10 nM of the trk-receptor blocker K252a shows that a 4 day exposure to the trk-receptor blocker increases the effect of FGF-2 on the Na+ current density. (iii) Effects of an incubation of neuron-enriched cultures with FGF-2 are increased by a co-incubation with antibodies against NT-3. Bars represent peak Na+ current densities elicited by step depolarizations to −12 mV starting from holding potentials of −77 mV. Numbers in bar charts indicate numbers of cells recorded from. The database for every column is derived from three to six different preparations. Error bars represent means ± s.e., *p < 0.05, **p < 0.01, ***p < 0.005. (b) Schematic to illustrate the results from figures 1 to 3; although both NT-3 and FGF-2 upregulate the Na+ current density the pathway activated by NT-3 inhibits the pathway activated by FGF-2. (Online version in colour.)
(b). Effects of proteins secreted from microglia
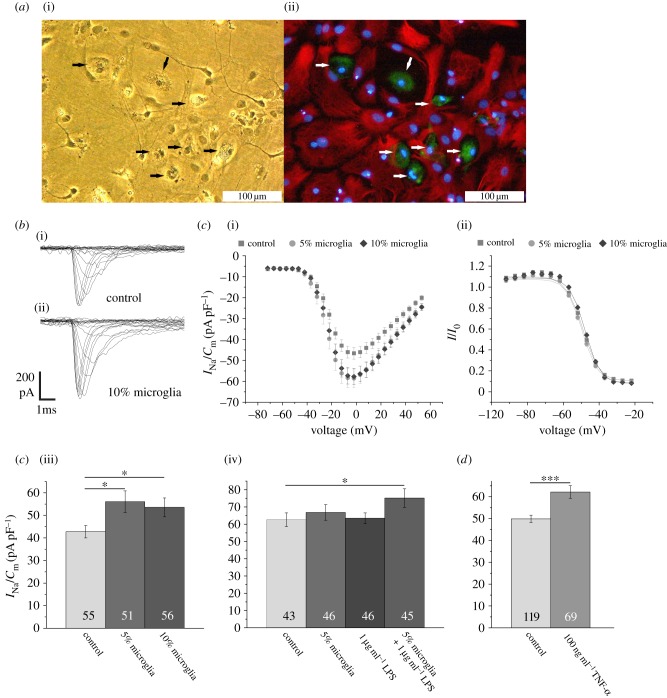
Microglia plays a major role in immune reactions in the central nervous system. As in inflammatory conditions after a period of apathy in many cases, neuronal hyperexcitability and epileptic seizures have been observed [45,46], we also tested here whether the presence and/or activation of microglia might contribute to Na+ current regulation. To study such effects we co-cultured hippocampal neurons with microglia obtained from whole brain glial cell cultures (figure 4a). In a first series of experiments, we added 5 and 10% microglia to neuron-enriched cultures and recorded Na+ current densities. As shown in figure 4a, these cultures contained a surplus of OX42 positive cells, which showed the characteristic morphology of activated amoeboid microglia. The presence of microglia for 4 days in the culture medium increased the Na+ current density without affecting the voltage dependence of activation and inactivation (figure 4c). The effect of the presence of microglia could be enhanced by stimulating the microglia by addition of 1 µg ml−1 LPS into the culture medium (figure 4c). As, most likely, microglia-secreted factors could account for the upregulation of the Na+ current density, we then tested the effects of an incubation of neuron-enriched cultures with TNF-α, which has been shown to be induced by LPS treatment and secreted from microglia (e.g. [20]). As shown in figure 4d, similar to the addition of microglia, an incubation of the cultures with 100 ng ml−1 TNF-α resulted in an upregulation of the Na+ current density.
Figure 4.
Influence of microglia and treatment with TNF-α on Na+ current density. (a)(i) Phase contrast picture and (ii) fluorescence microphotograph of a culture stained for OX42 positive microglia (green) (indicated by arrows), GFAP positive astrocytes (red) and nuclei with Hoechst 33258 (blue). (b) Family of original currents recorded from (i) control neuron and (ii) neuron cultured in the presence of 10% microglia. (c)(i) Mean current–voltage relationships for recordings from control cells and cells cultured in the presence of 5 and 10% microglia. (ii) Note that a pre-incubation with microglia does not influence the steady-state inactivation of the Na+ currents. (iii) Effects of the addition of 5 and 10% microglia on mean peak Na+ current densities. (iv) Effects of the addition of 1 µg ml−1 lipopolysaccharides to control cultures or cultures containing 5% microglia on mean peak Na+ current densities. (d) Effect of the addition of 100 ng ml−1 TNF-α for 4 days on Na+ current densities. Bars represent peak Na+ current densities elicited by step depolarizations to −12 mV starting from holding potentials of −77 mV. Numbers in bar charts indicate numbers of cells recorded from. The database for every column is derived from three to 10 different preparations. Error bars represent means ± s.e., *p < 0.05, ***p < 0.005. (Online version in colour.)
4. Discussion
The release of small molecular weight neurotransmitters, such as dopamine as well as acetylcholine, has been implicated in short-term plasticity of Na+ currents evoked by the activation of phosphorylation cascades [47]. Here we extend the concept of functional neuronal plasticity by providing evidence that the release of growth factors from glial cells, including astrocytes and microglia, can regulate neuronal Na+ current density and thus influence their excitability in a time range of several days. This adds, we believe, a novel aspect of glial influences on neuronal function.
In addition to our previous finding that FGF-2, which is released from astrocytes after stimulation with T3 [12], upregulates the Na+ current density, we here observed that NT-3 can evoke similar effects in hippocampal neurons. This corresponds to the finding of Al-Hadlaq et al. [18] that NT-3-treated geniculate ganglion neurons show increased action potential rise times and amplitudes. To our surprise the trk-receptor blocker K252a as well as antibodies neutralizing NT-3 did not inhibit, but potentiated the Na+ current regulation by T3. Our result is in line with the finding that K252a can upregulate Na+ currents in PC12 cells incubated in conditioned medium from sciatic nerve [48]. These observations can be explained by the assumption that several signal pathways converge at some point to fine-tune Na+ current density in neuronal membranes, such that the regulation of the Na+ current density by FGF-2 is inhibited by that of NT-3 (see figure 3b for a schematic illustration). In a different study, the FGF-2-induced proliferation of cortical precursor cells was inhibited by NT-3, showing a similar inhibitory effect of NT-3 on FGF-2 action [49]. Inhibitory effects of NT-3 are also suggested by a study by Yang et al. [50] showing that excessive astrocyte-derived NT-3 may exert an inhibitory effect on neuronal neurite outgrowth. A further mutual interaction of FGF-2 and NT-3 has been described in the retina, where FGF-2 release from Müller cells could rescue photoreceptors damaged by illumination. In this case, however, the effect was inhibited by NGF and enhanced by NT-3 [51]. Further interactions of FGF-2 and NT-3 have been described during development of the cochlear ganglion [52].
Our present observations suggest that the composition of the cocktail of growth factors released by glia after stimulation with thyroid hormone [9] could fine-tune Na+ current density in neurons. The clinically observed variability of the susceptibility of the brains of different subjects to hypothyroidism might thus not only be explained by polymorphisms in thyroid hormone transporters in the blood–brain barrier [53] but might also result from variations in the growth factor composition released from astrocytes.
(a). Na+ current density and immune responses
As inflammatory processes in the central nervous system may lead to epileptic seizures [33,34,54] and because an upregulation of Na+ current density has been implicated in the development of neuropathic pain [55], we also tested whether inflammatory signals accompanying microglia activation could influence Na+ current density in hippocampal neurons. In fact, the addition of 5 or 10% surplus microglial cells to the culture for 4 days significantly increased the Na+ current density (figure 4b,c). An activation of microglia by LPS, as occurs during bacterial infections, induced a stronger regulation than observed in a co-culture with microglia alone (figure 4c). As it is known that microglia release factors such as interleukins and tumour necrosis factor upon activation by LPS (e.g. [20]), we tested whether pre-incubation with TNF-α for 4 days mimics the effect of LPS-stimulated microglia. Our finding that this is indeed the case (figure 4d) is in line with observations made by Chen et al. [56] that TNF-α is elevated and increases Na+ currents in dorsal root ganglia following nerve injury. Although more details remain to be worked out, our results show that the presence of activated microglial cells can increase the Na+ current density in hippocampal neurons and that TNF-α is a potential candidate factor that could mediate this response.
Here we have shown that T3-stimulated astrocytes as well as LPS-stimulated microglia upregulate Na+ current density in hippocampal neurons. The emerging picture of an upregulation of Na+ current density by factors released from glia becomes even more complex if one considers that astrocytes in turn secrete factors, like transforming growth factor-β, macrophage colony-stimulating factor and granulocyte/macrophage colony-stimulating factor, that act in concert to inactivate microglia [57]. It remains to be shown how the cocktail of factors secreted from astrocytes is regulated by T3 in concert with inflammatory signals that might modify the pattern of protein synthesis/or secretion from astrocytes.
(b). Mechanisms of protein secretion from glial cells
Our observations suggest that glial cells secrete proteins by stimuli such as thyroid hormone exposure of astrocytes or bacterial lipoproteins on microglia, which in turn lead to a regulation of the neuronal Na+ current density over a time course of several days. The question how hormonal or inflammatory stimuli trigger protein release from glial cells has so far not been conclusively answered. The most conspicuous mechanism might be the release of dense-core vesicles, such as shown to be involved in the release of various peptides, such as for example, BDNF release from neurons (e.g. [58]). This type of release, however, generally requires depolarization-induced intracellular Ca2+ transients, which are difficult to conceive for long-lasting T3 actions, for these are most likely mediated by nuclear receptors (e.g. [59]). Future experiments will be needed to find out whether alternative routes of constitutive release, which could, e.g., involve connexin-induced chemokine release from astrocytes [60], similar to ATP release through pannexin channels [61], could be involved. Furthermore, unconventional mechanisms of membrane translocation have been discovered for FGF-2 [62], which could also apply to its release from astrocytes. Finally, neuron–glia interactions have also been shown to involve the exchange of exosomes, shed from the membranes of donor cells and then internalized by the acceptor cells [63,64].
5. Conclusion
A large number of papers have led to the concept that astrocytes physically separate neurons from each other and remove substances such as excitatory glutamate or depolarizing extracellular potassium ions. Thus, by preventing spillover between adjacent neurons they prevent cross-excitation that would otherwise lead to fatal conditions of over-excitation, such as epilepsies and cell death. Having thus provided a safety margin to prevent positive feedback loops, our present observations indicate that astrocytes in addition release proteins that increase Na+ current density within a time course of days. An increase in Na+ current density has been shown to lead first of all to an increase in firing frequency [65]. In T3-treated hippocampal and cortical cultures, it additionally leads to an increase in action potential rise time and amplitude [14], which may altogether lead to an increase in the intensity of the response to a given stimulus and to increased transmitter release. Since increases in Na+ channel density in the membrane lead to increases in conduction velocity for channel densities up to 2000 channels µm−2 [66], this furthermore leads to an acceleration of information processing in the brain allowing animals to react faster to various challenges. As discussed in [67], this might be a mechanism by which thyroid hormone speeds up many functions of the nervous system, including speech and visual perception.
The functional role of factors released from microglia is less clear. However, in infectious diseases, transient epileptic episodes have been observed [46], which could have potentially been caused by excessive cytokine release from activated microglia [34], leading to an enhanced neuronal activity not only by a change in synaptic balance but also by an increase in Na+ current density. Such a mechanism might have also been involved in the development of seizures that occurred during immune reactions in 20% of patients during the course of the recent outbreak of a Shiga-toxin-producing Escherichia coli infection [45].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Marina Wotske, Sebastian Siebrecht, Verena Honke, Jos Philipp, Lars Klapal, Thomas Walter, Christina Ahrensmeier and Isabel Kohtz for contributing patch-clamp recordings performed during laboratory courses and undergraduate theses to the database of this publication; Heidrun Breuker for securing the supply of materials and maintenance of equipment; Meray Serdar for the preparation of the microglial cells; Denis Thatenhorst for helpful discussions; as well as Rolf Heumann for continuous support.
Ethical statement
Animals were bred in the animal house of the Faculty for Medicine at the Ruhr University and all procedures adhered to the German animal protection law.
Data accessibility
The datasets presented in this paper are included in the electronic supplementary material.
Funding statement
V.N. received a fellowship from the International Graduate School of Neuroscience at the Ruhr University Bochum.
Authors' contributions
I.D.D. designed the experiments, participated in data analysis and interpretation, and drafted the article. B.A.I. acquired and analysed most of the data, participated in the conception of the experiments, prepared the figures and participated in writing the manuscript. V.N., C.K and M.D.L. contributed to some of the data and the conception of the first part of this research. All authors approved the version to be published.
Competing interests
We have no competing interests.
References
- 1.Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. ( 10.1016/S0166-2236(98)01349-6) [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Segura LM, Chowen JA, Naftolin F. 1996. Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front. Neuroendocrinol. 17, 180–211. ( 10.1006/frne.1996.0005) [DOI] [PubMed] [Google Scholar]
- 3.Pfrieger FW, Barres BA. 1997. Synaptic efficacy enhanced by glial cells in vitro. Science 277, 1684–1687. ( 10.1126/science.277.5332.1684) [DOI] [PubMed] [Google Scholar]
- 4.Canolle B, Masmejean F, Melon C, Nieoullon A, Pisano P, Lortet S. 2004. Glial soluble factors regulate the activity and expression of the neuronal glutamate transporter EAAC1: implication of cholesterol. J. Neurochem. 88, 1521–1532. ( 10.1046/j.1471-4159.2003.02301.x) [DOI] [PubMed] [Google Scholar]
- 5.Newman EA. 2015. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Phil. Trans. R. Soc. B 370, 20140195 ( 10.1098/rstb.2014.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laube G, Veh RW. 1997. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 19, 171–179. () [DOI] [PubMed] [Google Scholar]
- 7.Fleidervish IA, Libman L, Katz E, Gutnick MJ. 2008. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc. Natl Acad. Sci. USA 105, 18 994–18 999. ( 10.1073/pnas.0803464105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima FRS, Trentin AG, Rosenthal D, Chagas C, Moura Neto V. 1997. Thyroid hormone induces protein secretion and morphological changes in astroglial cells with an increase in expression of glial fibrillary acidic protein. J. Endocrinol. 154, 167–175. ( 10.1677/joe.0.1540167) [DOI] [PubMed] [Google Scholar]
- 9.Trentin AG, Alvarez-Silva M, Moura Neto V. 2001. Thyroid hormone induces cerebellar astrocytes and C6 glioma cells to secrete mitogenic growth factors. Am. J. Physiol. Endocrinol. Metab 281, E1088. [DOI] [PubMed] [Google Scholar]
- 10.Perraud F, Labourdette G, Miehe M, Loret C, Sensenbrenner M. 1988. Comparison of the morphological effects of acidic and basic fibroblast growth factors on rat astroblasts in culture. J. Neurosci. Res. 20, 1–11. ( 10.1002/jnr.490200102) [DOI] [PubMed] [Google Scholar]
- 11.Rodnight RB, Gottfried C. 2013. Morphological plasticity of rodent astroglia. J. Neurochem. 124, 263–275. ( 10.1111/jnc.12087) [DOI] [PubMed] [Google Scholar]
- 12.Niederkinkhaus V, Marx R, Hoffmann G, Dietzel ID. 2009. Thyroid hormone (T3)-induced up-regulation of voltage-activated sodium current in cultured postnatal hippocampal neurons requires secretion of soluble factors from glial cells. Mol. Endocrinol. 23, 1494–1504. ( 10.1210/me.2009-0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potthoff O, Dietzel ID. 1997. Thyroid hormone regulates Na+ currents in cultured hippocampal neurons from postnatal rats. Proc. R. Soc. Lond. B 264, 367–373. ( 10.1098/rspb.1997.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann G, Dietzel ID. 2004. Thyroid hormone regulates excitability in central neurons from postnatal rats. Neuroscience 125, 369–379. ( 10.1016/j.neuroscience.2004.01.047) [DOI] [PubMed] [Google Scholar]
- 15.Lindholm D, et al. 1993. Neurotrophin-3 induced by tri-iodothyronine in cerebellar granule cells promotes Purkinje cell differentiation. J. Cell Biol. 122, 443–450. ( 10.1083/jcb.122.2.443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudge JS, Alderson RF, Pasnikowski E, McClain J, Ip NY, Lindsay RM. 1992. Expression of ciliary neurotrophic factor and the nerurotrophins – nerve growth factor, brain-derived neurotrophic factor and neurotrophin 3– in cultured rat hippocampal astrocytes. Eur. J. Neurosci. 4, 459–471. ( 10.1111/j.1460-9568.1992.tb00896.x) [DOI] [PubMed] [Google Scholar]
- 17.Condorelli DF, Salin T, Dell’ Albani P, Mudo G, Corsaro M, Timmusk T, Metsis M, Belluardo N. 1995. Neurotrophins and their trk receptors in cultured cells of the glial lineage and in white matter of the central nervous system. J. Mol. Neurosci. 6, 237–248. ( 10.1007/BF02736783) [DOI] [PubMed] [Google Scholar]
- 18.Al-Hadlaq SM, Bradley RM, MacCallum DK, Mistretta CM. 2003. Embryonic geniculate ganglion neurons in culture have neurotrophin-specific electrophysiological properties. Neuroscience 118, 145–159. ( 10.1016/S0306-4522(02)00814-X) [DOI] [PubMed] [Google Scholar]
- 19.Kreutzberg GW. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318. ( 10.1016/0166-2236(96)10049-7) [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Si QS, Kataoka K. 1999. Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci. Res. 35, 95–100. ( 10.1016/S0168-0102(99)00071-1) [DOI] [PubMed] [Google Scholar]
- 21.Hanisch U-K. 2002. Microglia as a source and target of cytokines. Glia 40, 140–155. ( 10.1002/glia.10161) [DOI] [PubMed] [Google Scholar]
- 22.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. 2009. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980. ( 10.1523/JNEUROSCI.4363-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kettenmann H, Kirchhoff F, Verkhratsky A. 2013. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18. ( 10.1016/j.neuron.2012.12.023) [DOI] [PubMed] [Google Scholar]
- 24.Robbins DS, Shirazi Y, Drysdale BE, Lieberman A, Shin HS, Shin ML. 1987. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J. Immunol. 139, 2593–2597. [PubMed] [Google Scholar]
- 25.Andrews T, Zhang P, Bhat NR. 1998. TNFα potentiates IFNγ-induced cell death in oligodendrocyte progenitors. J. Neurosci. Res 54, 574–583. () [DOI] [PubMed] [Google Scholar]
- 26.Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC. 2004. Interferon-γ expression in periventricular leukomalacia in the human brain. Brain Pathol. 14, 265–274. ( 10.1111/j.1750-3639.2004.tb00063.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann SA, Versmold B, Marx R, Stahlhofen S, Dietzel ID, Heumann R, Berger R. 2008. Corticosteroids reverse cytokine-induced block of survival and differentiation of oligodendrocyte progenitor cells from rats. J. Neuroinflammation 5, 39 ( 10.1186/1742-2094-5-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanisch U-K. 2013. Functional diversity of microglia—how heterogeneous are they to begin with? Front. Cell. Neurosci. 7, 65 ( 10.3389/fncel.2013.00065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun D, Newman TA, Perry VH, Weller RO. 2004. Cytokine-induced enhancement of autoimmune inflammation in the brain and spinal cord: implications for multiple sclerosis. Neuropathol. Appl. Neurobiol. 30, 374–384. ( 10.1111/j.1365-2990.2003.00546.x) [DOI] [PubMed] [Google Scholar]
- 30.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. 1997. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor α. Br. J. Pharmacol. 121, 417–424. ( 10.1038/sj.bjp.0701148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommer C, Schmidt C, George A. 1998. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp. Neurol. 151, 138–142. ( 10.1006/exnr.1998.6797) [DOI] [PubMed] [Google Scholar]
- 32.Parada CA, Yeh JJ, Joseph EK, Levine JD. 2003. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur. J. Neurosci. 17, 1847–1852. ( 10.1046/j.1460-9568.2003.02626.x) [DOI] [PubMed] [Google Scholar]
- 33.Sinha S, Patil SA, Jayalekshmy V, Satishchandra P. 2008. Do cytokines have any role in epilepsy?. Epilepsy Res. 82, 171–176. ( 10.1016/j.eplepsyres.2008.07.018) [DOI] [PubMed] [Google Scholar]
- 34.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. 2013. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36, 174–184. ( 10.1016/j.tins.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 35.Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. 2003. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 23, 8881–8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai J, Porreca F, Hunter JC, Gold MS. 2004. Voltage-gated sodium channels and hyperalgesia. Annu. Rev. Pharmacol. Toxicol. 44, 371–397. ( 10.1146/annurev.pharmtox.44.101802.121627) [DOI] [PubMed] [Google Scholar]
- 37.Brewer GJ, Cotman CW. 1989. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 494, 65–74. ( 10.1016/0006-8993(89)90144-3) [DOI] [PubMed] [Google Scholar]
- 38.McCarthy KD, de Vellis J. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902. ( 10.1083/jcb.85.3.890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinsimlinghaus K, Marx R, Serdar M, Bendix I, Dietzel ID. 2013. Strategies for repair of white matter: influence of osmolarity and microglia on proliferation and apoptosis of oligodendrocyte precursor cells in different basal culture media. Front. Cell. Neurosci. 7, 277 ( 10.3389/fncel.2013.00277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knüsel B, Hefti F. 1992. K-252 compounds: modulators of neurotrophin signal transduction. J. Neurochem. 59, 1987–1996. ( 10.1111/j.1471-4159.1992.tb10085.x) [DOI] [PubMed] [Google Scholar]
- 41.Kase H, Iwahashi K, Matsuda Y. 1986. K-252a, a potent inhibitor of protein kinase C from microbial origin. J. Antibiot. 39, 1059–1065. ( 10.7164/antibiotics.39.1059) [DOI] [PubMed] [Google Scholar]
- 42.Elliott LH, Wilkinson SE, Sedgwick AD, Hill CH, Lawton G, Davis PD, Nixon JS. 1990. K252a is a potent and selective inhibitor of phosphorylase kinase. Biochem. Biophys. Res. Commun. 171, 148–154. ( 10.1016/0006-291X(90)91369-4) [DOI] [PubMed] [Google Scholar]
- 43.Niederkinkhaus V. 2007. Thyroid hormone regulates voltage-gated sodium currents in postnatal rat hippocampal neurons via a secretion of soluble factors from glial cells. Dissertation, Ruhr-Universität, Bochum, Germany See http://deposit.ddb.de/cgi-bin/dokserv?idn=987289209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koizumi S, Contreras ML, Matsuda Y, Hama T, Lazarovici P, Guroff G. 1988. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J. Neurosci. 8, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnus T, et al. 2012. The neurological syndrome in adults during the 2011 northern German E. coli serotype O104 : H4 outbreak. Brain 135, 1850–1859. ( 10.1093/brain/aws090) [DOI] [PubMed] [Google Scholar]
- 46.Singhi P. 2011. Infectious causes of seizures and epilepsy in the developing world. Dev. Med. Child Neurol. 53, 600–609. ( 10.1111/j.1469-8749.2011.03928.x) [DOI] [PubMed] [Google Scholar]
- 47.Cantrell AR, Catterall WA. 2001. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat. Rev. Neurosci. 2, 397–407. ( 10.1038/35077553) [DOI] [PubMed] [Google Scholar]
- 48.Longart M, García L, Castillo C, Martínez JC, Medina R, Forsyth P, Malavé C. 2009. Sciatic nerve conditioned medium depleted of pro-NGF modulates sodium currents and neurite outgrowth in PC12 cells. Neuroscience 159, 550–558. ( 10.1016/j.neuroscience.2008.12.063) [DOI] [PubMed] [Google Scholar]
- 49.Jin L, Hu X, Feng L. 2005. NT3 inhibits FGF2-induced neural progenitor cell proliferation via the PI3K/GSK3 pathway. J. Neurochem. 93, 1251–1261. ( 10.1111/j.1471-4159.2005.03118.x) [DOI] [PubMed] [Google Scholar]
- 50.Yang Q, Feng B, Zhang K, Guo Y-Y, Liu S-B, Wu Y-M, Li X-Q, Zhao M-G. 2012. Excessive astrocyte-derived neurotrophin-3 contributes to the abnormal neuronal dendritic development in a mouse model of fragile X syndrome. PLoS Genet. 8, e1003172 ( 10.1371/journal.pgen.1003172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. 2000. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron 26, 533–541. ( 10.1016/S0896-6273(00)81185-X) [DOI] [PubMed] [Google Scholar]
- 52.Hossain WA, D'Sa C, Morest DK. 2008. Interactive roles of fibroblast growth factor 2 and neurotrophin 3 in the sequence of migration, process outgrowth, and axonal differentiation of mouse cochlear ganglion cells. J. Neurosci. Res. 86, 2376–2391. ( 10.1002/jnr.21685) [DOI] [PubMed] [Google Scholar]
- 53.van der Deure WM, et al. 2008. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin. Endocrinol. 69, 804–811. ( 10.1111/j.1365-2265.2008.03267.x) [DOI] [PubMed] [Google Scholar]
- 54.Galic MA, Riazi K, Pittman QJ. 2012. Cytokines and brain excitability. Front. Neuroendocrinol. 33, 116–125. ( 10.1016/j.yfrne.2011.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Gu J, Li Y-Q, Tao Y-X. 2011. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol. Pain 7, 16 ( 10.1186/1744-8069-7-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Pang R-P, Shen K-F, Zimmermann M, Xin W-J, Li Y-Y, Liu X-G. 2011. TNF-α enhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury. Exp. Neurol. 227, 279–286. ( 10.1016/j.expneurol.2010.11.017) [DOI] [PubMed] [Google Scholar]
- 57.Schilling T, Nitsch R, Heinemann U, Haas D, Eder C. 2001. Astrocyte-released cytokines induce ramification and outward K+ channel expression in microglia via distinct signalling pathways. Eur. J. Neurosci. 14, 463–473. ( 10.1046/j.0953-816x.2001.01661.x) [DOI] [PubMed] [Google Scholar]
- 58.Edelmann E, Leßmann V, Brigadski T. 2014. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76, 610–627. ( 10.1016/j.neuropharm.2013.05.043) [DOI] [PubMed] [Google Scholar]
- 59.Bernal J. 2007. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 3, 249–259. ( 10.1038/ncpendmet0424) [DOI] [PubMed] [Google Scholar]
- 60.Chen G, Park C-K, Xie R-G, Berta T, Nedergaard M, Ji R-R. 2014. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 137, 2193–2209. ( 10.1093/brain/awu140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahl G. 2015. ATP release through pannexon channels. Phil. Trans. R. Soc. B 370, 20140191 ( 10.1098/rstb.2014.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steringer JP, Müller H-M, Nickel W. 2014. Unconventional secretion of fibroblast growth factor 2—a novel type of protein translocation across membranes? J. Mol. Biol. 427, 1202–1210. ( 10.1016/j.jmb.2014.07.012) [DOI] [PubMed] [Google Scholar]
- 63.Agnati LF, Fuxe K. 2014. Extracellular-vesicle type of volume transmission and tunnelling-nanotube type of wiring transmission add a new dimension to brain neuro-glial networks. Phil. Trans. R. Soc. B 369, 20130505 ( 10.1098/rstb.2013.0505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fröhlich D, et al. 2014. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Phil. Trans. R. Soc. B 369, 20130510 ( 10.1098/rstb.2013.0510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madeja M. 2000. Do neurons have a reserve of sodium channels for the generation of action potentials? A study on acutely isolated CA1 neurons from the guinea-pig hippocampus. Eur. J. Neurosci. 12, 1–7. ( 10.1046/j.1460-9568.2000.00871.x) [DOI] [PubMed] [Google Scholar]
- 66.Hodgkin A. 1975. The optimum density of sodium channels in an unmyelinated nerve. Phil. Trans. R. Soc. Lond. B 270, 297–300. ( 10.1098/rstb.1975.0010) [DOI] [PubMed] [Google Scholar]
- 67.Dietzel ID, Mohanasundaram S, Niederkinkhaus V, Hoffmann GWJ, Reiners C, Blasl C, Bohr K. 2012. Thyroid hormone effects on sensory perception, mental speed, neuronal excitability and ion channel regulation. In Thyroid hormone (ed. Agrawal NK.), pp. 85–122, ch. 4 Rijeka, Croatia: InTech; ( 10.5772/48310) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this paper are included in the electronic supplementary material.