Abstract
Pulmonary alveolar proteinosis (PAP) is a rare pulmonary disease. Diagnosis is established by bronchoalveolar lavage (BAL), which has macroscopic ‘milky appearance’, and in the presence of typical computed tomography, findings are diagnostic of PAP but, however, the feature of periodic acid–Schiff-positive eosinophilic proteinaceous fluid raises the confidence of the diagnosis. We report late-onset PAP in a 10-year-old girl who had acid fast bacilli on an initial BAL examination, but was subsequently diagnosed as PAP.
INTRODUCTION
Pulmonary alveolar proteinosis (PAP) is a rare pulmonary disease. Although PAP can occur from newborn to 72 years, the mean age of patient is 51 years [1]. PAP is characterized by the intra-alveolar accumulation of surfactant lipids and proteins positive on periodic acid–Schiff (PAS) staining, impairing gas exchange and resulting in progressive respiratory insufficiency [2]. Two forms are encountered in the pediatric age group: congenital alveolar proteinosis which is fulminant and frequently fatal and a late-onset form which is generally less severe and similar to the adult form [3]. We report a case of late-onset PAP in a 10-year-old girl who had acid fast bacilli (AFB) on bronchoalveolar lavage (BAL) smear examination initially and masqueraded as pulmonary tuberculosis (TB).
CASE REPORT
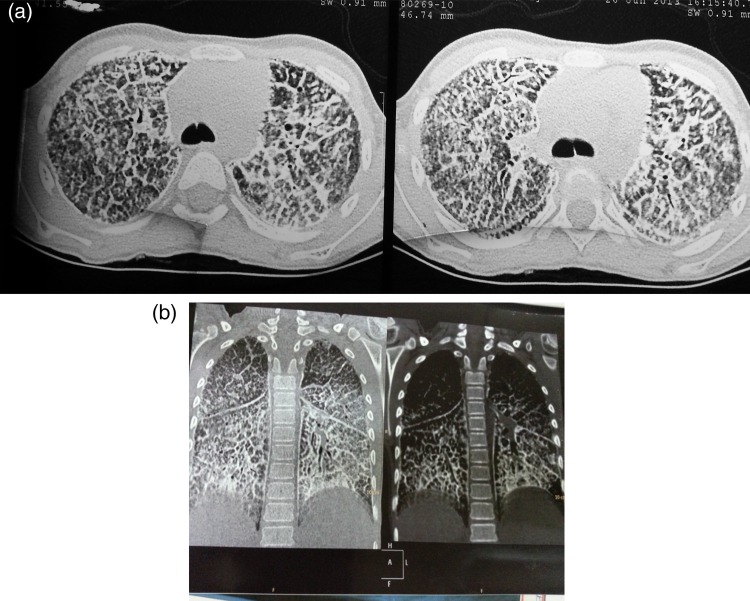
A 10-year-old girl presented with fever, cough and breathlessness with weight loss for a month. She had received anti-tuberculosis treatment (ATT) at the age of 11 months for 6 months, but the regimen was not known as no documentation was available. On presentation to us, weight was 16 kg and height was 127 cm. Heart rate was 96/min and respiratory rate was 34/min. She had grade 3 clubbing, but no clinical cyanosis. Her transcutaneous oxygen saturation while breathing room air was 98%. On auscultation, fine inspiratory crepitations were heard all over the chest and air entry was reduced bilaterally. Other systems were normal. Chest X-ray revealed a miliary pattern with infiltrates more on the basal area. Complete blood count, liver and renal functions, and arterial blood gas were normal. Erythrocyte sedimentation rate (ESR) was 9 mm at the end of first hour. Mantoux test and HIV Elisa were negative. BAL showed AFB on smear examination. She was started on ATT along with steroids. Her TB culture at the end of 6 weeks did not grow mycobacterium tuberculosis (MTB). At the end of 3 months, there was no improvement in the chest X-ray and child was suspected to have drug-resistant TB. Sputum was sent for TB culture, which did not grow any MTB. Hence, to search for another lung pathology, CT chest was done. High-resolution computed tomography (HRCT) scan of the chest revealed extensive interstitial septal thickening, suggestive of crazy-paving pattern noted in lower lobes which was suggestive of PAP (Fig. 1a and b). Serum lactate dehydrogenase (LDH) was raised (208 IU/l). BAL was repeated and BAL fluid macrophages showed PAS-positive material in the cytoplasm which was resistant to diastase treatment. BAL fluid was hazy macroscopically. In the same session, total lung lavage was performed with remarkable symptomatic benefit in the child. ATT was stopped after 6 months. Child's sibling chest X-rays were all normal. The child was discharged on hydroxychloroquine (5 mg/kg/day) and prednisolone (1 mg/kg/day), and child is under regular follow-up.
Figure 1:
(a and b) HRCT scan of the chest showing extensive interstitial septal thickening, suggestive of crazy-paving pattern in lower lobes.
DISCUSSION
PAP was first described by Rosen et al. in 1958 [4]. It is an unusual diffuse lung disease characterized by the accumulation of large amounts of a phospholipoproteinaceous material in the alveoli. In PAP, interruption of granulocyte–macrophage colony-stimulating factor (GM-CSF) signaling in the alveolar macrophage occurs, impairing the catabolism of surfactant by alveolar macrophages. This results in the intracellular buildup of membrane-bound, concentrically laminated surfactant aggregates and these along with cellular debris, fill up the alveoli, thus reducing the size of the available gas-exchange surface and eventually leading to the clinical syndrome [5]. Exertional progressive dyspnea is the most common presenting symptom followed by cough. Other symptoms are chest pain, hemoptysis, fever and malaise. Some patients do not present until they develop a supervening infection [2]. The findings on physical examination can be unremarkable, but there are inspiratory crackles in 50% of patients, cyanosis in 25% and digital clubbing in a small percentage [5]. We attribute the fever in our patient to secondary infection with MTB. The progression of the disease is very variable, ranging from asymptomatic forms diagnosed by chance or early-onset forms that progress rapidly to uncontrollable respiratory failure. BAL is the key to diagnosis [6]. BAL has a characteristic macroscopic ‘milky appearance’ and contains large, acellular, eosinophilic bodies in a diffuse background of granular material that stains with PAS, as well as elevated levels of surfactant proteins A, B and D [5], and is diastase-resistant [7]. The presence of anti-GM-CSF antibodies in blood or BAL fluid can serve as a diagnostic marker for adult sporadic type of PAP, but are not found in children and neonate with PAP [8]. Surfactant proteins and anti GM-CSF antibody titers were not done in our patient as these tests are not available in India; however, the child did have PAS-positive and diastase-resistant BAL fluid. Raised serum levels of LDH, tumor markers and mucin-like glycoprotein (KL-6) are often seen [2]. The classic radiological appearance of PAP is bilateral, symmetrical and perihilar airspace consolidation in a bat-wing distribution [3]. The radiological findings are often much more severe than the clinical abnormalities. HRCT shows patchy, ground-glass opacifications with superimposed interlobular septal and intralobular thickening, a pattern commonly referred to as ‘crazy paving’ [9] as was seen in our patient. The most effective treatment for PAP is the mechanical removal of the proteinaceous material via whole lung lavage, and initially proposed by Ramirez in 1965, is still being the only therapy that is really effective [10]. The major indications for whole lung lavage are symptomatic disease with dyspnea that limits activity and progressive deterioration of arterial oxygenation. Some considers PaO2, < 65 mmHG, P(A-a)O2 > 40 mmHG or a shunt fraction of >10–12% as thresholds for therapeutic lung lavage [11]. In our case, one lung lavage was done with a remarkable clinical improvement in terms of dyspnea. Other therapeutic trials have been proposed such as lung transplantation, GM-CSF administration and gene therapy [12]. The response to treatment with prednisolone and chloroquine has been inconsistent [12]. Through this case report, we emphasize the awareness of PAP among clinicians to avoid missed and delayed diagnosis.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Borie R, Danel C, Debray MP, Taille C, Dombret MC, Aubier M, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011;20:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax 2000;55:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbert JM, Costello P, Li W, Hoffman RM, Rogers RM. CT features of pulmonary alveolar proteinosis. Am J Roentgenol 2001;176:1287–94. [DOI] [PubMed] [Google Scholar]
- 4.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958;258:1123–42. [DOI] [PubMed] [Google Scholar]
- 5.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–39. [DOI] [PubMed] [Google Scholar]
- 6.Wang BM, Stern EJ, Schmidt RA, Pierson DJ. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest 1997;111:460–6. [DOI] [PubMed] [Google Scholar]
- 7.Lee KN, Levin DL, Webb WR, Chen D, Storto ML, Golden JA. Pulmonary alveolar proteinosis: high-resolution CT, chest radiographic and functional correlations. Chest 1997;111:989–95. [DOI] [PubMed] [Google Scholar]
- 8.Latzin P, Tredano M, Wust Y, de Blic J, Nicolai T, Bewig B, et al. Anti-GM-CSF antibodies in pediatric pulmonary alveolar proteinosis. Thorax 2005;60:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johkoh T, Itoh H, Muller NL, Ichikado K, Nakamura H, Ikezoe J, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 1999;211:155–60. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez J, Kieffer RF, Ball WC. Bronchopulmonary lavage in man. Ann Intern Med 1965;63:819–28. [DOI] [PubMed] [Google Scholar]
- 11.Khan A, Agarwal R. Pulmonary alveolar proteinosis. Respir Care 2011;56:1016–28. [DOI] [PubMed] [Google Scholar]
- 12.de Blic J. Pulmonary alveolar proteinosis in children. Paediatr Respir Rev 2004;5:316–22. [DOI] [PubMed] [Google Scholar]