Abstract
Agro-ecosystems constitute essential habitat for many organisms. Agricultural intensification, however, has caused a strong decline of farmland biodiversity. Organic farming (OF) is often presented as a more biodiversity-friendly practice, but the generality of the beneficial effects of OF is debated as the effects appear often species- and context-dependent, and current research has highlighted the need to quantify the relative effects of local- and landscape-scale management on farmland biodiversity. Yet very few studies have investigated the landscape-level effects of OF; that is to say, how the biodiversity of a field is affected by the presence or density of organically farmed fields in the surrounding landscape. We addressed this issue using the metacommunity framework, with weed species richness in winter wheat within an intensively farmed landscape in France as model system. Controlling for the effects of local and landscape structure, we showed that OF leads to higher local weed diversity and that the presence of OF in the landscape is associated with higher local weed biodiversity also for conventionally farmed fields, and may reach a similar biodiversity level to organic fields in field margins. Based on these results, we derive indications for improving the sustainable management of farming systems.
Keywords: organic farming, agricultural intensification, landscape heterogeneity, spatial scale, weeds, agroecology
1. Introduction
Agricultural landscapes occupy about 40% of all terrestrial ecosystems [1], providing habitat for many animal and plant species worldwide [2]. The intensification of agricultural practices has however resulted in a general decline of farmland species adapted to more extensive farming [3–5], in response to a mixture of local (field or farm levels) and regional (landscape) processes, such as increased use of pesticides [6] and fertilizers [7], shortened crop succession [3], landscape simplification [1] and territory specialization [8]. To mitigate this biodiversity decline, agri-environmental schemes (AESs) [9] and other policy initiatives were set up, often targeting reduced agrochemical applications [10]. Organic farming (OF), an AES under European regulation, is presented as a potential compromise between assuring food security and conserving biodiversity, thanks to the banishment of chemical and inorganic fertilizers, and higher crop diversity [11].
Many studies have assessed the potential biodiversity benefits of OF in comparison with conventional farming (CONV), but a general consensus is still lacking [11,12]. At the field level, an overall positive effect of OF was detected on plant species richness [13,14], though the response is highly taxon dependent [12]. However, due to lower yields, larger surfaces are needed to maintain food production under OF, hence the net balance between positive and negative impacts is still debated [12,15,16]. OF effects at the field level may further depend on surrounding landscapes [4,17,18]. Bengtsson et al. [19] proposed that OF benefits on biodiversity should increase linearly with agriculture intensification at the landscape scale. However, contrasted effects of landscape complexity have been reported [4,20–22]. Alternatively, Concepción et al. [23] proposed that landscape complexity may nonlinearly modify the biodiversity effects of field management, whereby below a minimal landscape complexity threshold, as well as above a saturation point, biodiversity will not increase with landscape complexity. Thus scale-dependent processes and the interplay between local and regional factors determining biodiversity loss under agricultural intensification must be further investigated [21,24].
In this context, studying ecological processes at the scale of the metacommunity [25] can be relevant to assess potential regional (i.e. landscape) effects on local community richness. Indeed, in highly heterogeneous and dynamic landscapes such as agro-ecosystems, dispersal is expected to be an essential driver that allows communities to persist in spite of landscape instability. Here, we hypothesize that local and regional processes interact in shaping biodiversity, such that landscape-scale processes may outcompete local processes. In other words, the presence of OF at the landscape scale could balance the field-level negative effects of conventional agricultural management through mass effect (species dispersing from favourable habitats in organic fields into surrounding conventional fields). To test this hypothesis, we used weed communities of winter wheat, the major crop in Europe and in France (approx. 10% of the total country area is cropped with wheat). Weeds represent the basic trophic component in agricultural food webs [26], but may induce crop yield loss [27]. Many weed species occur in both crop and non-crop areas [28,29], especially field edges where management practices are less intensive [30]. At the field scale, weeds strongly respond to OF: species richness may be about 70% higher and abundance doubled compared with CONV [12,31]. Furthermore, weed communities respond also to landscape-scale processes [32–35]. While almost all previous studies compared pairs of organic and conventional farming along a gradient of landscape complexity (e.g. [21]) or regions (e.g. [33]), here we used an unusually large dataset collected within a single landscape of 430 km² in which proportion of OF varies from 0 to more than 50% in 1 km² buffers. Using a spatially stratified design on 465 fields, we quantify the relative contribution of landscape- (proportion of OF in a 1 km buffer around a focal field) versus field-scale processes (organic or conventional management; field core versus field margin) on weed diversity at several spatial scales: within field, field, between fields and landscape. As OF systems are characterized by more diversified crop successions [36], which favours weed richness [37] as well as a clumped distribution of farms [38], we controlled for crop successions, field size, soil type, land use and semi-natural elements in our models to account for these confounding spatio-temporal effects.
2. Material and methods
(a). Study area
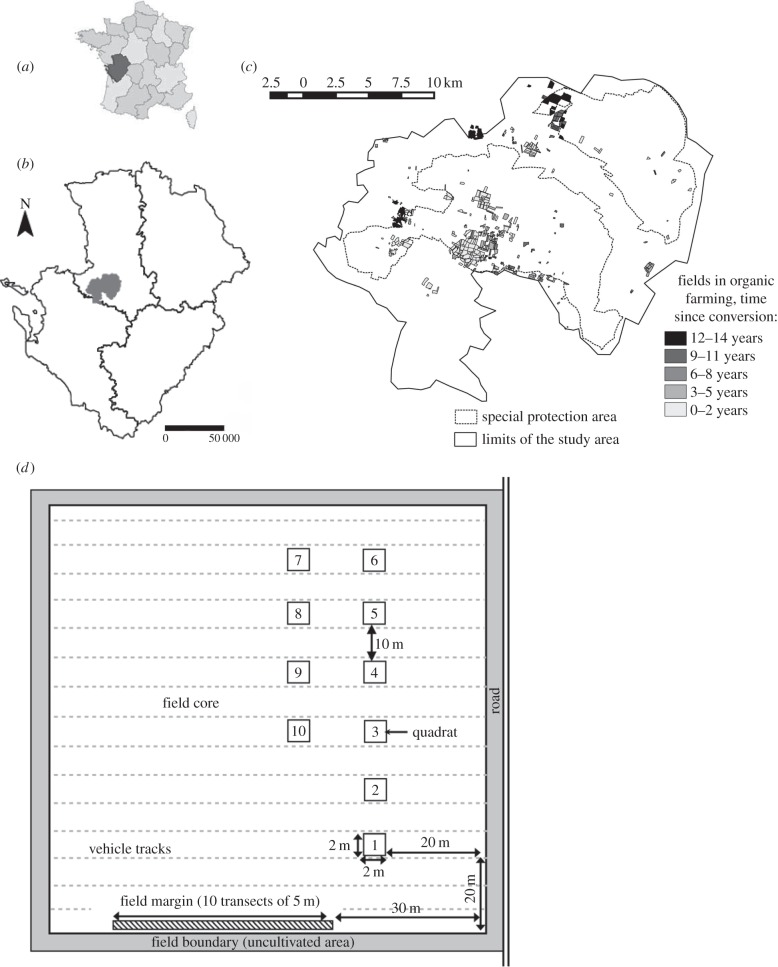
The study site (approx. 430 km2) is the LTER ‘Zone Atelier Plaine & Val de Sèvre’, located in central western France, Poitou-Charentes Region, France (46.23° N, 0.41° W; figure 1a,b). It is an agricultural landscape dominated by intensive cereal production, with an average field size of 3.7 ha. Since 1994, the land use for each of the about 14 000 fields has been recorded twice per year. Using eight crop categories, land use in 2010 consisted of 38.4% cereals (mainly winter wheat, 33.5%), 10.8% meadows and alfalfa, 12.1% sunflower, 8.6% corn, 8.7% oilseed rape, 2.8% pea, 2.3% ryegrass, 3.8% other crops; and 9.5% urban and 3.0% woodland (see electronic supplementary material, S3, for details). For this study, we selected fields situated in landscapes with at least 55% crop cover (grasslands included). In 2011, 18 out of about 450 farms used OF methods (410 arable fields, excluding grassland), corresponding to a surface of 15.7 km2 (3.7% of the study area; figure 1c), with farms having converted since 1–14 years ago (mean = 5.7 years).
Figure 1.
Location of the Plaine & Val de Sèvre study area and weed sampling design. (a) Geographical location of the Poitou-Charente region in France. (b) Location of the Plaine & Val de Sèvre study area in the Poitou-Charente region, department of Deux-Sèvres. (c) Distribution of OF in the study area; overall it covers 3.7% of the study area. (d) Sampling design of weeds (location of the quadrats and transects in a field). In each field, 10 quadrats of 4 m2 were sampled in the field core, and 10 transects of 5 m in the field margin.
(b). Weed sampling
Between 2009 and 2012, weed species were sampled in 465 wheat fields (see electronic supplementary materials S1 and S11 for details and species list, respectively), both in the field core and in the field margin. The latter is defined as the tilled zone between the field boundary and the first crop row (figure 1d). Over the years, field surveys varied slightly (either 32 quadrats of 4 m² in a star arrangement in the field core or 10 quadrats of 4 m² in a linear arrangement orthogonal to the tractor tracks and spaced by 10 m; figure 1d). In both protocols, the first quadrat was located at least 20 m from a field corner to avoid border effects. In field margin, transect started 30 m from the field margin. To homogenize sampling effort between the two protocols, species richness per field in field core was estimated over 10 quadrats using a bootstrap procedure in fields where the star arrangement protocol was applied (see electronic supplementary material S13 for details).
(c). Landscape analyses
Spatial data were treated using QGIS v. 1.7.3 (QGIS Development Team 2002–2010). The landscape was characterized by the proportion of each landscape component (forest, grassland and built area) and crop type (grouped in eight categories; see §2b), and the linear length of hedgerows, road/paths and rivers in buffer areas around each sampled field. The most relevant scale (buffer areas of six radii: 500, 1000, 1250, 1500, 2000 and 2500 m) at which landscape variables better explained weed diversity was selected using a model selection procedure based on the Akaike information criterion (AIC) [39]. The model most supported by the data (lowest AIC value; M3 in the following section) was the one at 1000 m. In this 1 km radius, the landscape around the focal fields was composed of 0–33% of grassland, 0–38% of forest, 0–42% of built area and 0–55% of OF with annual crops (see electronic supplementary material S3). A principal component analysis (PCA) was then conducted using the set of selected landscape variables (at the 1 km radius; see electronic supplementary material S9) to obtain a synthetic indicator of landscape complexity. The first PCA component (PC1, 25% of variance explained) summarized a gradient from simple landscapes (annual crops only and without any semi-natural elements) to more complex landscapes (mosaic of annual crops and semi-naturals components, with a large proportion of grasslands, hedgerows and built areas). The second axis (PC2, 15.3% of variance explained) opposed woodland and roads/paths.
(d). Multi-model selection in multiple regression analysis
We first investigated the effects of local (field) versus regional (landscape) parameters on weed diversity per field (considering the 10 quadrats, equivalent to the γ-plot used in the additive partitioning analysis; see below), using generalized additive mixed models (GAMMs; R package ‘gamm4’ [40,41]) to allow for nonlinear relationships. In all cases, these could be approximated to quadratic functions in GLMM (lme4 v. 1.1–6 [42] in R v. 3.1.0). We then used an information-theoretic multi-model selection framework to evaluate the support from the data for five competing models of increasing complexity. A first model (M0, the ‘baseline model’) investigated independent variables that were considered a priori as confounding factors acting on species richness, namely year (four-level factor) and date of sampling (in Julian day as quadratic polynomial), soil type (three-level factor), and field area (log-transformed). As the effect of date varied spatially (in field margin, species richness increased linearly throughout the season, but the effect was quadratic in field core), we included an interaction term between date and position in the field (field core or margin). To account for the survey design (repeated measures within each field and several fields per farmer), we used a nested random intercept structure [43,44], the ‘field ID’ (442 levels) nested within the ‘farmer ID’ (131 levels). This basic model structure was included in all the four other competing models. A second model (M1, ‘management type and position in the field model’) hypothesized that weed species richness varied consistently with the management type in the field (OF versus CONV, fitted as a two-level fixed-effect factor), the position of sampling within the field (margin versus core) and their interaction. The third model (M2, the ‘crop successions model’) aimed at disentangling direct (i.e. ban of herbicides) versus indirect effects (i.e. crop-succession diversity) of OF on weed species richness. In preliminary analysis, we tested 5- and 10-year successions, using the percentage of the eight crop categories in the succession (see electronic supplementary material S2 for details), and kept the 10-year successions in the analysis as we obtained the lowest AIC value for this duration. Then we tested the effect of the number of crops in the succession, and the effect of the preceding crop. As the presence of grassland and corn in the 10-year succession and preceding crop were the only variables supported by the data (lowest AIC values), we kept these as proxies of OF effect. The fourth model (M3, ‘landscape model’) aimed at investigating the effect of landscape complexity, modelled as PC1 and PC2. Finally, in the fifth model (M4, the ‘OF in the landscape model’), we added the proportion of OF in the landscape (percentage of the total area in the 1 km buffer around the sampled plot) including annual crops and grasslands (M4.a and M4.b) or annual crops only (M4.c and M4.d). We also tested the interaction between the percentage OF in the landscape and the position in the field (field core or margin).
The model selection procedure started with all two-way interactions and main effects, and was based on minimizing the AIC using the MuMIn library in R (v. 1.6.5) [45] and the dredge function to test all covariate combinations. All retained covariates of the lower-level models had to be included in the more complex competing models, thus the model selection procedure started with the baseline model (M0). For each model, we checked for spatial autocorrelation in the model residuals (using variograms in the geoR library v. 1.6–29 [46]); as none was found, we did not include a random effect for each point count [47]. To aid model convergence and to facilitate the interpretation, we mean-centred all numerical covariates and standardized variables by dividing by 2 s.d. [48].
(e). Additive partitioning analysis
We also analysed the effect of OF and the position in the field (field core or margin) on the α, β and γ components of diversity [49]. To avoid sample bias, we selected the same number of fields between organic and conventional fields (i.e. 77 fields in both cases) by randomly selecting the same number of conventional fields. The α-plot diversity corresponds to the mean number of species in the sampled unit (i.e. quadrat). The β-plot diversity corresponds to the difference between quadrats within a field and is calculated by the γ-plot minus the average of the α-plots, where γ-plot is the total species richness per field (sum of the 10 quadrats). The γ-field diversity is the total number of species found by class (e.g. in all the centres of organic fields). β-field diversity corresponds to the difference between fields (β-field = γ-field minus α-field), where α-field corresponds to species richness per field (so α-field = γ-plot). All analyses were undertaken first using all weed species, then repeated separately for the more common species and the less frequent species of the study area.
3. Results
Field size varied greatly across the 465 sampled fields (range 0.37–50.7 ha) and was to some extent related to management type (Welch two-sample test, mean OF = 6.7 ha, mean CONV = 5.4 ha, t = 1.34, p = 0.18). As expected, crop successions were more diverse in OF fields than in CONV ones, with a higher number of crops in 10-year successions (OF = 6.53, CONV = 5.06, t = 7.64, p < 0.001). There was a higher percentage of spring cereal, corn and other crops, and a reduced frequency of winter cereal, rape and sunflower in OF than in CONV, while the percentage of grasslands and alfalfa were similar (electronic supplementary material S2). Similarly, landscape composition at 1 km around the fields differed between OF and CONV fields, with more alfalfa, corn or pea around OF fields, and less hedgerows, winter wheat, rape or sunflower (electronic supplementary material S3). Furthermore, as OF fields are spatially aggregated, there were more OF around the OF sampled field than the CONV ones (electronic supplementary material S3).
In total, 175 weed species were detected (see electronic supplementary material S11), including 28 common species (present in more than 25% of the fields) and 104 less frequent species (present in less than 5% of the fields; no red-listed species were recorded). As expected, weed richness was significantly higher in OF fields than in CONV ones (by roughly 50%) and in field margin than in field core. Differences in weed richness between field core and margin were higher in CONV systems (electronic supplementary material S4).
(a). Relative effects of local farming practices versus landscape complexity on weed α-diversity
Overall, we found an increase in the goodness of fit of the competing models (electronic supplementary material S12), suggesting contributing effects of local (management type and position in the field, M1, and crop-succession diversity, M2) and landscape (M3) on weed species richness. Adding the percentage of grassland and corn in the 10-year succession and the preceding crop type increased the goodness of fit of the model (electronic supplementary material S12), having a positive effect on weed richness, but it did not really affect the variation explained by OF (4.28% and 4.09% of the variation is explained by OF without and with crop succession, respectively), suggesting that the main effect of OF was not due to the differences in crop sequences.
Landscape complexity (modelled as PC1 and PC2) had no significant effects on weed richness. However, the percentage of alfalfa and the length of road/paths in a 1 km buffer around the fields had a positive effect, as did the landscape percentage of OF (electronic supplementary material S5). Moreover, the variance explained by the farming system (OF versus CONV) at the local scale was nearly halved when the percentage of OF fields in the landscape was included in the models (2.28% in M4.b model versus 4.51% in the landscape model; electronic supplementary material S12). Overall, the fixed effects in these two final models explained around 35% of the variation compared with the null model (electronic supplementary material S12).
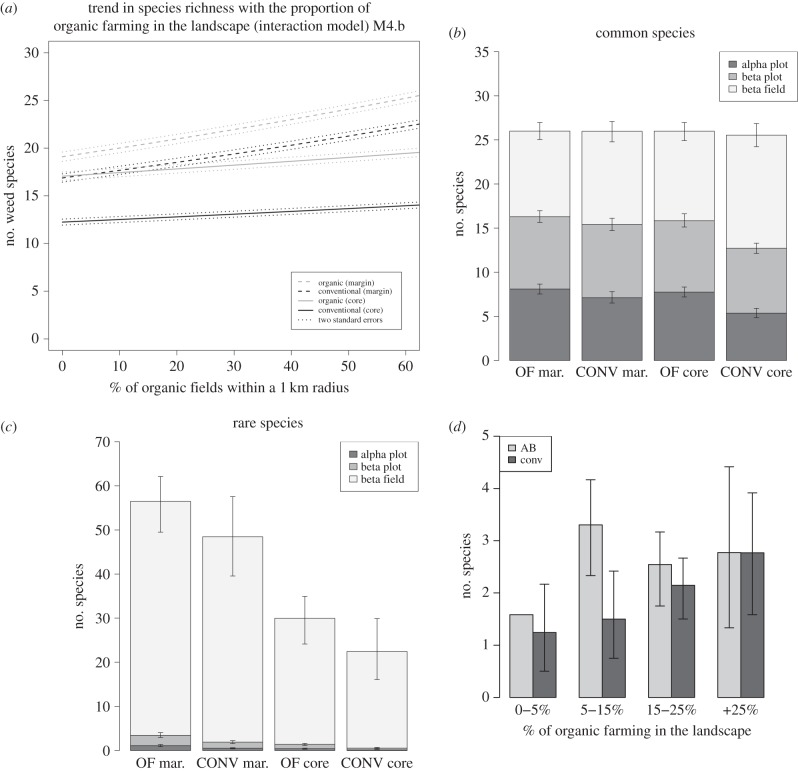
Importantly, all final models predicted an increase of species richness with the percentage of OF in the landscape (both for OF and CONV fields and both in field margin and field core), but the interaction models (M4.b and M4.c) further indicated that species richness was especially increased in the field margin: a field margin in a CONV field surrounded by OF fields had a higher weed richness than a field margin in an OF field surrounded by CONV fields (see electronic supplementary materials S5 and S8). Indeed, model M4.b predicted an increase from 12.4 to 13.6 species for the core of a conventional field whether surrounded by 0 or 50% OF, whereas the increase was from 17.4 to 21.2 in its field margin. For an OF field, the increase was from 17.1 to 18.8 (0 to 50% OF in the landscape) in the field core and 19.5 to 23.7 in field margin (figure 2a).
Figure 2.
Predictions of the final model for weed species richness (α-field diversity), biodiversity partitioning (α, β, γ) of species richness for the common and less frequent species, and observed mean species richness of less frequent species in field margins (α-field diversity), depending on the % of OF in the landscape. (a) Model predictions for the response of species richness (α-field diversity) to the proportion of OF in the landscape (model M4.b) in both organic and conventional fields, and both in field margins and field cores. The model shows an increase of species richness both in OF and conventional fields, and both in field cores and margins with the % of OF in the landscape but this response is stronger for field margins. (b) Additive partitioning approach of biodiversity (more common species). The figure shows the mean α, β and γ diversity for organic and conventional fields (core and margin) with the same number of fields per category (74 fields). One thousand repetitions were done by bootstrapping and we calculated the mean and the 95% CI for species richness by class on these repetitions. This figure shows that if γ-diversity for abundant species seems equal between field cores and margins and OF and conventional fields, we observe that α-diversity of conventional field cores appears lower. (c) Additive partitioning approach of biodiversity (less frequent species). One thousand repetitions were done by bootstrapping and we calculated the mean and the 95% CI for species richness by class on these repetitions (74 fields). We observe that the diversity of rare species is mostly explained by β-field diversity, and that diversity (both α and β) appears lower in field core than in field margin and in conventional than in OF fields. (d) Mean diversity of the field margins for less frequent species depending on the percentage of OF in the landscape. The species richness of each class was calculated on the same number of fields (12 fields). One thousand repetitions were done by bootstraping, and we calculated the mean and the 95% confidence interval for species richness by class on these repetitions. We observe that diversity increase with the percentage of OF in the landscape, both for OF and conventional fields.
(b). Diversity partitioning: effect of organic farming on β-diversity
In the 74 fields of each class, we found a γ-diversity of 118 species in field margins and 90 in field cores for OF, compared with 110 and 82, respectively, in CONV fields; of all species, 40 were only found in OF fields while 22 were only found in CONV. The additive partitioning approach indicated that the largest part of the diversity was due to the β-field diversity (i.e. diversity between fields; electronic supplementary material S10), especially for less frequent species (figure 2c). For the less frequent species, γ-diversity in field margin was higher in OF (56 species) than in CONV (48 species), with a similar difference in field core (30 versus 22 species; figure 2c). The α-field diversity of less frequent species increased with the percentage of OF in the surrounding landscape, especially in field margins (figure 2d). A similar trend was observed in field cores of conventional fields (electronic supplementary material S6). For the common species (figure 2b), we did not observe any differences in γ-diversity between OF and CONV or between field margin and field core, suggesting similar species pools (electronic supplementary material S7). Diversity components of common species varied between the core field in CONV versus OF fields, with a higher contribution of the β-field diversity and lower α-plot and β-plot diversity in CONV (figure 2b), suggesting that common weeds were less frequent in CONV, leading to differences in the between-fields diversity. Altogether, these results support the positive effect of OF in the landscape on weed diversity, an effect larger in the field margins than in the core, and larger also for less frequent than for common species.
4. Discussion
(a). Weed diversity in organic and conventional wheat fields
Weed community composition is strongly affected by application of herbicides, fertilization and mechanical weed control, the latter being mostly used in organic farms [50–53]. OF fields in general harbour more insect-pollinated plants [54], forbs [55], and rare or threatened weeds [56,57], and fewer nitrophilous species [31,56], while conventional fields have fewer broad-leaved species due to the use of auxin herbicides to control them [58], and more herbicide resistant weeds, in particular grasses [59]. Though in some cases OF may not increase weed species richness [60,61], our results agree with most previous studies [11,12,33], indicating a positive effect on weed species richness (roughly +30% in the latter studies compared with +48.9% in the field core and +30% in the field margin in this study). We also found that magnitude of the difference between field core and margin was higher in CONV than in OF, in accordance with Gabriel et al. [33] and other studies that demonstrated that field boundaries can act as refugia for many weeds species including species threatened by agricultural intensification [29,62]. Our results support that the release of herbicides and the combination of less intense agricultural practices (e.g. weed harrow, reduced use of fertilizers) in OF fields may favour weed species that are not adapted to conventional systems either because of their sensitivity to chemical control or a high level of nitrogen [37,50,54]. A greater proportion of grassland in the succession may also explain this pattern, as the presence of grassland (and alfafa) tends to increase weed diversity while decreasing the relative abundance of annual weed species [63]. Therefore, at the local scale, both the agricultural practices associated with OF and the field history (crop succession) seem to act on weed richness.
(b). Regional effects are driven by the amount of organic farms in the landscape
Several studies have demonstrated the role of landscape in shaping weed communities [4,23,64–66], though in some cases this was only detected in OF and not in CONV fields [33], or even not supported [67]. In all these studies, regional effects were accounted for by semi-natural elements. In our study, we did not observe a landscape complexity effect. Instead, we found a strong landscape effect of OF that can even exceed local effects of field management. Gabriel et al. [33] also found a beneficial effect of OF in the landscape, however in the latter study the beneficial effect was only found in OF fields, contrary to our results showing positive effects for both OF and CONV fields (especially in field margins). In Gabriel et al.'s [33] study, conventional farms surrounded by organic farms used more synthetic fertilizers and herbicides than conventional farms surrounded mainly by conventional farms, possibly removing the landscape effect on CONV fields. This difference between the two studies may highlight the filtering effect of conventional management (especially chemical fertilization and herbicides) in field cores that prevent species richness from equalling that of OF, conversely in field margins where farming practices are less intensive. In addition, despite a large range of landscape complexity around focal fields, local effects (OF versus CONV) did not vary with landscape complexity, as also found by Winqvist et al. [21], and contrary to Concepción et al. [23], weed richness was not higher in intermediate landscape complexity but increased linearly with the percentage of OF in the landscape, as predicted by Bengtsson et al. [19].
(c). The role of organic fields in sustaining metacommunity dynamics
We showed that differences in weed richness between OF and CONV systems were mostly explained by the higher diversity of less frequent species in OF fields, suggesting that the main effect of OF at the landscape scale on species richness acts through the effect on less frequent species in field margins. Higher values of diversity and higher density of weeds in the seed bank of organic fields have already been reported, both in field cores and margins [50]. However, the main proposed factor determining seed bank size was crop seed origin from organic farms, which would favour the entry of weed seeds, but this argument cannot explain the increased weed diversity in CONV fields found in our study. We alternatively suggest that spatio-temporal flows of seeds influence weeds in local communities (i.e. semi-natural or crop fields) by generating mass effect [68] and source–sink dynamics [69,70]. Such dynamics involve interactions among local communities at large scales (i.e. the agricultural landscape), as in a metacommunity [25]. Among the metacommunity paradigms, the ‘species-sorting’ and ‘mass effects’ require that different patches have different conditions and be sufficiently connected to allow local coexistence of species with different performances and competitive abilities [25]. Therefore, mass effect through dispersal from field margins could act at the field scale, as previously proposed by Poggio et al. [71], while heterogeneous habitats provided by variation in farming systems across the agricultural landscape may ensure weed regional coexistence through species sorting, as suggested by Perronne et al. [35]. Spatial dispersal is not recognized as the main process involved in weed landscape dynamics, with temporal dispersion through the seed bank typically suggested as the main process as a buffer memory of past infestations [72]. However, weed species spatial dispersal by farming practices has long been present in the agroecosystem [73]. Based on our results, we propose that the persistence of species (especially the less frequent ones) in agricultural landscapes relies on two different strategies, both belonging to the storage effect [74], in response to the high disturbance regime typical of crop successions in intensively farmed landscapes. Species with high dispersal rates will benefit from variation in the occurrence of habitat disturbances across the agricultural landscape (i.e. a spatial storage effect), while other less frequent species will have a high persistence rate in the seed bank, allowing them to respond to temporal variation in habitat disturbances. The role of OF within the metacommunity dynamic would thus be twofold. First, as less intensively disturbed habitat, OF enhances the diversity of less frequent species through a temporal storage effect. Second, species loss in more intensively disturbed habitats (i.e. conventional fields) would be compensated by a spatial storage effect allowing for dispersal. Interestingly, some evidence for the storage effect hypothesis has recently been provided for weed coexistence. García De León et al. [75], in a long-term experiment, showed that the variation of climatic conditions can modify interspecific competition, for species sharing similar resource requirement (fertilization type and level) but differing by the adaptation to climate, allowing coexistence between these species to be maintained, and suggesting the importance of storage effects to maintain diversity. Moreover, using simulations, Bianchi et al. [18] showed that the response of organisms to the landscape proportion of OF may depend on the movement ability of the organisms (see also [76]), as well as on the degree of spatial aggregation of OF fields, especially at intermediate levels of proportion of OF.
5. Conclusion
Our results suggest that a major benefit of OF systems lies in the persistence, at the landscape scale, of less frequent species (see also [6,77,78]) through a metacommunity effect: OF fields, and field margins of both management types, provide habitats for less frequent weed species [56,57], and high density of OF fields enhances weed diversity in farmland landscapes. Thus, landscape heterogeneity per se is not sufficient for-maintaining regional weed diversity, but rather the finer-grain heterogeneity and availability of ruderal habitats (characteristic of OF), acting as refugia for annual plants, is the key driver. Improving such habitats may have a lower effect on crop production (i.e. less frequent species are in field margins and are rarely abundant in the field core), but may support other ecological services such as pollination [26,79,80]. Our results also suggest that biodiversity and crop production may be supported in landscapes with less intensively farmed fields according to a land-sharing strategy, although further studies incorporating weed abundance (rather than just richness) need to be conducted.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Roncorini, E. Cadet and T. Fanjas for carrying the main part of fieldwork. For very useful comments, we thank Jan Bengtsson and an anonymous reviewer, as well as the associate editor Colin Osborne.
Authors' contributions
V.B. conceived and coordinated the study. V.B., S.G., L.B. and L.H. designed the study. L.H. and H.M. carried out part of fieldwork. H.M. managed the datasets. L.H. and S.G. drafted the manuscript. L.H., L.B., S.G. and V.B. carried out the statistical analyses. All authors gave final approval for publication and contributed to the writing.
Funding
We acknowledge ANR AGROBIOSE, BIODIAVGRIM and DYNARURABIO for funding the study.
References
- 1.Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL. 2011. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 14, 101–112. ( 10.1111/j.1461-0248.2010.01559.x) [DOI] [PubMed] [Google Scholar]
- 2.Pimentel D, Stachow U, Takacs DA, Brubaker HW, Dumas AR, Meaney JJ, Onsi DE, Corzilius DB. 1992. Conserving biological diversity in agricultural/forestry systems. Bioscience 42, 354–362. ( 10.2307/1311782) [DOI] [Google Scholar]
- 3.Benton TG, Vickery J, Wilson JD. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18, 182–188. ( 10.1016/S0169-5347(03)00011-9) [DOI] [Google Scholar]
- 4.Batáry P, Báldi A, Kleijn D, Tscharntke T. 2011. Landscape-moderated biodiversity effects of agri-environmental management: a meta-analysis. Proc. R. Soc. B 278, 1894–1902. ( 10.1098/rspb.2010.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storkey J, Meyer S, Still KS, Leuschner C. 2012. The impact of agricultural intensification and land-use change on the European arable flora . Proc. R. Soc. B 279, 1421–1429. ( 10.1098/rspb.2011.1686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyvönen T. 2007. Can conversion to organic farming restore the species composition of arable weed communities? Biol. Conserv. 137, 382–390. ( 10.1016/j.biocon.2007.02.021) [DOI] [Google Scholar]
- 7.Bischoff A, Mahn E-G. 2000. The effects of nitrogen and diaspore availability on the regeneration of weed communities following extensification. Agric. Ecosyst. Environ. 77, 237–246. ( 10.1016/S0167-8809(99)00104-8) [DOI] [Google Scholar]
- 8.Stoate C, Boatman N, Borralho R, Carvalho CR, de Snoo GR, Eden P. 2001. Ecological impacts of arable intensification in Europe. J. Environ. Manage. 63, 337–365. ( 10.1006/jema.2001.0473) [DOI] [PubMed] [Google Scholar]
- 9.Henle K, et al. 2008. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe: a review. Agric. Ecosyst. Environ. 124, 60–71. ( 10.1016/j.agee.2007.09.005) [DOI] [Google Scholar]
- 10.Barzman M, Dachbrodt-Saaydeh S. 2011. Comparative analysis of pesticide action plans in five European countries. Pest Manage. Sci. 6, 1481–1485. ( 10.1002/ps.2283) [DOI] [PubMed] [Google Scholar]
- 11.Hole DG, Perkins AJ, Wilson JD, Alexander IH, Grice PV, Evans AD. 2005. Does organic farming benefit biodiversity? Biol. Conserv. 122, 113–130. ( 10.1016/j.biocon.2004.07.018) [DOI] [Google Scholar]
- 12.Tuck SL, Winqvist C, Mota F, Ahnström J, Turnbull LA, Bengtsson J. 2014. Land-use intensity and the effects of organic farming on biodiversity: a hierarchical meta-analysis. J. Appl. Ecol. 51, 746–755. ( 10.1111/1365-2664.12219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller R, et al. 2005. Benefits of organic farming to biodiversity vary among taxa. Biol. Lett. 1, 431–434. ( 10.1098/rsbl.2005.0357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson RH, Pearce S, Morris RJ, Symondson WOC, Memmott J. 2007. Plant diversity and land use under organic and conventional agriculture: a whole-farm approach. J. Appl. Ecol. 44, 792–803. ( 10.1111/j.1365-2664.2007.01292.x) [DOI] [Google Scholar]
- 15.de Ponti T, Rijk B, van Ittersum MK. 2012. The crop yield gap between organic and conventional agriculture. Agric. Syst. 108, 1–9. ( 10.1016/j.agsy.2011.12.004) [DOI] [Google Scholar]
- 16.Gabriel D, Sait SM, Kunin WE, Benton TG. 2013. Food production vs. biodiversity: comparing organic and conventional agriculture. J. Appl. Ecol. 50, 355–364. ( 10.1111/1365-2664.12035) [DOI] [Google Scholar]
- 17.Concepción ED, et al. 2012. Interactive effects of landscape context constrain the effectiveness of local agri-environmental management. J. Appl. Ecol. 10, 1365–2664. ( 10.1111/j.1365-2664.2012.02131.x) [DOI] [Google Scholar]
- 18.Bianchi FJJA, Ives AR, Schellhorn NA. 2013. Interactions between conventional and organic farming for biocontrol services across the landscape. Ecol. Appl. 23, 1531–1543. ( 10.1890/12-1819.1) [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson J, Ahnström J, Weibull A. 2005. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J. Appl. Ecol. 42, 261–269. ( 10.1111/j.1365-2664.2005.01005.x) [DOI] [Google Scholar]
- 20.Rundlöf M, Smith HG. 2006. The effect of organic farming on butterfly diversity depends on landscape context. J. Appl. Ecol. 43, 1121–1127. ( 10.1111/j.1365-2664.2006.01233.x) [DOI] [Google Scholar]
- 21.Winqvist C, et al. 2011. Mixed effects of organic farming and landscape complexity on farmland biodiversity and biological control potential across Europe. J. Appl. Ecol. 48, 570–579. ( 10.1111/j.1365-2664.2010.01950.x) [DOI] [Google Scholar]
- 22.Batáry P, Sutcliffe L, Dormann CF, Tscharntke T. 2013. Organic farming favours insect-pollinated over non-insect pollinated forbs in meadows and wheat fields. PLoS ONE 8, e54818 ( 10.1371/journal.pone.0054818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Concepción ED, Díaz M, Baquero RA. 2008. Effects of landscape complexity on the ecological effectiveness of agri-environment schemes. Landsc. Ecol. 23, 135–148. ( 10.1007/s10980-007-9150-2) [DOI] [Google Scholar]
- 24.Lüscher G, Jeanneret P, Schneider MK. 2014. Responses of plants, earthworms, spiders and bees to geographic location, agricultural management and surrounding landscape in European arable fields. Agric. Ecosyst. Environ. 186, 124–134. ( 10.1016/j.agee.2014.01.020) [DOI] [Google Scholar]
- 25.Leibold MA, et al. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. ( 10.1111/j.1461-0248.2004.00608.x) [DOI] [Google Scholar]
- 26.Marshall EJP, Brown VK, Boatman ND, Lutman PJW, Squire GR, Ward LK. 2003. The role of weeds in supporting biological diversity within crop fields. Weed Res. 43, 77–89. ( 10.1046/j.1365-3180.2003.00326.x) [DOI] [Google Scholar]
- 27.Oerke EC. 2006. Crop losses to pests. J. Agric. Sci. 144, 31–43. ( 10.1017/S0021859605005708) [DOI] [Google Scholar]
- 28.Alignier A, Bretagnolle V, Petit S. 2012. Spatial patterns of weeds along a gradient of landscape complexity . Basic Appl. Ecol. 13, 328–337. ( 10.1016/j.baae.2012.05.005) [DOI] [Google Scholar]
- 29.Fried G, Petit S, Dessaint F, Reboud X. 2009. Arable weed decline in Northern France: crop edges as refugia for weed conservation. Biol. Conserv. 142, 238–243. ( 10.1016/j.biocon.2008.09.029) [DOI] [Google Scholar]
- 30.Wilson PJ, Aebischer NJ. 1995. The distribution of dicotyledonous arable weeds in relation to distance from the field edge. J. Appl. Ecol. 32, 295–310. ( 10.2307/2405097) [DOI] [Google Scholar]
- 31.Hyvönen T, Ketoja E, Salonen J, Jalli H, Tiainen J. 2003. Weed species diversity and community composition in organic and conventional cropping of spring cereals. Agric. Ecosyst. Environ. 97, 131–149. ( 10.1016/s0167-8809(03)00177-8) [DOI] [Google Scholar]
- 32.Gabriel D, Roschewitz I, Tscharntke T, Thies C. 2006. Beta diversity at different spatial scales: plant communities in organic and conventional agriculture . Ecol. Appl. 16, 2011–2021. ( 10.1890/1051-0761(2006)016[2011:BDADSS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 33.Gabriel D, Sait SM, Hodgson JA, Schmutz U, Kunin WE, Benton TG. 2010. Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecol. Lett. 13, 858–869. ( 10.1111/j.1461-0248.2010.01481.x) [DOI] [PubMed] [Google Scholar]
- 34.Gaba S, Chauvel B, Dessaint F, Bretagnolle V, Petit S. 2010. Weed species richness in winter wheat increases with landscape heterogeneity. Agric. Ecosyst. Environ. 138, 318–323. ( 10.1016/j.agee.2010.06.005) [DOI] [Google Scholar]
- 35.Perronne R, Le Corre V, Bretagnolle V, Gaba S. 2015. Stochastic processes and crop types shape weed community assembly in arable fields. J. Veget. Sci. 26, 348–359. ( 10.1111/jvs.12238) [DOI] [Google Scholar]
- 36.Lechenet M, Bretagnolle V, Bockstaller C, Boissinot F, Petit M-S, Petit S, Munier-Jolain NM. 2014. Reconciling pesticide reduction with economic and environmental sustainability in arable farming. PLoS ONE 9, e97922 ( 10.1371/journal.pone.0097922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero A, Chamorro L, Sans FX. 2008. Weed diversity in crop edges and inner fields of organic and conventional dryland winter cereal crops in NE Spain. Agric. Ecosyst. Environ. 124, 97–104. ( 10.1016/j.agee.2007.08.002) [DOI] [Google Scholar]
- 38.Gabriel D, Carver SJ, Durham H, Kunin WE, Palmer RC, Sait SM, Stagl S, Benton TG. 2009. The spatial aggregation of organic farming in England and its underlying environmental correlates. J. Appl. Ecol. 46, 323–333. ( 10.1111/j.1365-2664.2009.01624.x) [DOI] [Google Scholar]
- 39.Burnham KP, Anderson DR. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 40.Scheipl F. 2009. amer: additive mixed models with lme4 See http://CRAN.R-project.org/package.
- 41.Wood SN. 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 99, 673–686. ( 10.1198/016214504000000980) [DOI] [Google Scholar]
- 42.Bates D, Maechler M, Bolker B. 2014. lme4: linear mixed-effects models using S4 classes R package version 1.1–6. See http://cran.r-project.org/web/packages/lme4/index.html.
- 43.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-Plus. New York, NY: Springer. [Google Scholar]
- 45.Barton K. 2011. MuMIn: multi-model inference. R package v. 1.6.5 Vienna, Austria: R Foundation for Statistical Computing; See http://CRAN.R-project.org/package=MuMIn. [Google Scholar]
- 46.Ribeiro JRPJ, Diggle PJ. 2001. geoR: a package for geostatistical analysis. See http://cran.r-project.org/web/packages/geoR/index.html.
- 47.Betts MG, Ganio LM, Huso MMP, Som NA, Huettmann F, Bowman J, Wintle BA. 2009. Comment on ‘Methods to account for spatial autocorrelation in the analysis of species distributional data: a review’. Ecography 32, 374–378. ( 10.1111/j.1600-0587.2008.05562.x) [DOI] [Google Scholar]
- 48.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. ( 10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 49.Crist TO, Veech JA, Gering JC, Summerville KS. 2003. Partitioning species diversity across landscapes and regions: a hierarchical analysis of α, β, and γ diversity. Am. Nat. 162, 734–743. ( 10.1086/378901) [DOI] [PubMed] [Google Scholar]
- 50.José-María L, Sans FX. 2011. Weed seedbanks in arable fields: effects of management practices and surrounding landscape. Weed Res. 51, 631–640. ( 10.1111/j.1365-3180.2011.00872.x) [DOI] [Google Scholar]
- 51.Doucet C, Weaver SE, Hamill AS, Zhang J. 1999. Separating the effects of crop rotation from weed management on weed density and diversity . Weed Sci. 47, 729–735. [Google Scholar]
- 52.Hyvönen T, Salonen J. 2002. Weed species diversity and community composition in cropping practices at two intensity levels—a six-year experiment. Plant Ecol. 159, 73–78. ( 10.1023/A:1015580722191) [DOI] [Google Scholar]
- 53.Hawes C, Squire GR, Hallett PD, Watson CA, Young M. 2010. Arable plant communities as indicators of farming practice. Agric. Ecosyst. Environ. 138, 17–26. ( 10.1016/j.agee.2010.03.010) [DOI] [Google Scholar]
- 54.Hald A. 1999. Weed vegetation (wild flora) of long established organic versus conventional cereal fields in Denmark. Ann. Appl. Biol. 134, 307–314. ( 10.1111/j.1744-7348.1999.tb05269.x) [DOI] [Google Scholar]
- 55.Moreby SJ, Aebischer NJ, Southway SE, Sotherton NW. 1994. A comparison of the flora and arthropod fauna of organically grown winter wheat in Southern England. Ann. Appl. Biol. 125, 13–27. ( 10.1111/j.1744-7348.1994.tb04942.x) [DOI] [Google Scholar]
- 56.Rydberg NT, Milberg P. 2000. A survey of weeds in organic farming in Sweden. Biol. Agric. Hortic. 18, 175–185. ( 10.1080/01448765.2000.9754878) [DOI] [Google Scholar]
- 57.Van Elsen T. 2000. Species diversity as a task for organic agriculture in Europe. Agric. Ecosyst. Environ. 77, 101–109. ( 10.1016/s0167-8809(99)00096-1) [DOI] [Google Scholar]
- 58.Kudsk P, Streibig JC. 2003. Herbicides: a two-edged sword. Weed Res. 43, 90–102. ( 10.1046/j.1365-3180.2003.00328.x) [DOI] [Google Scholar]
- 59.Heap I. 1997. The occurrence of herbicide-resistant weeds worldwide . Pest. Sci. 51, 235–243. () [DOI] [Google Scholar]
- 60.Kleijn D, Berendse F, Smit R, Gilissen N. 2001. Agri-environment schemes do not effectively protect biodiversity in Dutch agricultural landscapes. Nature 413, 723–725. ( 10.1038/35099540) [DOI] [PubMed] [Google Scholar]
- 61.Weibull AC, Östman Ö, Granqvist Å. 2003. Species richness in agroecosystems: the effect of landscape, habitat and farm management. Biodivers. Conserv. 12, 1335–1355. ( 10.1023/A:1023617117780) [DOI] [Google Scholar]
- 62.Smart SM, Bunce RGH, Firbank LG, Coward P. 2002. Do field boundaries act as refugia for grassland plant species diversity in intensively managed agricultural landscapes in Britain? Agric. Ecosyst. Environ. 91, 73–87. ( 10.1016/S0167-8809(01)00259-6) [DOI] [Google Scholar]
- 63.Meiss H, Médiène S, Waldhardt R, Caneill J, Bretagnolle V, Reboud X, Munier-Jolain N. 2010. Perennial lucerne affects weed community trajectories in grain crop rotations. Weed Res. 50, 331–340. ( 10.1111/j.1365-3180.2010.00784.x) [DOI] [Google Scholar]
- 64.Gabriel D, Thies C, Tscharntke T. 2005. Local diversity of arable weeds increases with landscape complexity. Perspect. Plant. Ecol. Evol. Syst. 7, 85–93. ( 10.1016/j.ppees.2005.04.001) [DOI] [Google Scholar]
- 65.Duelli P, Obrist MK. 2003. Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl. Ecol. 4, 129–138. ( 10.1078/1439-1791-00140) [DOI] [Google Scholar]
- 66.Kleijn D, Sutherland WJ. 2003. How effective are European agri-environment schemes in conserving and promoting biodiversity? J. Appl. Ecol. 40, 947–969. ( 10.1111/j.1365-2664.2003.00868.x) [DOI] [Google Scholar]
- 67.Armengot L, José-María L, Blanco-Moreno JM, Romero-Puente A, Sans FX. 2011. Landscape and land-use effects on weed flora in Mediterranean cereal fields. Agric. Ecosyst. Environ. 142, 311–317. ( 10.1016/j.agee.2011.06.001) [DOI] [Google Scholar]
- 68.Shmida A, Wilson MV. 1985. Biological determinants of species diversity. J. Biogeogr. 12, 1–20. ( 10.2307/2845026) [DOI] [Google Scholar]
- 69.Holt RD. 1985. Density-independent mortality, non-linear competitive interactions, and species coexistence. J. Theor. Biol. 116, 479–493. ( 10.1016/S0022-5193(85)80084-9) [DOI] [Google Scholar]
- 70.Pulliam HR. 1988. Sources, sinks and population regulation. Am. Nat. 132, 652–661. ( 10.1086/284880) [DOI] [Google Scholar]
- 71.Poggio SL, Chaneton EJ, Ghersa CM. 2010. Landscape complexity differentially affects alpha, beta, and gamma diversities of plants occurring in fencerows and crop fields. Biol. Conserv. 143, 2477–2486. ( 10.1016/j.biocon.2010.06.014) [DOI] [Google Scholar]
- 72.Bàrberi P, Cozzani A, Macchia M, Bonari E. 1998. Size and composition of weed seedbank under different management systems for continuous maize cropping. Weed Res. 38, 319–334. ( 10.1046/j.1365-3180.1998.00098.x) [DOI] [Google Scholar]
- 73.Benvenutti S. 2007. Weed seed movement and dispersal strategies in the agricultural environment. Weed Biol. Manage. 7, 141–157. ( 10.1111/j.1445-6664.2007.00249.x) [DOI] [Google Scholar]
- 74.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Sys. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 75.García De León D, Storkey J, Moss SR, González-Andújar JL. 2014. Can the storage effect hypothesis explain weed co-existence on the Broadbalk long-term fertiliser experiment? Weed Res. 54, 1365–3180. ( 10.1111/wre.12097) [DOI] [Google Scholar]
- 76.Schellhorn NA, Bianchi FJJA, Hsu CL. 2014. Movement of entomophagous arthropods in agricultural landscapes: links to pest suppression. Annu. Rev. Entomol. 59, 559–581. ( 10.1146/annurev-ento-011613-161952) [DOI] [PubMed] [Google Scholar]
- 77.Aavik T, Liira J. 2010. Quantifying the effect of organic farming, field boundary type and landscape structure on the vegetation of field boundaries. Agric. Ecosyst. Environ. 135, 178–186. ( 10.1016/j.agee.2009.09.005) [DOI] [Google Scholar]
- 78.Pywell RF, Heard MS, Bradbury RB, Hinsley S, Nowakowski M, Walker KJ, Bullock JM. 2012. Wildlife-friendly farming benefits rare birds, bees and plants. Biol. Lett. 8, 772–775. ( 10.1098/rsbl.2012.0367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isaacs R, Kirk AK. 2010. Pollination services provided to small and large highbush blueberry fields by wild and managed bees . J. Appl. Ecol. 47, 841–849. ( 10.1111/j.1365-2664.2010.01823.x) [DOI] [Google Scholar]
- 80.Bretagnolle V, Gaba S. 2015. Weeds for bees: a review . Agron. Sustain. Dev. ( 10.1007/s13593-015-0302-5) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.